Mechanism of the Entomotoxic Activity Induced by Araucaria Angustifolia Methanolic Extract in Nauphoeta Cinerea Lobster Cockroaches
Abstract
Araucaria angustifolia (Bert.) O. Kuntze (Araucariaceae), known as "Parana pine", is a conifer found in Southern of Brazil and it needles are used traditionally as natural insecticide and acaricide. The aim of this study was to investigate the insecticide activity of A. angustifolia needles Nauphoeta cinerea as behavior model and neuromuscular preparations. A crude methanolic extract of A. angustifolia (AAME) was prepared and the cockroaches were treated with AAME sub-lethal doses (200 and 400 µg/g of) or quercetin (40 µg/g) to investigate the insect cholinesterase, insecticide, grooming activity and neurotoxicity. The AAME was subjected to a HPLC analysis which suggested the presence of several chemical compounds, including quercetin. We have demonstrated the insecticidal activity of AAME and one of its chemical secondary metabolites, quercetin, against N. cinerea. The quercetin presence at AAME was determined by HPLC. After 24 hrs, AAME and quercetin at 800 and 80 µg/g, respectively, were effective to kill all cockroaches injected. Both AAME (200 µg/g) and quercetin (40 µg/g) at sub-lethal doses induced a significant blockage of insect acetylcholinesterase activity. AAME and quercetin also increased the grooming activity (138.11 ± 5 s/30 min and 230 ± 5 s/30 min), respectively and were able to complete inhibit the cockroach twitch tension in 120 min of recording. These results point out to a complex mechanism of insecticide activity of AAME, related at least in part to the presence of quercetin and at both central and peripheral insect nervous system.
Keywords
Araucaria angustifolia, Dopaminergic neurotransmission, Entomotoxic activity, Glutamatergic neurotransmission, Neurotoxicity
Introduction
In order to compete against the continuous threat provoked by different phytophagous insects, plants have developed a vast range of defense mechanisms that comprehend from morphological and structural characteristics to the synthesis of chemical compounds [1,2]. With the aim of repel or kill the aggressor, one of the most efficient mechanisms utilized by plants is the synthesis of secondary metabolites, such as saponins, tannins and flavonoids. The production of these chemical compounds can be both, passive or even wounded-induced [3,4]. Therefore, most part of the insecticide effect induced by natural vegetal compounds is due to the selective interaction between two or more secondary metabolites with insect nervous system [5,6]. In this respect, analogues of secondary metabolites can interfere at insect nervous system, with cellular signaling systems or vital enzymes, which include neurotransmitter synthesis, storage, release, binding, reuptake, receptor activation and function, and enzymes involved in signal transduction and blockage of metabolic pathways [7].
Araucaria angustifolia (Bert.) O. Kuntze (Araucariaceae), known as "Parana pine", is a conifer found mainly in southern Brazil (Rio Grande do Sul, Paraná and Santa Catarina states) [8,9]. Its wood has economic importance as a raw material for paper and pulp production [10]. Due to the high content of phenolic compounds, its knot powder has been used as a partial substitute for phenolic resins [11,12]. Previous ethnopharmacological research on this plant showed that A. angustifolia needles are used traditionally as natural insecticide [13] and acaricide [14] in rural communities of southern Brazil. However, to the best of our knowledge, the present study is likely to be the first suggesting the chemical constitution associated to the mechanism of insecticide activity of A. angustifolia extracts.
The aim of this study was to investigate the insecticide activity of A. angustifolia needles methanolic extract, using Nauphoeta Cinerea in vivo behavior models and neuromuscular preparation. The composition of methanolic extract was evaluated by UV spectrophotometry and the presence of quercetin was evaluated by HPLC methods.
Materials and Methods
Reagents and solutions
All chemicals and reagents used were of the highest purity and were obtained from Sigma-Aldrich, Merck, Roche, Life Technologies or Bio-Rad. Methanol HPLC grade was purchased from Tedia (Fairfield, OH, USA). Tested solutions were prepared daily by dilution in insect saline immediately before use. We called insect saline a carbonate-buffered solution prepared essentially as described by Collins and Miller [15], with the following composition in mM: 200.17 NaCl, 10.73 KCl, 0.996 MgSO4, 3.40 CaCl2, 2.14 NaHCO3 and 0.083 NaH2PO4 (pH 6.9 adjusted with 2.0 N NaOH). All drugs were administered at the third abdominal hemocoel segment, at a final volume of 20 µl, by means of a Hamilton syringe. Experiments were performed at controlled room temperature (22-25 ℃).
Experimental animals
All experiments were performed on adult male Nauphoeta cinerea cockroaches (3-4 months after adult molt). To estimate the actual doses of different compounds to be assayed in vivo, two hundred animals were previously weighted, given a final body weight of 0.5 ± 0.03 g. The animals were reared at laboratory conditions of controlled temperature (22-25 ℃) on a 12 h:12 h (light:dark cycle). All cockroaches were provided with water and dog chow ad libitum. Prior to the analysis of neurophysiological parameters, the minimum lethal dose of A. angustifolia Methanolic Extract (AAME) and its secondary metabolite quercetin were determined essentially as described by Kagabu, et al. [16].
Plant material
Araucaria angustifolia (Bert) O. Kuntze needles were collected in the rural area of São Gabriel, Rio Grande do Sul state, Brazil (30º20'18.63"S-54º19'16.83"W). After appropriate identification by a plant taxonomist, a voucher specimen was deposited (register number: HBEI 085) at Bruno Edgar Irgang Herbarium (HBEI).
Extract preparation
One kilogram of A. angustifolia needles was collected, dried in a ventilated stove at 60 ℃ and powdered in a knife mill (Marconi, Model MA-680, Piracicaba, SP and Brazil). Dried and powdered material (500 g) was extracted exhaustively with MeOH (4 × 500 ml) at room temperature. After filtration through a fine filter, the solvent was removed by rotary evaporation (Model R-220, BÜCHI Labortechnik AG, Postfach, Switzerland) under reduced pressure at < 45 ℃. The resulted semi-solid extract was lyophilized and stored at -4 ℃ until identification protocols.
Identification of extract secondary metabolites
Preliminary investigation on A. angustifolia Methanolic Extract (AAME) (15 µl, 20 µg) was performed by Thin-Layer Chromatography (TLC) on silica sheets (60F254, aluminum backed, 200 µm layer thickness, 8.0 × 5.0 cm, Merck). The presence of flavonoids and phenolic acids was investigated using the adequate development systems and revealers [17]. After development, the sheets were air dried and sprayed with the indicators in a fume hood.
Total phenolic content
The total phenolic content was measured by spectrophotometry using the Folin-Ciocalteu method, with modifications [18]. Briefly, 1.0 ml of 1.0 N Folin-Ciocalteu reagent was added to a 1.0 ml of sample and this mixture was allowed to stand for 2-5 min before the addition of 2.0 ml of 20% Na2CO3. The solution was then allowed to stand for 10 minutes before reading at 750 nm in spectrophotometer using 1 cm quartz cells. The total polyphenol content was expressed as milligram of Gallic acid equivalent per milliliter (mg GAE/ml) of each extract. The samples were analyzed in triplicate.
High Performance Liquid Chromatography (HPLC)
The results obtained on TLC sheets were complemented by HPLC analysis, which was performed on a Prominence Liquid Chromatograph Shimadzu instrument equipped with a LC-20AT pump, SIL-20A autosampler, SPD-20AT PDA detector and CTO-20A column oven (Shimadzu Corporation, Kyoto, Japan). LC Solution V. 1.24 SP1 system software was used to control the equipment and to evaluate the obtain Ed-data. The assay was conducted using a reverse-phase technique. The analyses of methanolic extract and quercetin were performed according to the method proposed by De Souza, et al. [19], which describes an isocratic elution protocol with a flow rate of 0.6 ml/min. The mobile phase was composed by a mixture of methanol and 0.16 M phosphoric acid (53:47, v/v), being prepared daily, filtered through a 0.45 µm membrane Millipore filter (Milford, MA, USA) and sonicated before use. The wavelength of the DAD detector was set to 362 nm. An ODS-Hypersil Thermo Scientific C18 column (250 × 4.6 mm × 5 µm) (Bellefonte, United States) was used. The HPLC system was operated at 25 ℃. The injection volume was 20 µl.
For samples analysis, the extracts were diluted in a solution of ethanol in a proportion of 1:100 (v/v). Quercetin authentic standard was prepared by dilution in ethanol at a concentration of 10 µg/ml. A co-injection was performed by adding the quercetin to the extract sample. All solutions were filtered through a 0.45 µm membrane filter from Millipore before injection.
Video-mounting apparatus for biological assays
For each specific biological assay, the activities were recorded during 30 min by using a video-camera (Panasonic coupled to a 50 mm Carl-Zeisslens) connected or not to an eyepiece of microscopy (Olympus, model SZ51, Germany). The camera had a frame-by-frame (60/s) and was connected to a PC (Infoway, Itautec, Brazil). Video movies were latter analyzed using a HD Writer AE 2.6T system (Panasonic) with variable speed control.
Assay for insecticide activity
The insecticidal assay against adult N. cinerea, was carried out as described by Kagabu, et al. [16]. Various concentrations of AAME and quercetin, a common phenolic compound in A. angustifolia seeds and barks [20,21], were dissolved in 20 µl insect saline and injected between the third and the fourth abdominal segments of N. cinerea. All the experiments were performed in triplicate. Five insects were used to test each dose and were kept at 22-25 ℃ for 24 hrs after injection. After this period, the minimum dose at which three or more insects were considered killed was taken as the Minimum Lethal Dose (MLD in µg). Paralyzed insects were also counted as having died.
Assay for insect cholinesterase activity
The in vitro inhibition of AchE was evaluated according to the assays described by Ellman, et al. [22] and modified by Franco, et al. [23]. The whole amount of protein was measure according to Bradford [24]. In brief, three cockroaches were injected with AAME (200 and 400 µg/g animal weight), quercetin (40 µg/g) and the well known cholinesterase inhibitor trichlorfon (40 µg/g), thirty minutes before the acetylcholinesterase analysis. The animals were previously anesthetized by chilling at -5 ℃ and their brains collected after cuticle removal. The material was mixed with 750 µl of Kpi buffer, composed of 1 M K2HPO4 and 1M KH2PO4, pH 7.0 (500 rpm/5 min/4 ℃) and 400 µl of supernatant was collected. Fifty microliter of this sample was added to 50 ml of 50 mM DTNB, 500 ml Kpi (pH 8.0) and 2.5 ml acetylcholine. The reaction was measured during 60 seconds (s) at 412 nm using a UV-Visible Spectrophotometer (model Evolution 60S, Thermo scientific, New Hampshire, USA) and analyzed by the software VISION lite (Thermo scientific).
Grooming activity
The grooming behavior of cockroaches was monitored in an opaque plastic box (29 cm × 18 cm × 13 cm) with a clear plastic cover [25] and was recorded with a camera for later analysis of motion duration. The duration of continuous grooming in seconds was measured for a 30 min period immediately following treatment. Animals had never been in the testing box previously, and it was therefore a novel environment in all cases. The temperature in the testing room was maintained at 25-30 ℃. Testing was performed 2-8 hrs after the beginning of the light cycle. Control cockroaches were injected with saline [25].
In vivo cockroach metathoracic coxal-adductor nerve-muscle preparation
To analyze peripheral neurotoxicity, in vivo cockroach metathoracic coxal-adductor muscle preparation was used [26]. Animals were immobilized by chilling and mounted, ventral side up, in Lucite holder covered with 1.0 cm soft rubber that restrained the body and provided a platform to which the metathoracic coxae could be firmly attached using entomologic needles. The left leg was then tied in the medial joint with a dentistry suture line connected to a 1.0 g force transducer (AVS Instruments, São Carlos, SP and Brazil). The transducer was mounted in a manipulator that allowed adjustment of muscle length. The exoskeleton was removed from over the appropriated thoracic ganglion. Nerve 5, which includes the motor axon to the muscle, was exposed and a bipolar electrode inserted to provide electrical stimulation. The nerve was stimulated at 0.5 Hz/5 ms, with twice the threshold during 120 min. The nerve was covered with mineral oil to prevent dryness. Twitch tension were recorded, digitalized and retrieved using a computer based software AQCAD (AVS Instruments, São Carlos, SP and Brazil). Data were further analyzed using the software ANCAD (AVS Instruments, São Carlos, SP and Brazil).
Data statistical analysis
The results were expressed as the mean ± SEM and were analyzed using Analysis of Variance (One-Way ANOVA), followed by Tukey's test as a post hoc. A p-value ≤ 0.01 or p-value ≤ 0.05 indicated significance. Statistics and graphs were made using the Software Origin Pro 8.6 (Origin Lab Corporation, MA, USA).
Results
Chemical investigation of Araucaria angustifolia methanolic extract
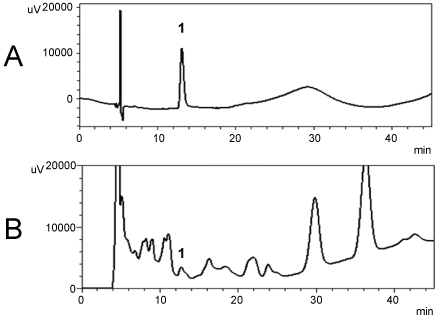
Preliminary phytochemical investigation of AAME by thin layer chromatography suggested the presence of quercetin (data not shown), which was confirmed by evaluation of the chromatograms obtained by HPLC (Figure 1). The methanolic extract was analyzed by HPLC and compared with an authentic standard. The authentic solution of quercetin showed a chromatographic peak at 12.695 min, as can be observed in (Figure 1A). The methanolic extract submitted to chromatographic separation, presented a complex chemical profile and several substances were detected in the retention time ranging from 7.5 to 20 min (Figure 1B). The AAME presented a peak at the same retention time of quercetin standard, 12.695 min and this allowed us to suggest the presence of quercetin on plant material studied.
Total phenolic content
In order to determine the total phenolic content in the studied plant material, the quantitative assay was applied according to the standardized protocol described for spectrophotometric determination. The results demonstrated a mean content of 23.27 mg GAE/ml of extract (RSD = 1.03%), illustrating a high content of phenolic in the methanolic extract of A. angustifolia.
Insecticide activity of AAME and quercetin
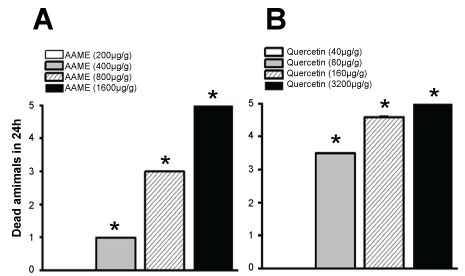
To determine the insecticidal activity of A. angustifolia, four doses of the AAME were assayed 200, 400, 800 and 1600 µg/g of animal weight. After 24 hrs, the dose of 800 µg/g of animal weight was considered as the Minimum Lethal Dose (MLD) (Figure 2A). Quercetin was also assayed at 40, 80, 160 and 320 µg/g of animal weight and showed to be lethal at 80 µg/g (Figure 2B). Once MLDs were identified, all the following biological protocols were carried out using sub-lethal concentrations.
Effect of sub-lethal doses of AAME and quercetin on brain AChE activity
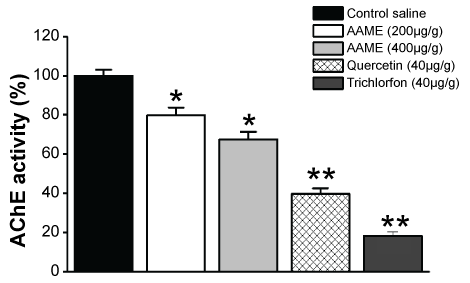
The analysis of AChE activity of cockroach brain homogenates before and after injection of different sub-lethal concentrations of AAME (200 and 400 µg/g of animal weight), quercetin (40 µg/g of animal weight) and trichlorfon (40 µg/g of animal weight), revealed a dose-dependent enzyme inhibition. The control of AChE activity with saline was 185 ± 3 nmol TNB/min/mg protein. At 200 µg/g of animal weight AAME decreased the AChE activity to 148.5 ± 6 nmol TNB/min/mg protein (n = 3). When AAME at 400 µg/g of animal weight was incubated, there was also a significant decrease of AChE activity (125.5 ± 3 nmol TNB/min/mg protein; n = 3) compared to control of saline. In addition, a significant increase in AChE inhibition was observed when animals were treated with quercetin at 40 µg/g of animal weight (73.5 ± 5 nmol TNB/min/mg protein; n = 3). Trichlorfon (40 µg/g of animal weight) administration resulted in an AChE inhibition (24 ± 6 nmol TNB/min/mg protein; n = 3) (Figure 3).
Effect of sub-lethal doses of AAME and quercetin on grooming activity
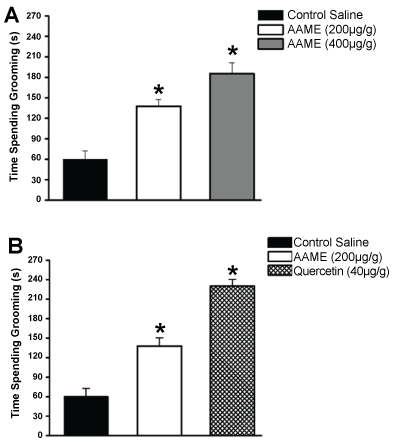
In saline injected cockroaches, the mean time of continuous grooming was 60 ± 8 s/30 min (n = 30). We found that only the manipulation of the animal and the introduction of the rod of the syringe does not significantly interfere with the normal behavior of animals (67.5 ± 12 s/30 min; n = 28). All cockroaches treated with AAME showed a dose-dependent increase in grooming activity. At 200 µg/g of animal weight, AAME induced a significant increase in grooming activity (138.11 ± 5 s/30 min; n = 28). With the highest dose assayed (400 µg/g of animal weight), the AAME increased even more the grooming parameters (185 ± 8 s/30 min; n = 30) (Figure 4A). Quercetin was assayed at 40 µg/g of animal weight, and increased the time spending grooming. This arose induced by quercetin in grooming activity was higher than the previously tested concentrations of AAME, (230 ± 5 s/30 min; n = 31) (Figure 4B).
Effect of cholinergic modulators on quercetin-induced grooming activity
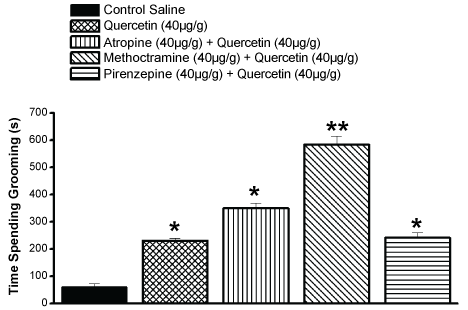
Atropine (40 µg/g of animal weight), a non-selective muscarinic cholinergic receptor inhibitor, was assayed alone in grooming activity (76 ± 2 s/30 min) and no alteration was observed in comparison with control grooming levels (75 ± 14 s/30 min). The injection of atropine (40 µg/g of animal weight) combined with quercetin (40 µg/g of animal weight) significantly increased the grooming levels to over the control values of quercetin (350 ± 3 s/30 min; n = 30) (Figure 5). When a selective inhibitor of M2-M3 cholinergic receptor, methoctramine (40 µg/g of animal weight), was combined with quercetin (40 µg/g of animal weight) the effect on the grooming pattern was pronounced (600 ± 8 s/30 min; n = 29) (Figure 5). However, pirenzepine (40 µg/g of animal weight), a selective M1-cholinergic blocker, added 15 min earlier to quercetin (40 µg/g of animal weight) induced no significant alteration in the grooming levels in comparison with quercetin alone (230 ± 5 s/30 min; n = 30) (Figure 5).
Effect of different pharmacological on quercetin-induced grooming activity
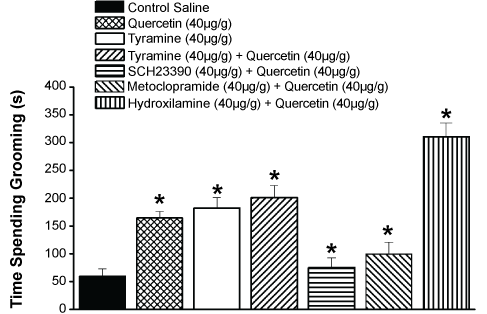
Since Dopamine (DA) activity is mediated by dopamine receptors at pre- and postsynaptic neuronal membrane (e.g., D2 and D1 receptors families), the protocols described below aimed to verify the influence of dopaminergic modulators on quercetin-induced grooming activity. Thus, metoclopramide (40 µg/g of animal weight), a DA-D2 receptor antagonist, injected 15 min before quercetin (40 µg/g of animal weight) inhibited significantly the quercetin-induced grooming activity (100 ± 12 s/30 min; n = 30) (Figure 6). When the SCH 23390 (40 µg/g of animal weight), a selective DA-D1 receptor blocker was administrated 15 min earlier to quercetin (40 µg/g of animal weight) there was also a significant inhibition of grooming levels, even below the metoclopramide treatment values (90 ± 6 s/30 min; n = 30) (Figure 6). Dopamine release depends on calcium entrance at nerve terminals in order to induce exocytosis [27]. Nitric oxide is thought to be involved in dopamine release by increasing the opening probability of L-type calcium channels [28]. We used the NO donor hydroxylamine, in order to verify the influence of NO cascade in the quercetin-induced grooming increase. Thus, hydroxylamine (40 µg/g of animal weight) alone induced a slight increase of grooming activity compared to control saline values (75 ± 8 s/30 min). However, the application of hydroxylamine (40 µg/g of animal weight) 15 min prior quercetin (40 µg/g of animal weight) induced a significant increase in grooming activity in comparison with quercetin alone (325 ± 15 s/30 min; n = 30) (Figure 6). In order to verify the influence of catecholamine in the quercetin-induced increase of grooming behavior we used tyramine, an agonist of tyramine receptors at insect nerve terminals. Tyramine (40 µg/g of animal weight) alone induced a high increase of grooming activity compared to control saline values (182 ± 8 s/30 min, n = 30). The grooming activity was increased compared with quercetin control when tyramine (40 µg/g of animal weight) was applied 15 min prior quercetin (40 µg/g of animal weight) (204 ± 15 s/30 min; n = 30) (Figure 6).
Neuromuscular blockade induced by AAME and quercetin in in-vivo cockroach nerve-muscle preparation
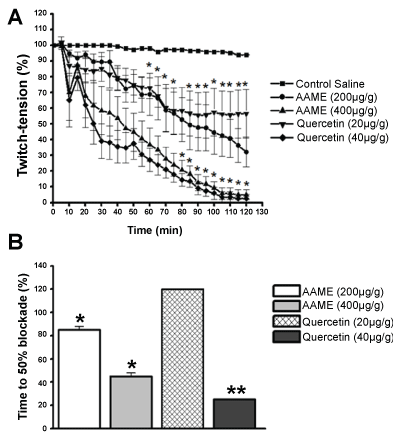
To analyze the effect of AAME and quercetin on cockroach peripheral nervous system, we used the in vivo metathoracic coxal-abductor nerve-muscle preparation. The administration of insect saline did not interfere with neuromuscular responses during 120 min recordings (n = 6) (Figure 7). The injection of AAME (200 and 400 µg/g of animal weight) induced a dose and time-dependent neuromuscular blockade in 120 min recordings. AAME at 200 µg/g of animal weight induced a blockade of twitch tension in 82 min equal to 50 ± 9% (n = 6) and 68 ± 6% in 120 min recordings (Figure 7). The injection of the highest concentration of AAME (400 µg/g of animal weight) induced 50 ± 5% and 95 ± 3% inhibition of the twitches in 45 and 120 min recordings, respectively (n = 6) (Figure 7).
The quercetin was assayed (20 and 40 µg/g of animal weight) and the same dose and time-dependent effect was observed at neuromuscular parameters. At the lowest concentration assayed (20 µg/g of animal weight), there was only 45 ± 15% neuromuscular blockade in 120 min recordings (n = 6) compared with control saline (Figure 7). When the highest concentration was assayed, 50 ± 13% inhibition of the twitch tension at 25 min and complete inhibition of the muscle strength at 120 min was observed (n = 6) (Figure 7). In all preparations, the increase of the frequency of electrical stimulation was unable to recovery the neuromuscular function.
Discussion
In this study, we have demonstrated for the first time the insecticide activity of Araucaria angustifolia needles extract and one of its constituent quercetin. The toxic activity induced by the AAME was related to a neurotoxic action, probably by the inhibition of insect Acetylcholinesterase (AChE). In this regard, a number of plants have been ascribed as having insecticide activity by acting mainly on insect nervous system [29-32], however, few studies have demonstrated a direct correlation between inhibition of insect AChE and toxicity [33]. Acetylcholinesterase plays an important role in cholinergic synapses that is essential for insects and higher animals [34]. Inhibition of AChE causes accumulation of acetylcholine at the synapses, resulting in a state of permanent stimulationof postsynaptic membrane, which results in ataxia, i.e., general lack of coordination in the neuromuscular system and eventual death [35,36]. We also investigated the composition of secondary metabolites in the AAME. We found a great amount of phenolic compounds, such quercetin in the extract. Phenolic compounds are involved on essential functions and interfere in several biological systems, mainly due their known antioxidant potential with hydrogen-donating radical scavenger properties [37,38]. Furthermore, quercetin was identified in the crude extract by comparison with the quercetin authentic standard. In our experimental conditions, quercetin has also demonstrated inhibitory actions on cockroach brain AChE, corroborating the previous literature for mammals [39] and insects [17]. This observation reinforces the relevance of quercetin in the insecticide activity of A. angustifolia. Therefore, to the best of our knowledge, it is the first time that the quercetin was assayed for insecticide activity in cockroaches. This may improve the biotechnological applications of the AAME, especially because quercetin is an important antioxidant compound of humans dietary [40].
AAME showed a lethal effect to cockroaches at relatively moderated concentrations, confirming the traditional knowledge [13]. Many plant extracts have demonstrated insecticidal activity [7] and their mode of action and side effects for the insecticide activities have been studied [1,41-43]. In this respect, insecticides exert a broad range of effects on insects and other arthropods, such as neuroexcitation resulting in hyperactivity, tremor and rigid paralysis due to energy depletion and neuromuscular fatigue, while neuroinhibition results in immobility and paralysis because of possible oxygen deprivation and/or reduced respiratory capacity that ultimately leads to death [44,45]. Because these effects is important to investigate behavioral patterns in insects to elucidate the mode of action of novel and conventional insecticides and their response in the environment by minimizing their contact with the toxic material [46].
The effects induced by AAME and quercetin on grooming activity showed to be similar. In insects aneural center involved in grooming behavior is not well identified but, it was demonstrated that Dopamine (DA) may act as the main neurotransmitter associated with this response [25]. Signaling by extracellular DA is regulated by several mechanisms, and a Muscarinic (M) cholinergic mechanism probably underlies the activation of serotoninergic/dopaminergic interneurons [47]. Indeed, in our experimental conditions, the previous treatment with atropine, an unspecific blocker of muscarinic receptors, followed by quercetin increased the quercetin-induced grooming behavior. Therefore, where muscarinic stimulation is effective in insects brain, behavioral changes are evoked by forskolin, an activator of Adenylate Cyclase (AC); 8-Br-cAMP-activating Protein Kinase A (PKA); and 3-isobuty-1-methylxanthine, leading to the accumulation of endogenously generated cAMP through inhibition of phosphodiesterases. This suggests that the mAChRs mediated-excitation occurs by stimulating the AC/cAMP/PKA pathway. Because the inhibition of AC, PKA or PLC by various individually applied substances entirely suppressed muscarine-evoked behavior parameters in insects, activation of both pathways, AC/cAMP/PKA and PLC/IP3/diacylglycerine, appeared to be necessary to mediate the excitatory effects of mAChRs [48]. Furthermore, activation of insect mAChRs by muscarinic agonists demonstrated to increase cytosolic IP3 and Ca2+ concentrations, showing selectivity for antagonists that are similar to vertebrate M1 and M3 subtypes but distinct from M2 AChRs [49,50]. Our data agreed with the literature, since pirenzepine counteract the quercetin-induced increase in grooming activity.
In addition, at nerve terminals, Phosphatidylinositol (PI) 3-kinase is involved in DA transporter activation and the degradation of (IP3) [51]. Quercetin inhibit phosphatidylinositol 3-kinase [52], reinforcing that the AAME modulation of insect behavior is likely to involve the increase of IP3 signaling, following activation of mAChRs.
We also investigated the influence of tyramine and hydroxylamine on quercetin-induced increase of cockroach grooming behavior. Tyramine activates Tyramine receptors (TyrRs) at insect central nervous system, modulating insect behavior by increasing cytosolic Ca2+ and IP3 signaling [53]. However, in our experimental conditions, tyramine was unable to arise quercetin-induced increase of grooming behavior, suggesting that TyrRs are probably not involved in this effect. Regarding hydroxylamine, a Nitric Oxide (NO) regenerator [54]. NO is a membrane-permeant messenger molecule generated from the amino acid L-arginine andcan activate soluble guanylyl cyclase leading to the formation of Cyclic GMP (cGMP) in target cells [55]. NO is an atypical neurotransmitter since it is not packaged in synaptic vesicles, rather it diffuses from its site of production and moves readily through cell membranes. The principal function of NO is activating the heterodimeric heme protein, the Soluble Guanylyl Cyclase (sGC). The activation of guanylyl cyclase in insect neurons is related to excitatory process of neurotransmission by modulation of intracellular Ca2+ stores [55]. We suggest that hydroxylamine potentiate the quercetin-induced increase in grooming behavior in cockroaches by a synergism in Ca2+ signaling pathway at dopaminergic nerve terminals.
In addition, it was investigated if the insecticide effect of AAME was due to a direct action on cockroach peripheral nervous system. We confirmed a significant blockage of neuromuscular twitches by AAME and quercetin using in vivo cockroach nerve-muscle preparations. In neuromuscular junctions in insect, glutamate is the main excitatory neurotransmitter and GABA(A,B) is the main inhibitory one. The increase of neuromuscular blockade induced by both AAME and quercetin resemble drugs that act direct on receptor ion channels. In these junctions, there are mainly two types of ionotropic receptors. The first is the NMDA receptor which is activated by the excitatory neurotransmitter glutamate and the second is GABAA receptor which is activated by the inhibitory neurotransmitter gamma-aminobutyric-acid [56]. Which receptor AAME and quercetin is preferable targeting was not investigated, however, blockage of NMDA and/or activation of GABAA receptors cannot be disregarded. Some plant natural compounds are able to modulate both NMDA [57] and GABA receptors [58]. In murine, quercetin and its derivatives can indeed induce selective inhibitory actions on NMDA receptors [59].
Conclusion
In this paper we demonstrated the insecticidal activity of A. angustifolia methanolic extract, using N. cinerea as experimental model. This result confirms the traditional knowledge for A. angustifolia as a natural insecticide. The insecticidal activity of AAME was mainly related to its inner anticholinesterase activity in insects, but also at a direct action of the AAME chemical compounds on insect neuromuscular junctions. The anticholinesterase activity of the AAME is probably dueto the presence of quercetin in its constitution. Anti-AChE activity initiates a cascade of events that probably begins with the activation of insect muscarinic receptors. This process causes a modulation of insect behavior by changing the dopaminergic pathways at insect central nervous system. The inhibitory actions of AAME and quercetin at insect neuromuscular junction should be exploited in a future detailed investigation, but inhibition of NMDA receptors is suggested.
Acknowledgments
The authors thank the Brazilian Antarctic Program through CNPq (process no. 574018/2008, FAPERJ (process no. E-26/170.023/2008) Ministry of science and Technology-MCT, Ministry of Environment-MMA, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES Edital Toxinologia 063/2010 and CIRM through INCT-APA for financial support. T.C. was granted by PROBIC-FAPERGS and CAPES fellowships. We also would like to thank Dr. Jeferson Luis Franco and Ana Paula Zemolin for technical assistance related to the acetylcholinesterase activity analysis.
Conflict of Interests
The authors declare that there is no conflict of interests regarding this work.
References
- Vandenborre G, Smagghe G, Van Damme EJ (2011) Plant lectins as defense proteins against phytophagous insects. Phytochemistry 72: 1538-1550.
- Carrazoni T, de Avila Heberle M, Perin AP, et al. (2016) Central and peripheral neurotoxicity induced by the Jack Bean Urease (JBU) in Nauphoeta cinerea cockroaches. Toxicology 368-369: 162-171.
- Bown AW, Macgregor KB, Shelp BJ (2006) Gamma-aminobutyrate: Defense against invertebrate pests? Trends Plant Sci 11: 424-427.
- Pavarini DP, Pavarini SP, Niehues M, et al. (2012) Exogenous influences on plant secondary metabolite levels. Animal Feed Science and Technology 176: 5-16.
- Adeyemi MM, Adebote DA, Amupitan JO, et al. (2010) Antifeedant activity of quercetin isolated from the stem bark of Bobgunnia madagascariensis (desv.) J.H. Kirkbr and Wiersema. (Caesalpiniaceae). Aust J Basic & Appl Sci 4: 3342-3346.
- Nishida R (2014) Chemical ecology of insect-plant interactions: ecological significance of plant secondary metabolites. Biosci Biotechnol Biochem 78: 1-13.
- Rattan RS (2010) Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Protection 29: 913-920.
- Handro W (1986) Araucaria (Araucaria spp.). In: YPS Bajaj, Biotechnology in Agriculture and Forestry. Springer-Verlag, New York, 310-315.
- Kersten RA, Borgo M, Galvão F (2015) Floresta Ombrófila Mista: Aspectos fitogeográficos, ecológicos e métodos de estudo. In: Eisenlohr PV, Felfili JM, Melo MMR, Andrade LA, Meira Neto JAA, Fitossociologia no Brasil: métodos e estudos de casos. Viçosa: Editora UFV, 156-182.
- Lorenzi H (1992) Árvores brasileiras: Manual de identificação e cultivo de plantas arbóreas nativas do Brasil. Plantarum, 378.
- Anderegg RJ, Rowe JW (1974) Lignans, the major component of resin from Araucaria angustifolia knots. Holzforschung 28: 171-175.
- Campello JDP, Fonseca SF (1975) Diterpenes from Araucaria angustifolia. Phytochemistry 14: 2299-2300.
- Arcego MSC (2005) Plantas Medicinais no Controle de Doenças no Gado Leiteiro. São João da Urtiga 1-11.
- Castro KNC (2009) Evaluation in vitro of brazilian pine tree extract for cattle tick control. Rev Bras Agroecol 4: 2575-2578.
- Collins C, Miller T (1977) Studies on the action of biogenic amines on cockroach heart. Journal of Experimental Biology 67: 1-15.
- Kagabu S, Murase Y, Imai R, et al. (2007) Effect of substituents at the 5-position of the pyridine ring of imidacloprid on insecticidal activity against Periplaneta americana. Pest Manag Sci 63: 75-83.
- Pontual EV, Napoleao TH, Dias de Assis CR, et al. (2012) Effect of Moringa oleifera flower extract on larval trypsin and acetylcholinesterase activities in Aedes aegypti. Arch Insect Biochem Physiol 79: 135-152.
- Nurmi K, Ossipov V, Haukioja E, et al. (1996) Variation of total phenolic content and individual low-molecular-weight phenolics in foliage of mountain birch trees (Betula pubescens ssp.tortuosa). J Chem Ecol 22: 2023-2040.
- De Souza KC, Schapoval EE, Bassani VL (2002) LC determination of flavonoids: Separation of quercetin, luteolin and 3-O-methylquercetin in Achyrocline satureioides preparations. J Pharm Biomed Anal 28: 771-777.
- Cordenunsi BR, De Menezes Wenzel E, Genovese MI, et al. (2004) Chemical composition and glycemic index of Brazilian pine (Araucaria angustifolia) seeds. J Agric Food Chem 52: 3412-3416.
- Seccon A, Rosa DW, Freitas RA, et al. (2010) Antioxidant activity and low cytotoxicity of extracts and isolated compounds from Araucaria angustifolia dead bark. Redox Rep 15: 234-242.
- Ellman GL, Courtney KD, Andres V, et al. (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology 7: 88-95.
- Franco JL, Posser T, Mattos JJ, et al. (2009) Zinc reverses malathion-induced impairment in antioxidant defenses. Toxicol Lett 187: 137-143.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248-254.
- Weisel-Eichler A, Haspel G, Libersat F (1999) Venom of a parasitoid wasp induces prolonged grooming in the cockroach. J Exp Biol 202: 957-964.
- Full R, Stokes D (1998) Energy absorption during running by leg muscles in a cockroach. J Exp Biol 201: 997-1012.
- Rozov A, Burnashev N, Sakmann B, et al. (2001) Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J Physiol 531: 807-826.
- Buyukuysal RL (1997) Effect of nitric oxide donors on endogenous dopamine release from rat striatal slices. I: Requirement to antioxidants in the medium. Fundam Clin Pharmacol 11: 519-527.
- Bloomquist JR (1996) Ion channels as targets for insecticides. Annu Rev Entomol 41: 163-190.
- Richards AG, Cutkomp LK (1945) The cholinesterase of insect nerves. Journal of Cellular and Comparative Physiology 26: 57-61.
- Soderlund DM, Knipple DC (1995) Actions of insecticides on sodium channels: multiple target sites and site-specific resistance. In: JM Clark, Molecular Action of Insecticides on Ion Channels. American Chemical Society, Washington, USA, 97-108.
- Duke SO, Cantrell CL, Meegapala KM, et al. (2010) Natural Toxins for Use in Pest Management. Toxins (Basel) 2: 1943-1962.
- Hieu TT, Kim SI, Ahn YJ (2012) Toxicity of Zanthoxylum piperitum and Zanthoxylum armatum oil constituents and related compounds to Stomoxys calcitrans (Diptera: Muscidae). J Med Entomol 49: 1084-1091.
- Fournier D, Mutero A (1994) Modification of acetylcholinesterase as a mechanism of resistance to insecticides. Comp Biochem Phys 108: 19-31.
- Aygun D, Doganay Z, Altintop L, et al. (2002) Serum acetylcholinesterase and prognosis of acute organophosphate poisoning. J Toxicol Clin Toxicol 40: 903-910.
- Singh K, Singh DK (2000) Toxicity to the snail Limnaea acuminata of plant-derived molluscicides in combination with synergists. Pest Management Science 56: 889-898.
- Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20: 933-956.
- Urquiaga I, Leighton F (2000) Plant polyphenol antioxidants and oxidative stress. Biol Res 33: 55-64.
- Jung M, Park M (2007) Acetylcholinesterase inhibition by flavonoids from Agrimonia pilosa. Molecules 12: 2130-2139.
- Cartea ME, Francisco M, Soengas P, et al. (2010) Phenolic compounds in Brassica vegetables. Molecules 16: 251-280.
- Chaidir, Hiort J, Nugroho BW, et al. (1999) New insecticidal rocaglamide derivatives from flowers of Aglaia duperreana (Meliaceae). Phytochemistry 52: 837-842.
- Hiort J, Chaidir, Bohnenstengel FI, et al. (1999) New insecticidal rocaglamide derivatives from the roots of Aglaia duperreana. J Nat Prod 62: 1632-1635.
- Michiels K, Van Damme EJ, Smagghe G (2010) Plant-insect interactions: what can we learn from plant lectins? Arch Insect Biochem Physiol 73: 193-212.
- Scharf MB, Baumann M, Berkowitz DV (2003) The effects of sodium oxybate on clinical symptoms and sleep patterns in patients with fibromyalgia. J Rheumatol 30: 1070-1074.
- de França SM, Breda MO, Barbosa DRS, et al. (2017) The Sublethal Effects of Insecticides in Insects, Biological Control of Pest and Vector Insects. In: Vonnie DC Shields, Agricultural and Biological Sciences. InTech, 358.
- von Keyserlingk DG, Niemann K, Wasel J, et al. (1985) A new method in computer-assisted imaging in neuroanatomy. Acta Anat (Basel) 123: 240-246.
- Buhl E, Schildberger K, Stevenson PA (2008) A muscarinic cholinergic mechanism underlies activation of the central pattern generator for locust flight. J Exp Biol 211: 2346-2357.
- Wenzel B, Elsner N, Heinrich R (2002) mAChRs in the grasshopper brain mediate excitation by activation of the AC/PKA and the PLC second-messenger pathways. J Neurophysiol 87: 876-888.
- Honda H, Tomizawa M, Casida JE (2007) Insect muscarinic acetylcholine receptor: pharmacological and toxicological profiles of antagonists and agonists. J Agric Food Chem 55: 2276-2281.
- Millar NS, Baylis HA, Reaper C, et al. (1995) Functional expression of a cloned Drosophila muscarinic acetylcholine receptor in a stable Drosophila cell line. J Exp Biol 198: 1843-1850.
- Carvelli L, Moron JA, Kahlig KM, et al. (2002) PI 3-kinase regulation of dopamine uptake. J Neurochem 81: 859-869.
- Nanua S, Zick SM, Andrade JE, et al. (2006) Quercetin blocks airway epithelial cell chemokine expression. Am J Respir Cell Mol Biol 35: 602-610.
- Farooqui T (2007) Octopamine-mediated neuromodulation of insect senses. Neurochem Res 32: 1511-1529.
- Antoine MH, Ouedraogo R, Sergooris J, et al. (1996) Hydroxylamine, a nitric oxide donor, inhibits insulin release and activates K+ATP channels. European Journal of Pharmacology 313: 229-235.
- Bicker G (2001) Nitric oxide: An unconventional messenger in the nervous system of an orthopteroid insect. Arch Insect Biochem Physiol 48: 100-110.
- Osborne RH (1996) Insect neurotransmission: Neurotransmitters and their receptors. Pharmacol Ther 69: 117-142.
- Karangwa C, Esters V, Tits M, et al. (2007) Characterization of the neurotoxicity induced by the extract of Magnistipula butayei (Chrysobalanaceae) in rat: Effects of a new natural convulsive agent. Toxicon 49: 1109-1119.
- Wang Z, Kai L, Day M, et al. (2006) Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron 50: 443-452.
- Mehdizadeh M, Taghi Joghataei M, Nobakht M, et al. (2010) The beneficial effect of the flavonoid quercetin on behavioral changes in hemi-Parkinsonian rats. Basic and Clinical Neuroscience 1: 30-32.
Corresponding Author
Chariston André Dal Belo, PhD, Interdisciplinary Center for Biotechnology Research (CIPBIOTEC), Federal University of Pampa (UNIPAMPA), Av. Antônio Trilha, 1847-Centro, São Gabriel-RS, Brazil, Tel: +55-55-3232-0850.
Copyright
© 2017 Carrazoni T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.