Evidences towards a Genetic Differentiation and Karyotype Evolution of the Sedges Cyperus odoratus L and C Ligularis L (Cyperaceae)
Abstract
Cyperaceae is composed of pioneer species and presents a complex taxonomy, with genera like Cyperus presenting specific complexes. The aim of this study was to analyze the genetic diversity between the broadly distributed sedges Cyperus ligularis L. and C. odoratus L using DNA fingerprinting markers and cytogenetic features, focusing on collection sites from an Atlantic Forest reserve and its surroundings in the city of Recife (Pernambuco, Brazil). Both the significative genetic diversity (GST = 0.363) and the low gene flow (Nm = 0.877) were found and indicated a clear genetic differentiation between the two species. Moreover, the clustering analysis showed the presence of two distinct genetic groups, also suggesting no evidence of hybridization between C. ligularis and C. odoratus. Regarding the phenogram topology, the species are also two clear different lineages, and although all individuals of C. odoratus were collected in nearby plots, they showed a greater intraspecific genetic variability than that observed among individuals of C. ligularis, which were sampled in a wider geographic range. However, the variation in chromosome numbers within the two species showed an opposite pattern, indicating higher karyotype stability in C. odoratus. Furthermore, the lowest genetic differentiation in C. ligularis may reflect a founder effect associated with the ability to disperse seeds and colonize multiple areas. Further field observations, as well as an analysis of reproductive biology, should enrich the understanding of the genetic structure of the populations investigated and the role of these plants in successional processes.
Keywords
Hybridization, DNA amplification fingerprinting, Genetic differentiation, Agmatoploidy
Introduction
The family Cyperaceae (sedges) presents several taxonomic uncertainties with the presence of some species complexes, most of them described for the genus Carex, such as Carex flava and Carex macrocephala species complexes [1-3] among others. For other Cyperaceae genera, there are few records of species complexes as seen in Rhynchospora (e.g. Rhynchospora glomerata) [4]. Regarding the genus Cyperus, which is among the largest genera of the family, is considered complex, with most of the controversial groups inhabiting the tropical regions [5], mainly in Brazil, with about 100 species registered in varied vegetation types [6]. In the most recent phylogenetic approaches, based on morphology and molecular data [7,8] Cyperus was not recovered as a monophyletic group, being divided in the monophyletic clade "Cyperus C4" and the paraphyletic clade "Cyperus C3". The complexity of the genus Cyperus is also seen at the infrageneric relationships level, for instance, in the C. rigens group [8].
It has been suggested that some degree of cross-species hybridization might occur between sympatric populations of the widely distributed group of articulated spikes, which comprises highly variable species as Cyperus ligularis L. and C. odoratus L [9]. However, analyzing the internal anatomy of leaves of these two species [10] did not find any hybridization evidence. Species delimitation analyzes in Cyperaceae have been clarifying intraspecific relationships within difficult genera by accessing DNA fingerprinting profiles and karyotype features, as in the case of species of Carex and Schoenoplectus [11-13]. Therefore, we relied on a genetic characterization of sympatric individuals of both C. ligularis and C. odoratus, by means of DNA Amplification Fingerprinting (DAF) and cytogenetic analyses, to access their diversity and access the relationship between both species.
Materials and Methods
Plant material
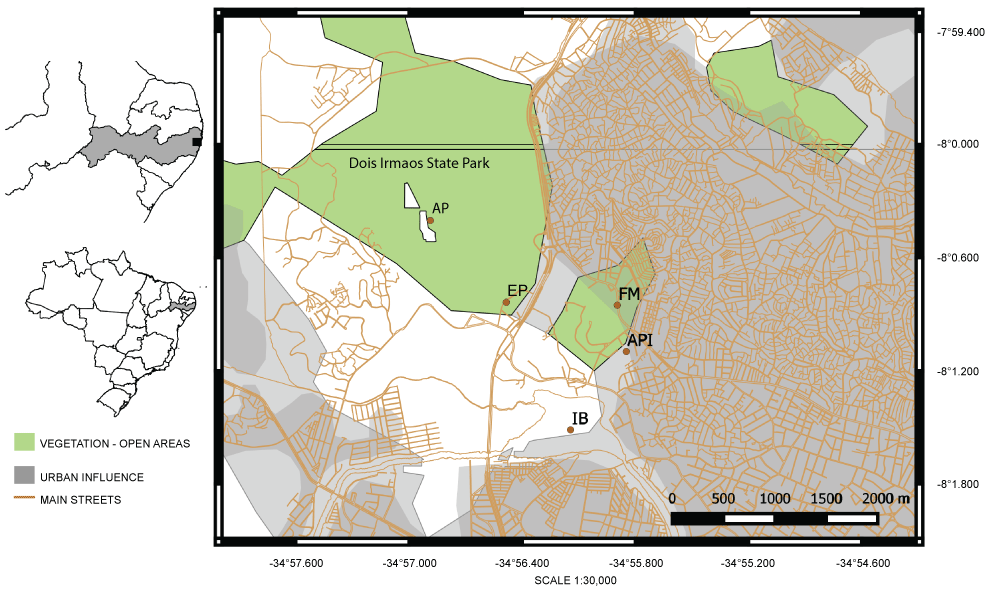
For molecular analysis, young leaves were collected from 40 individuals of C. odoratus (20) and C. ligularis (20) populations in five collection plots in open spaces and on the border of an urban fragment of the Atlantic Forest fragment (Table 1), with a minimum distance of three meters among individuals (Figure 1). For cytogenetic analyses, young root tips of both species were collected, pre-treated with 2 mM 8-hidroxiquinolein for 4 h at 18 °C, and then fixed in ethanol: acetic acid (3:1) for 4-6 h at room temperature (ca. 25 °C).
Molecular data analyzes
DNA extraction procedures followed the CTAB protocol as described by [14], with minor modifications. The DAF analysis was carried out following [15] with modifications introduced by [16,17]. The PCR reactions with a final volume of 15 L consisted of 0.5 ng/μL DNA template, 1.5 μL 10 × PCR buffer, 2.5 mM MgCl2, 10 mM dNTP-mix (Fermentas), 20 μM primer (Table 2) and 1.0 U Taq polymerase (Fermentas). The DNA amplifications were performed in an Eppendorf Mastercycler Gradient thermocycler, using a program consisting of 2 min initial denaturation step (95 °C), followed by 40 cycles of 15 s at 95 °C, 1 min at 35 °C and 2 min at 72 °C, and the reactions were completed by a final extension step of 7 min at 72 °C. PCR products were separated by electrophoresison 1.8% agarose gel and stained with 0.5 g/mL ethidium bromide.
The selection of the most informative primers was performed from aninitial set of 50 DAF primers (Table 2) tested on four random individuals (two of each species), using as parameters the number of amplified loci and polymorphic bands. The 10 most informative primers were used to amplify the DNA of the 40 individuals of both species. The genotyping process resulted in a binary matrix of presence (1) and absence (0) of clear amplified DNA fragments.
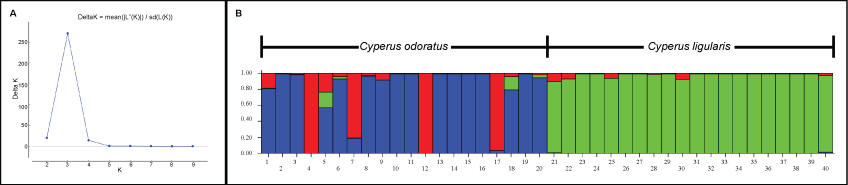
In order to check how much close the species are the genetic dissimilarity analysis was performed according to the Jaccard's coefficient [18] using DAR win 5.0 program [19], to generate a phenogram based onthe weighted neighbor-joining algorithm (2000 replications of boots trap). The genetic differentiation between the two species (here represented by two populations) was accessed by GST, a statistical index which is appropriate to analysis where the contribution of genetic drift among population's differences is not of interest [20]. Moreover, according to [21], the genetic differentiation among populations is directly related to the migration, which was accessed through the number of migrants (Nm). The GST and Nm were calculated using the Pop gene 3.2 program [22] based on 1000 simulations. The genetic clusters were verified to assure whether there is evidence of interspecific hybridization between the two species. For this purpose, the software Structure 2.3.3 [23] was used through the Bayesian method where the K value was identified and analyzed to obtain the level of genetic mixing between the two species. The interaction between individuals was measured based on 600,000 replicates (60,000 in the burning stage), using the admixture model. The number of genetic clusters was inferred through the index described by Evanno, et al. [24].
Cytogenetic analyses
Slides were prepared as described by [25], with modifications. Root tips were digested in an aqueous solution of 2% cellulose (Calbiochem 21947) and 20% pectinase (Sigma) solution for 90 min at 37 °C. Meristems were squashed in 45% acetic acid and frozen in liquid nitrogen to remove the coverslip. Then, slides were aged for three days at room temperature, stained with CMA (0.5 mg/mL, 1 h) and DAPI (2 g/mL, 30 min), mounted in McIlvaine's buffer (pH 7.0): glycerol (1:1, v/v) and stored for another three days, according to [26]. After image capture of ca. 100 cellsof each species (with a Leica DMLB epifluorescence and an Olympus SP350 camera), slides were destained in ethanol:acetic acid (3:1, v/v) for 30 min, followed by immersion in ethanol for 1 h. Then, chromosome preparations were hydrolyzed in 5 N HCl at room temperature for 20 min and stained with 2% Giemsa over 15 min and mounted with Entellan (Merck), for further image acquisition. Silver nitrate (AgNO3) impregnation of interphase nuclei followed the protocol described by [27], with the modifications described in [28]. Thirty microliters of 50% silver nitrate were added to the preparations, which were covered with a piece of nylon mesh (24 × 32 cm), incubated at 37 °C for 40 min in a moisture chamber, being subsequently washed in distilled water and mounted with Entellan. For the chromosome counting and the silver nitrate impregnation approaches, atleast 100 and 1000 cells were analyzed per species, respectively.
Results and Discussion
A total of 172 polymorphic bands were observed at intra and interspecific levels (Table 3). The C. odoratus population presented the highest polymorphism level, represented by 124 polymorphic loci (Table 3). According to [29], for a reliable estimation of genetic diversity with dominant markers, at least 50 polymorphic loci are necessary for both resolving tree topologies and differentiating populations. The authors conclude that there is a direct relationship between the consistency of the genetic diversity analysis and the number of obtained polymorphic loci. Therefore, our results for the two analyzed Cyperus species are well supported, considering the relatively high number of sampled loci, despite the low number of sampled individuals.
Genetic dissimilarity and clustering analyzes
As previously mentioned, there are evidences of natural interspecific hybridization among sympatric populations of related Cyperus species [30], as suggested for C. odoratus and C. ligularis by [9]. Therefore, considering this hypothesis, we analyzed the genetic dissimilarity between these two species.
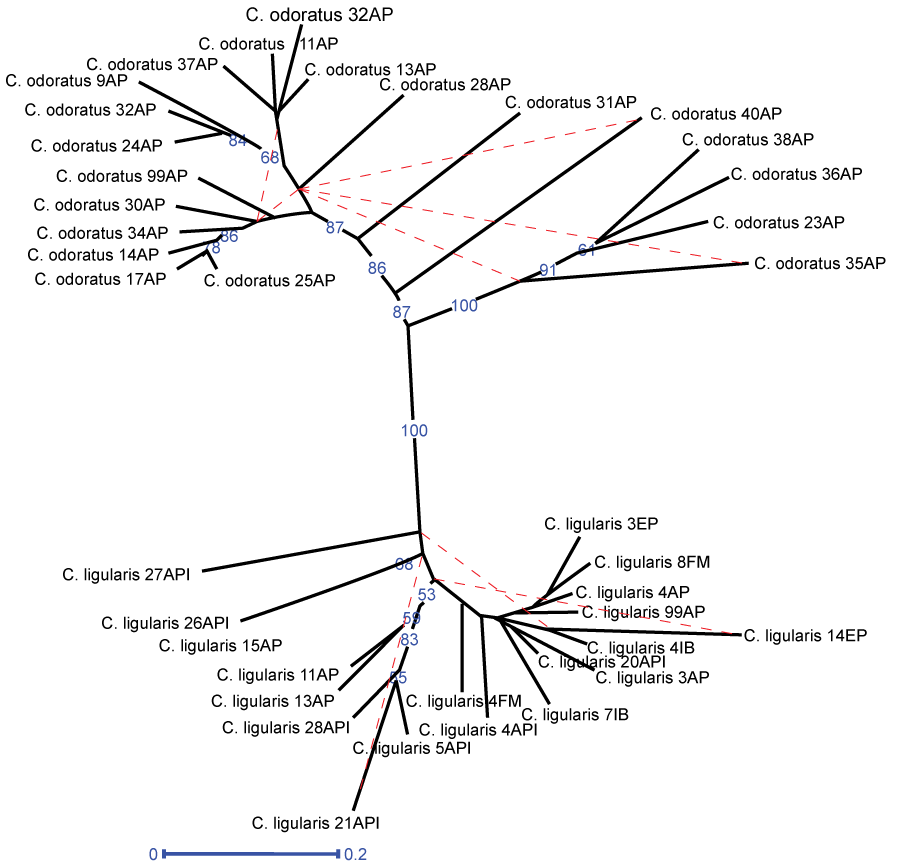
The analysis of population genetic differentiation (GST) and gene flow (Nm) between the two sampled species revealed a clear differentiation between the two species, with GST = 0.363 and Nm = 0.877, considering that values of GST > 0.25 [31] and Nm < 1 indicate a favorable scenario for genetic differentiation [32]. Moreover, the Bayesian clustering analysis also indicated no evidence of hybridization between the species, although some individuals of C. ligularis and C. odoratus presented subtle evidences of ancestral mixing, which also may be associated with the highly polymorphic nature of the DAF markers. Interestingly, three genetic clusters were observed, being two distinct groups between C. odoratus and C. ligularis (Figure 2). In addition, the neighbor-joining phenogram (Figure 3) showed two main clades clearly separating C. odoratus and C. ligularis in different clusters, confirming the ITS-based molecular phylogeny by [8] and the internal anatomy analysis by Martins and Alves [10], respectively.
Regarding the intra specific relationships, the individuals of C. odoratus presented a higher level of differentiation when compared with individuals of C. ligularis (Table 3). The clustering data also makes this clear, where C. odoratus presents two predominant genetic clusters (Figure 2-clusters red and blue) showing a higher level of genetic differentiation. Moreover, a higher level of genetic differentiation was also observed in the neighbor-joining tree (Figure 3), once several subclades with good support were separated from each other. All specimens of C. odoratus were collected in the vicinity of two dykes in the Dois Irmãos natural reserve. In turn, the C. ligularis individuals were found in various habitats, including the same as C. odoratus, and in different heavily perturbed open areas located nearby (Figure 1 and Figure 3). The variability observed for C. odoratus may be related to higher levels of intraspecific polymorphism due toseedling recruitment, as previously reported for C. papyrus L. [33]. On the other hand, the lower genetic differentiation observed in C. ligularis may be related to vegetative reproduction and/or a more efficient seed dispersion, which occurs by ornithochory, mirmechory, anemochory and mainly hydrochory [34], thus enhancing gene flow and reducing spatial aggregation among individuals [33].
Karyotype analysis
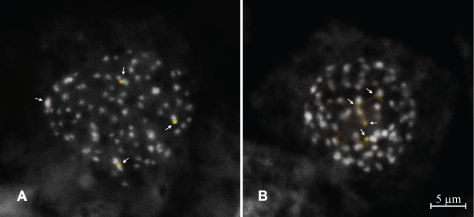
Both species presented an intrapopulational variation in chromosome numbers, as it has been reported for other Cyperaceae genera) [11,35,36]. For C. odoratus, two chromosome numbers were observed: 2n = 72 (in 67% of the cells) and 2n = 76 (in 33% of the cells). On the other hand, C. ligularis showed a higher chromosome number variation, in which the diploid number ranged from 2n = 66 to 2n = 88, with 2n = 82 as the most frequent one (40% of the cells). For both species, the CMA/DAPI banding always revealed two pairs of CMA+/DAPI- signals (Figure 4), which were associated with the two satellited chromosome pairs, also revealed by the silver nitrate impregnation (data not shown).
Contrasting with the significant amount of published data on the karyological features of some genera of Cyperaceae, such as Carex, Eleocharis and Rhynchospora [35,37-39] very little is known about the chromatin organization in Cyperus species. In fact, to the best of our knowledge, this is the first time that heterochromatic regions are evidenced in chromosomes of Cyperus species by means of fluorochrome banding. Consequently, it is hard to know if the coincident number of nucleolar organizer regions (NORs), which were evidenced by both CMA/DAPI and silver nitrate staining, indicates some conserved feature within the genus, as reported for other groups of Cyperaceae [38]. Thus, even sharing the same number of active NORs, the different ranges of chromosome number variation among sympatric individuals of C. odoratus and C. ligularis reinforces the assumption of a clear separation of the two species.
The high degree of chromosome number variation in Cyperaceae members is widely attributed to the occurrence of holokinetic chromosomes in all members of the family, as well as in Juncaceae, its sister group. Thus, the presence of diffuse kinetochores may facilitate a faster karyotypic evolution by means of chromosome fission/fusion and/or translocations [36,40]. As demonstrated for Carex species and herein observed in the sampled populations, it seems that there is no correlation between geographic range, chromosome number variation and genetic diversity in these species with holokinetic chromosomes [36,40].
Conclusions
The natural interspecific hybridization is not rare in Cyperaceae, where species of several genera as Carex, Schoenoplectus and Isolep is present species with hybrid origin [41-43]. Although the morphology-based suggestion of hybridization between C. odoratus and C. liguraris [9] our molecular markers and cytogenetic analyses showed no evidence of hybridization.
Regarding the genetic differentiation, C. odoratus surprisingly presents higher levels than C. ligularis, whichis likely a result of a long period of evolution [44] where the genetic diversity could be preserved by local vegetative regrowth of individuals. In the case of C. ligularis, the dispersal syndrome of the seeds, mainly by hydrochory and related with the colonization ability under various environmental conditions, seems to play a major role in keeping the gene flow among individuals from different localities. However, the chromosome numbers showed a different picture, with C. odoratus less variable than C. ligularis, probably indicating a faster karyotypic evolution in the last species.
These results should be associated with more field observations and specific analysis of reproductive biology, in order to enrich the knowledge concerning the dynamics and structure of the studied populations, as well as the role of these species in the successional processes and environmental restoration.
Acknowledgments
The authors thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for financial support.We are also grateful to Dois Irmãos State Park (Recife, PE, Brazil) for permission to carry out collections and the Recife City Hall for the logistic support of the Environmental Brigade.
References
- Hedren M (2004) Species delimitation and the partitioning of genetic diversity-an example from the Carex flava complex (Cyperaceae). Biodivers Conserv 13: 293-316.
- King MG, Roalson EH (2009) Discordance between phylogenetics and coalescent-based divergence modelling: exploring phylogeographic patterns of speciation in the Carexmacrocephala species complex. Mol Ecol 18: 468-482.
- Jimenez-Mejias P, Luceno M, Martin-Bravo S (2014) Species Boundaries within the Southwest Old World Populations of the Carexflava Group (Cyperaceae). Syst Botany 39: 117-131.
- Naczi RF, Moyer RD (2017) Revision of the Rhynchospora glomerata species complex, focusing on the taxonomic status of R. leptocarpa (Cyperaceae). Brittonia 69: 114-126.
- Simpson DA, Furness CA, Hodkinson TR, et al. (2003) Phylogenetic relationships in Cyperaceae subfamily Mapanioideae inferred from pollen and plastid DNA sequence data. Am J Bot 90: 1071-1086.
- Alves M, Heer SM, Trevisan R, et al. (2014) Cyperaceae In: Lista de Especies da Flora do Brasil. Jardim Botanico do Rio de Janeiro.
- Larridon I, Bauters K, Reynders M, et al. (2013) Towards a new classification of the giant paraphyletic genus Cyperus (Cyperaceae): phylogenetic relationships and generic delimitation in C4 Cyperus. Bot J Linn Soc 172: 106-126.
- Reid CS, Carter R, Urbatsch LE (2014) Phylogenetic insights into New World Cyperus (Cyperaceae) using nuclear ITS sequences. Brittonia 66: 292-305.
- Luceno M, Alves MV (1997) Clave de los generos de ciperaceas de Brasil y novedades taxonomicas y corologicas en la familia. Candollea 52: 185-191.
- Martins S, Alves M (2009) Anatomical features of species of Cyperaceae from northeastern Brazil. Brittonia 61: 189-200.
- Hipp AL, Rothrock PE, Reznicek AA, et al. (2007a) Chromosome number changes associated with speciation in sedges: a phylogenetic study in Carex section Ovales (Cyperaceae) using AFLP data. Aliso 23: 193-203.
- Esselman EJ, Enders TA, Smith M, et al. (2012) Examination of hybridization relationships between Schoenoplectus hallii and S. saximontanus (Cyperaceae) using ISSR markers. Phytoneuron 36: 1-9.
- Gizaw A, Wondimu T, Mugizi TF, et al. (2016) Vicariance, dispersal, and hybridization in a naturally fragmented system: the afro-alpine endemics Carexmonostachya and C. runssoroensis (Cyperaceae). Alp Bot 126: 59-71.
- Weising K, Nybom H, Wolff K, et al. (2005) DNA Finger printing in Plants, Principles, Methods, and Applications. (2nd edn), CRC Press, USA.
- Caetano-Anolles G, Bassam BJ, Gresshoff PM (1991) DNA amplification fingerprinting using very short arbitrary oligonucleotide primers. Nature Biotechnology 9: 553-557.
- Winter P, Benko-Iseppon AM, Huttel B, et al. (2000) A linkage map of the chickpea (Cicer arietinum L.) genome based on recombinant inbred lines from a C. arietinumx C. reticulatumcross: localization of resistance genes for fusarium wilt races 4 and 5. Theor Appl Genet 101: 1155-1163.
- Benko-Iseppon AM, Winter P, Huttel B, et al. (2003) Molecular markers closely linked to fusarium resistance genes in chickpea show significant alignments to pathogenesis-related genes located on Arabidopsis chromosomes 1 and 5. Theor Appl Genet 107: 379-386.
- Perrier X, Flori A, Bonnot F (2003) Methods of data analysis. In: Hamon P, Seguin M, Perrier X, et al. Genetic diversity of cultivated tropical plants. Science Publishers, Montpellier, France, 31-63.
- Perrier X, Jacquemoud-Collet JP (2006) DARwin software
- Holsinger KE, Weir BS (2009) Fundamental concepts in genetics: genetics in geographically structured populations: defining, estimating and interpreting FST. Nat Rev Genet 10: 639-650.
- Wright S (1931) Evolution in Mendelian populations. Genetics 16: 97-159.
- Yeh FC, Yang R, Boyle T (2001) Popgene version 1.32 Microsoft Window-based Freeware for Population Genetic Analysis. University of Alberta and Centre for International Forestry Research.
- Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155: 945-959.
- Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software structure: a simulation study. Mol Ecol 14: 2611-2620.
- Benko-Iseppon AM, Morawetz W (2000) Cytological comparison of Calyceraceae and Dipsacaceae with special reference to their taxonomic relationships. Cytologia 65: 123-128.
- Schweizer D, Ambros P (1994) Chromosome banding. In: Gosden JR, Methods in molecular biology, chromosome analysis protocols. NJ: Humana Press Totowa, 97-112.
- Hizume M, Sato S, Tanaka A (1980) A highly reproducible method of nucleolus organizing regions staining in plants. Stain Technol 55: 87-90.
- Vasconcelos S, Souza AA, Gusmao CL, et al. (2010) Heterochromatin and rDNA 5S and 45S sites as reliable cytogenetic markers for castor bean (Ricinus communis, Euphorbiaceae). Micron 41: 746-753.
- Hollingsworth PM, Ennos RA (2004) Neighbor joining trees, dominant markers and population genetic structure. Heredity 92: 490-498.
- Chozin MA, Yasuda S (1991) Possibility of natural hybridization between Cyperus iria L. and Cyperus microiria Steud. J Weed Sci Tech 36: 282-289.
- Wright S (1978) Evolution and genetics of populations. The theory of gene frequencies. (2nd edn), University of Chicago Press, London.
- Almeida FS, Sodre LM, Contel EP (2003) Population structure analysis of Pimelodusmaculatus (Pisces, Siluriformes) from the Tiete and Paranapanema Rivers (Brazil). Genet Mol Biol 26: 301-305.
- Triest L, Sierens T, Terer T (2014) Diversity and fine-scale spatial genetic structure of Cyperus papyrus populations in Lake Naivasha (Kenya) using microsatellite markers. Hydrobiologia 737: 131-144.
- Linder HP, Rudall PJ (2005) Evolutionary history of Poales. Annu Rev Ecol Syst 36: 107-124.
- Roalson EH (2008) A synopsis of chromosome number variation in the Cyperaceae. Bot Re 74: 209-236
- Escudero M, Hipp AL, Luceno M (2010) Karyotype stability and predictors of chromosome number variation in sedges: a study in Carex section Spirostachyae (Cyperaceae). Mol Phylogenet Evol 57: 353-363.
- Vanzela AL, Guerra M (2000) Heterochromatin differentiation in holocentric chromosomes of Rhynchospora (Cyperaceae). Genet Mol Biol 23: 453-456.
- Silva CR, Quintas CC, Vanzela AL (2010) Distribution of 45S and 5S rDNA sites in 23 species of Eleocharis (Cyperaceae). Genetica 138: 951-957.
- Cabral G, Marques A, Schubert V, et al. (2014) Chiasmatic and achiasmatic inverted meiosis of plants with holocentric chromosomes. Nat Commun 5: 50-70
- Chung KS, Weber JA, Hipp AL (2011) Dynamics of chromosome number and genome size variation in a cytogenetically variable sedge (Carexscoparia var. scoparia, Cyperaceae). Am J Bot 98: 122-129.
- Pedersen AT, Nowak MD, Brysting AK, et al. (2016) Hybrid origins of Carexrostratavar. borealis and C. stenolepis, two problematic taxa in Carexsection Vesicariae (Cyperaceae). PLoS One 12: e0171398.
- Yano O, Ikeda H, Hoshino T (2010) Molecular and cytological studies of an interspecific hybrid in the genus Schoenoplectus (Cyperaceae). Acta Phytotax Geobot 60: 141-149.
- Yano O, Tanaka N, Ito Y (2016) Molecular evidence for a natural hybrid between Isolepis crassiuscula and Isolepis lenticularis (Cyperaceae) in New Zealand. N Z J Bot 54: 433-445.
- Sheng HM, An LZ, Chen T, et al. (2006) Analysis of the genetic diversity and relationships among and within species of Hippophae (Elaeagnaceae) based on RAPD markers. Plant Syst Evol 260: 25-37.
Corresponding Author
Ana Maria Benko-Iseppon, Laboratório de Genética e Biotecnologia Vegetal, Departamento de Genética, CCB, Universidade Federal de Pernambuco, Avenida da Engenharia s/n, CEP 50740-600, Recife-PE, Brazil.
Copyright
© 2017 Cruz GAS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.