Development of a Stationary Chomatography Radionuclide Generator of Technetium-99M
Keywords
Target, Irradiation, Sorbent, Column, Generator Tehnetium-99m, Eluent, Elate, Radiochemical Purity
Abbreviations
99m Tc - radionuclide technetium-99 metastable; Eg - energy of gamma rays, keV; E b - beta radiation energy, keV; T 1/2 - radionuclide half-life, hour Ci - measure of radioactivity, curie; 98 MoO 3 - molybdenum oxide labeled with the Mo-98 isotope; Al 2 O 3 - g-aluminum oxide; r - sorbent particle size, mm; pH - pH value; N - normality of the solution; M - molarity of solution; D - radionuclide distribution coefficient; FA - fuel assembly (nuclear fuel); TcO 4- - pertechnetate anion ( 99m Tc); OSGI - industry standard calibrated source; r.p. - radiochemical purity of the eluate, %.
Introduction
Radionuclide technetium-99m is used for diagnostics and scintigraphy (scanning) of the thyroid and salivary glands, brain, and radionuclide angiocardiography, and also ventriculography. For example, myocardial perfusion scanning plays an important role in diagnosing and making therapeutic decisions in heart disease. For example, technetium-99m sestamibi or technetium-99m tetrofosmin radiopharmaceuticals are often used to scan myocardial perfusion in SPECT (single photon ejection computed tomography) techniques [1].
Elution of the daughter nuclide 99m Tc from it`s the portable generators or “milking” of the generator is expediently performed every 6 hours twice a day. Since the half-life of 99 Mo is 66 hours, the supply of the original product is depleted to an insufficient level in about one week, so the generator must be replaced with a fresh generator. Meanwhile, for large cities with a population of one million people, where there are several dozen clinics, it may be more promising to manufacture a stationary generator with an activity of 15-20 Ci (555-740 GBq) in a radiochemical laboratory and centrally deliver the finished preparation of sodium pertechnetate solution ( 99m Tc) to clinics. In this case, the problems associated with the operation of generators, with ensuring radiation safety in clinics, are positively solved, and production costs are also reduced.
The purpose of this work is to study the radiochemical foundations of the chromatographic stationary radionuclide generator Tc-99m, to develop a radiochemical scheme for the manufacture of a stationary Tc-99m generator and to manufacture pilot models of the stationary Tc-99m generator.
In this work, a scheme of radiochemical technology for manufacturing a stationary radionuclide generator 99 Mo/ 99m Tc of the chromatographic type on sorbent of aluminum oxide is developed, which makes it possible to obtain the radionuclide 99m Tc in the form of a solution of sodium pertechnetate ( 99m Tc) with high specific activity and radiochemical purity for the needs of nuclear medicine.
Materials and Methods
Molybdenum oxide crystalline grade "special purity" and isotopically enriched in Mo-98 molybdenum trioxide were used as a target for irradiation at the WWR-SM reactor.
In the experiments hydrochloric acid, sulfuric acid, caustic soda, sodium chloride, sodium nitrate, disubstituted sodium phosphate - chemically pure reagents were used. To obtain the parent radionuclide molybdenum-99 by neutron irradiation of the WWR-SM reactor, samples with the natural isotopic composition of molybdenum (MoO 3 ) and enriched in the isotope 98 Mo with an enrichment in the isotope Mo-98 of at least 97% were used.
Aluminum oxide (Al 2 O 3 ) of the “for chromatography” type with a r = 0.1-0.2 mm was used as a sorbent for stationary chromatographic columns. The sorbent was activated by preliminary treatment with a 3 M hydrochloric acid solution during 1 hour, then washed with distilled water until the wash water reaches the value of pH = 2.0, than obtained product dried at a temperature of 120 °C during 3 hours. To carry out experiments in dynamic modes, generator columns of various volumes (25-200 ml) with ordinary glass taps were used.
Radiometric and spectrometric measurements of the activity of samples and solutions were carried out on a measuring complex consisting of a DGDK-EMS-666/V detection unit, an NTA-1024 pulse analyzer, an Aspect SU-01P gamma spectrometer with a Ge-Li detector of the DGDK-120 type, and used beta-gamma spectrometer "Progress BG(II)" BDEB 3-2U with software "Progress 5". Static experiments were carried out in chemical glass beakers with periodic mixing of the liquid and solid phases. Experiments in the dynamic mode were carried out in special glass columns made of molybdenum glass, equipped with communications, consisting of an eluent line (inlet) and an eluate line (outlet). The yield of 99m Tс was studied by washing sorbents in generator columns containing the adsorbed parent radionuclide 99 Mo.
Static experiments on the study of the sorption of molybdenum on aluminum oxide weighing 1g were made by periodic mixing of the aqueous and solid phases in 50 ml glasses. The volume of the aqueous phase was 3-5 ml; the contact time was 1 hour.
The distribution coefficient of 99 Mo under static sorption conditions ( D Mo ) was calculated by the formula:
Where: A s and A w are radioactivity 99 Mo in the solid phase and the aqueous (water) phase, impulses/sec; V w is water phase volume, ml; M is mass of dry sorbent, g.
The distribution coefficient of 99m Tc under static sorption conditions ( D Tc ) was calculated by the formula:
Where: activities of initial and final solutions (before and after sorption), impulses/sec; V - is water phase volume, ml; mis mass of dry sorbent, g.
Laboratory experiments in dynamic mode were carried out in columns with drain cocks in the lower part made of ordinary glass with a volume of 5-20 ml. Industrial generators with significant Mo activity were tested in columns with a volume of 5 ml (small columns) and 200 ml (large columns) of molybdenum glass. The generator columns with the sorbent in two ways were loaded: 1) 99 Mo was initially sorbed statically in a beaker, then the sorbent with 99 Mo was transferred into the column; 2) Sorption of 99 Mo was carried out directly in the column in dynamic mode with a preliminary filled sorbent by the method of descending and ascending chromatography.
The calculation of the output of the daughter radionuclide Tc and the slip of the parent radionuclide Mo from the generator column under dynamic conditions was carried out according to the following formulas
where: A ed is the total radioactivity of the 99m Tc radionuclide in the eluate, imp/sec; A rd is the equilibrium radioactivity of the daughter radionuclide in the 99 Mo- 99m Tc system, imp/sec.
Results of the Study of the Chemical Forms of Molybdenum in Solutions
Table 1 shows the main nuclear characteristics of 99 Mo, 99m Tc and 99 Tc in a 99 Mo/ 99m Tc generator system.
Passing a saline solution through a generator column with adsorbed 99 Mo leads to the elution of soluble 99m Tc, as well as 99 Tc. Since 99 Tc cannot be separated from 99m Tc, this by-product, which is part of the eluate, has no medical benefit and is not a contaminant. The eluate will also include a small amount of 99 Mo and some aluminum, the latter two substances being contaminants that must be limited according to IAEA standard [1].
Normal molybdates (MoO 4 ) -2 in neutral and alkaline solutions are formed [2]:
The study of the forms of molybdenum in its solutions was a very important condition for choosing an appropriate system for the sorption of the parent radionuclide 99 Mo on the sorbent (Al 2 O 3 ).
In acidic solutions (pH 3÷4) complex ions of molybdenum phosphomolybdate (5,6) and isopolymolybdate (7) are formed according to the following formulas:
Here must be taken into account that with a stronger acidification of the solution (pH < 2.0), the formation of slightly soluble molybdic acid can occur, which precipitates [3]:
The chemical form of molybdenum in solutions depends on the acidity of the aqueous phase, and this factor can affect the completeness of molybdenum sorption. The study of the dependence of molybdenum sorption on the hydrogen index (pH) showed that the most optimal pH values are 2.0-3.0 (Table 2).
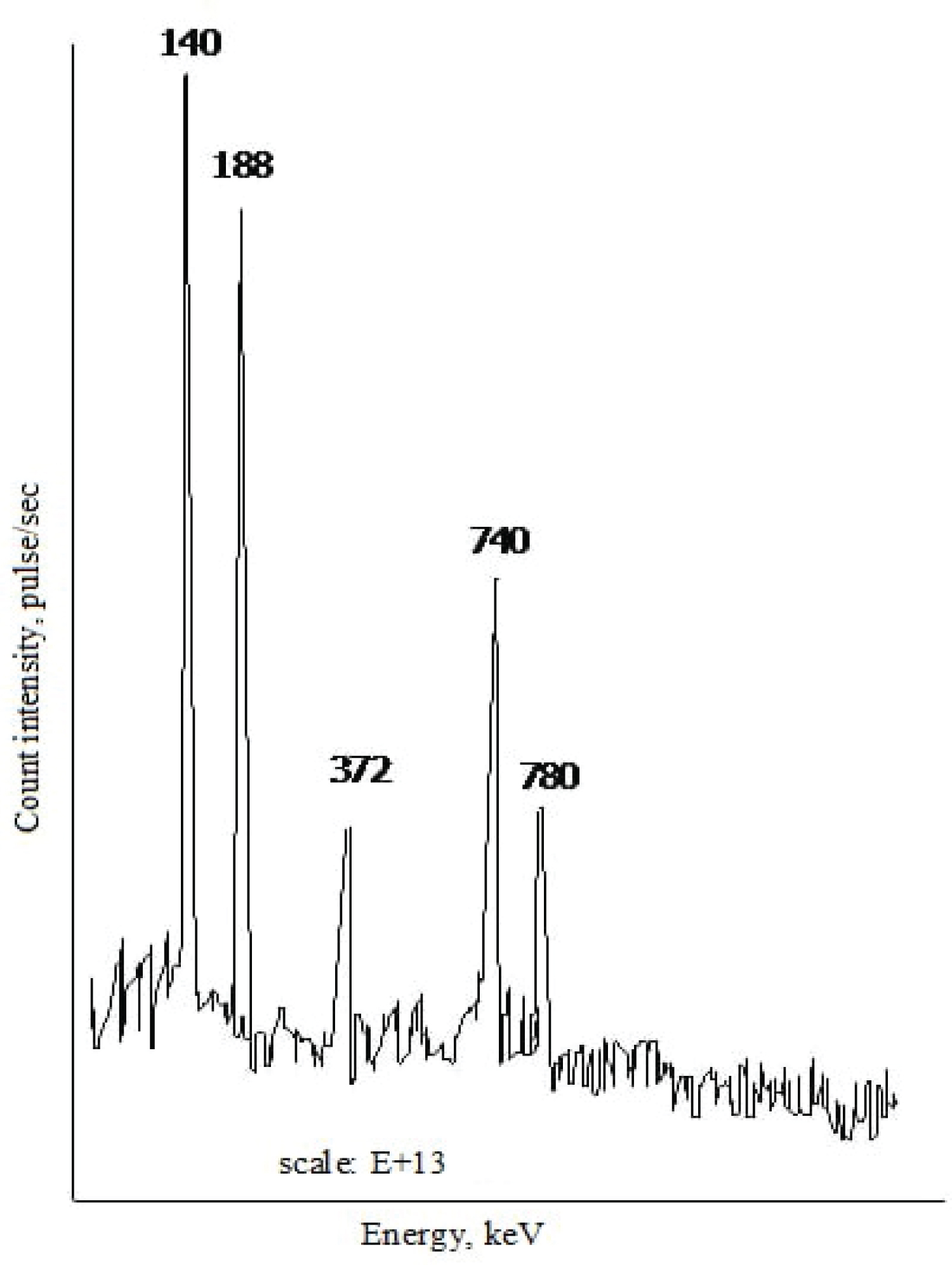
Figure 1 shows the gamma spectrum of an equilibrium 99 Mo- 99m Tc mixture, which shows the characteristic 99 Mo gamma lines with gamma radiation energies of 188 keV, 372 keV, 740 keV and 780 keV, as well as 99m Tc gamma lines with an energy of 140 keV.
The chemical form of the molybdenum-98 carrier and the non-carrier 99 Mo parent radionuclide in solution depends on the properties of the sorbent and, therefore, it is important for the manufacture of the 99 Mo/ 99м Tc generator system. For research, the adsorption-chromatographic version of the 99m Tc generator was chosen as the most promising, because aluminum oxide is a radiation resistant sorbent of natural origin.
Аs an eluent due to its isotonic properties a solution of 0.9% NaCl was used, which is one of the main requirements for medical generators. The output of 99m Tc from generator systems is calculated relative to the activity of the accumulated daughter radionuclide 99m Tс:
Where: A ( 99m Tc) and A ( 99 Mo) activities of the daughter 99 m Tc and parent 99 Mo radionuclides at the time of elution; 0.875 - coefficient taking into account the decay of the parent radionuclide according to the scheme:
Preliminary activation of aluminum oxide makes it possible, on average, to increase the sorption of molybdenum on the sorbent by 13% (Table 3).
The study of molybdenum sorption on Al 2 O 3 , both in static and dynamic modes, showed that the maximum capacity of the sorbent is 75-80 mg Mo/g Al 2 O 3 (Figure 2). Taking into account that a high concentration of molybdenum in the solid phase can lead to contamination of the eluate with 99 Mo impurities, for the manufacture of stationary generators, the Mo concentration was limited to 25-30 mg/g Al 2 O 3 . At these values, the content of 99 Mo in the eluates did not exceed 10 –2 %. The optimal concentration of molybdenum in an aqueous solution for carrying out the sorption process turned out to be 15-20 mg/ml. In this case, there is a more uniform distribution of 99 Mo on the sorbent and the loss of 99 Mo in the sorption process (molybdenum slippage) is reduced.
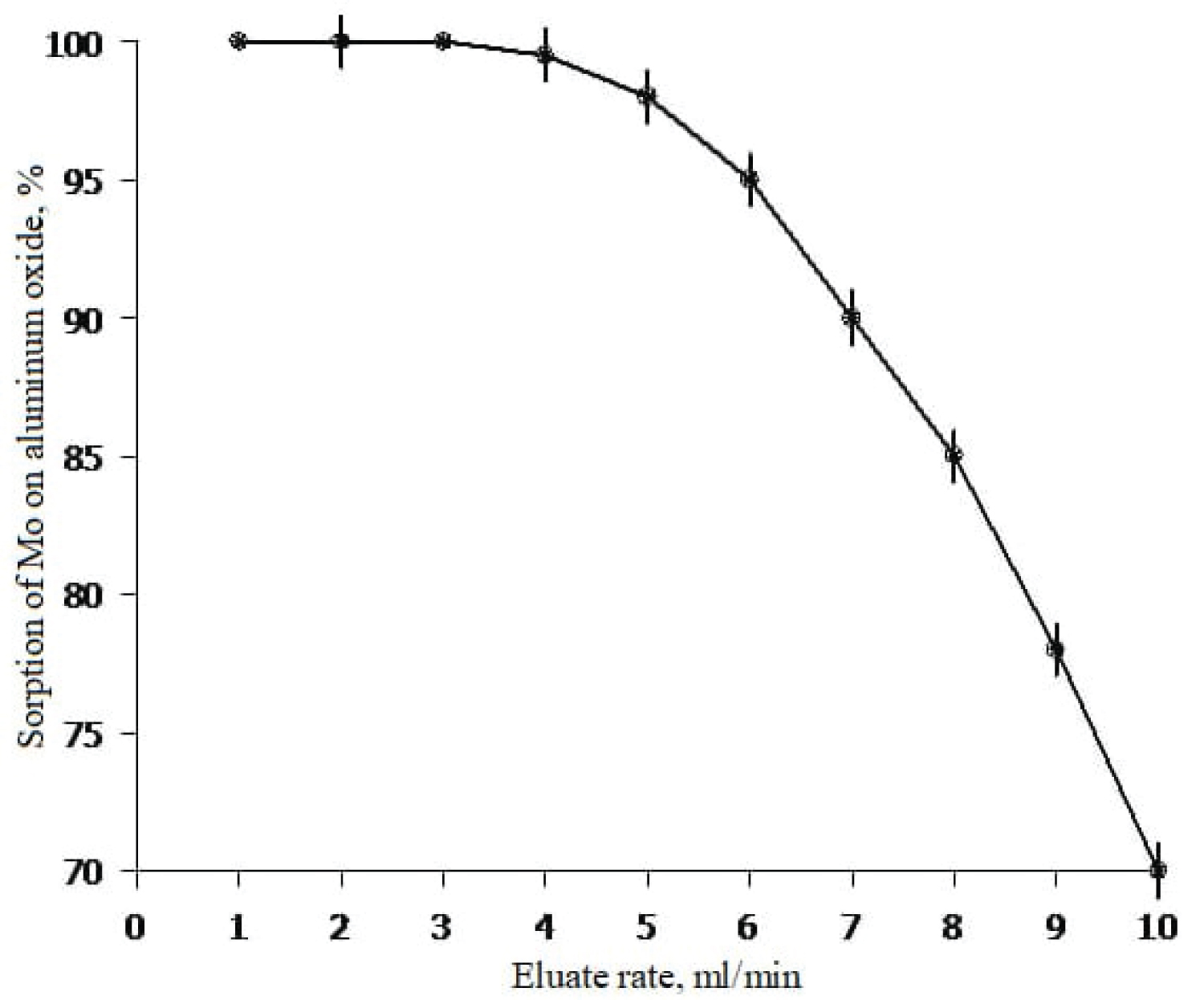
For experiments in the dynamic mode, a glass column with an inner diameter of 33 mm and a height of 200 mm was made, which had a glass filter and a drain cock in the lower part to release the liquid phase. Activated alumina pre-moistened with distilled water and acidified with hydrochloric acid solution to pH 2-3 was placed in the column. The mass of aluminum oxide in the column was 110g. Sorption and elution were carried out according to the principle of descending chromatography. To study the effect of the rate of passage of a molybdenum solution through a layer of sorbent on its sorption, a special experiment was set up, the result of which is shown in Figure 3. The study of this dependence made it possible to determine the optimal flow rate of the solution, which turned out to be 2-4 ml/min (Figure 3).
In dynamic modes, the value of molybdenum sorption also affects the rate of passage of the solution through the sorbent layer of the generator column. Under experimentally selected conditions (the height of the sorption layer of the column is 160 mm, the Mo concentration in the isopolymolybdate solution is 20 mg/ml, the pH of the solution is 2.0, the mass of the sorbent is 110g, the rate of transmission of the isopolymolybdate solution through the aluminium oxide is »2 ml/min), sorption of 99 Mo was carried out on the sorbent of the generator column. Under such conditions, the sorption of molybdenum was more than 99.0%, the total amount of sorbed molybdenum was 3.55g.
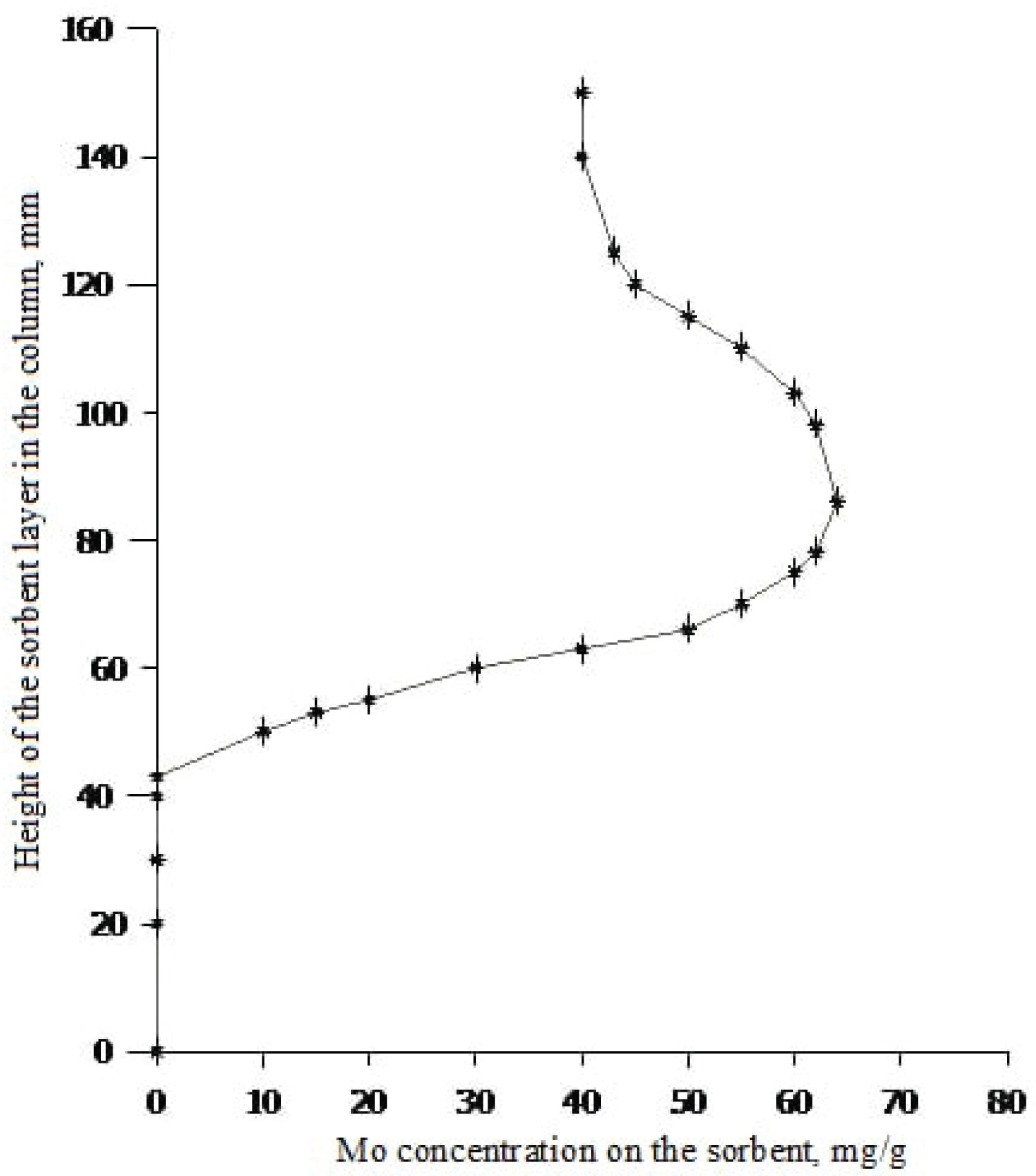
To study the distribution of molybdenum in the sorption layer of the generator column after sorption of 99 Mo, the column was broken by layer-by-layer removal of the mass of the sorbent by 5.0g of the column. By measuring the radioactivity of each layer, the amount and strength of molybdenum in each plate along the entire length of the column is increased (Figure 4).
The Figure 4 shows that the maximum concentration of molybdenum on alumina is 65 mg/g. Molybdenum on 72% of alumina with an average sorbent load of 30 mg/g was sorbed. The lower part of the generator column with 28% alumina (from 0 to 43 mm) is completely free of molybdenum with a free volume of the generator column of 77 ml. The main part of 99 Mo is concentrated in the upper part of the column, while the lower layer of the sorbent, remaining free, serves as a filter layer for retaining 99 Mo. A parallel experiment showed the same result: The main part of molybdenum is in the upper part of the generator column with a total sorbent weight of 66g (60%) with an average molybdenum concentration of 46.3 mg/g; in the middle part of the column there is up to 243 mg of molybdenum with a sorbent weight of 13g (12%) with an average molybdenum concentration of 18.7 mg/g; in the lower part of the column with a sample of aluminum oxide 31g (28%), molybdenum is completely absent and this is very important as a filter layer to retain the parent radionuclide 99 Mo.
Results of Production of Experimental Stationary Generators and Their Study
Samples of stationary generators were both laboratory (low-active) and pilot-industrial (up to 10 Ci for molybdenum-99 per generator). The manufacture and testing of pilot industrial generators with greater activity was carried out in hot chambers of technological lines at the «Radiopreparat» enterprise at the Institute of Nuclear Physics of the Academy of Sciences of the Republic of Uzbekistan.
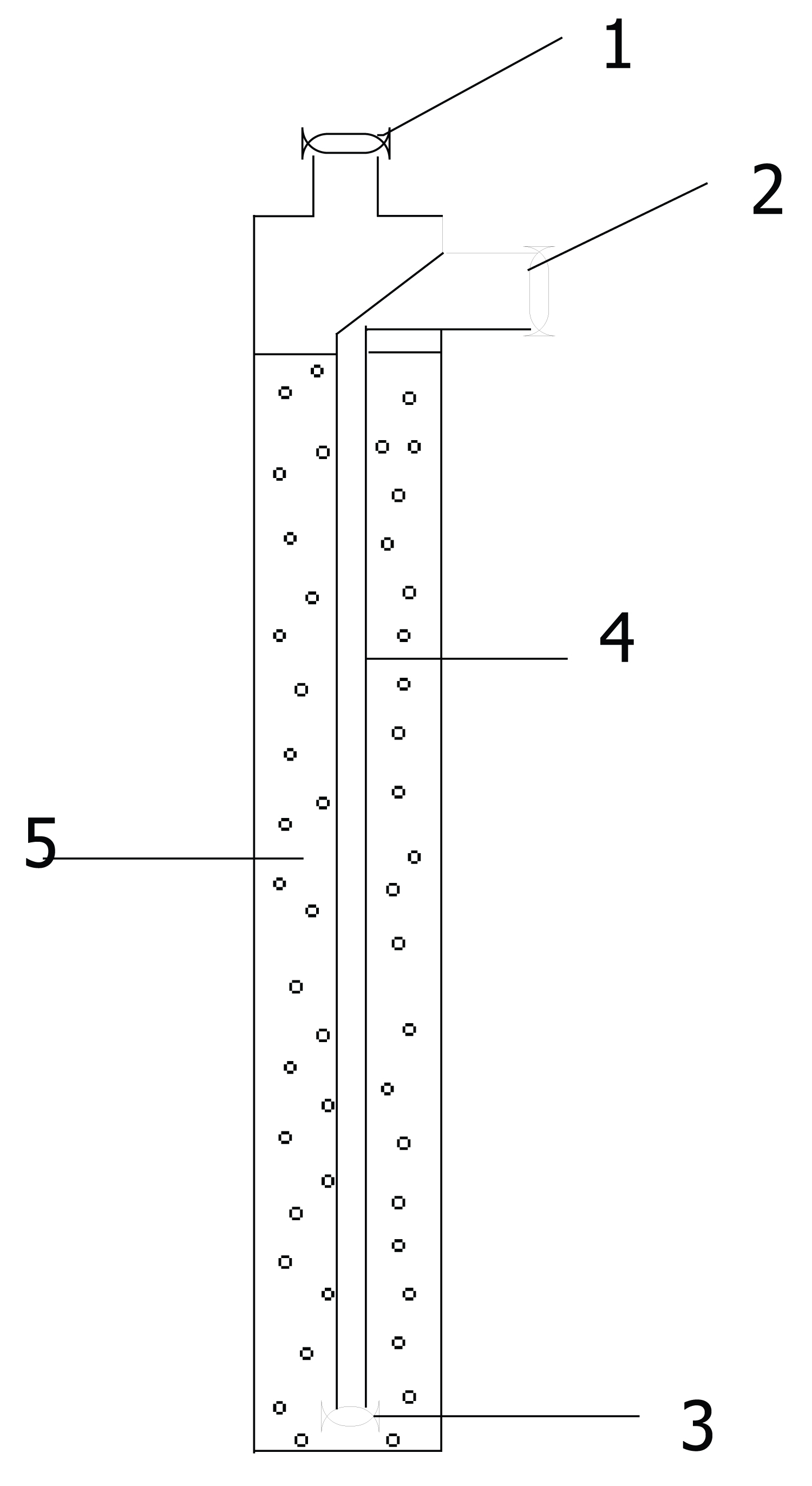
Figure 5 shows a schematic representation of a stationary generator. The generator is a molybdenum glass chromatographic column 25 mm in diameter, filled with oxide, 99 Mo adsorbed on it. The column has: An inlet pipe 1, through which oxide is loaded, passing a radioactive solution of 99 Mo and a solution for elution of 99m Tc (eluent line); outlet pipe 2, through which both wash solutions and 99m Tc eluate are supplied (eluate line); inner tube 4 with glass filter 3; sorbent (aluminum oxide) for sorption of Mo 5. Rubber caps with silicone capillaries inserted into them are put on the inlet and outlet pipes. The rate of supply of solutions to the generator column is controlled by a peristaltic pump and is 5-8 ml/min, with eluting 99m Tc and 1-4 with sorption of 99 Mo.
Discussion of Technical Characteristics of Stationary Generators Tc-99m
To develop experimental industrial stationary generators Tс-99m, we studied the sorption of molybdenum by the method of descending elution in dynamic mode in a column with dimensions of 25 mm in diameter and 350 mm in height. The height of the sorbent layer in the column is 280-285 mm; The amount of sorbed molybdenum is 99% at an average sorbent load 30 mg/g. The results of these experiments allowed us to determine the optimal dimensions of the generator column, the conditions for performing the processes of Mo-99 sorption on the sorbent of the generator column, and the required weight of the sorbent in the column and molybdenum, taking into account the nominal activity of industrial generators - more than 10 Сi for molybdenum-99.
Stationary generators of technetium-99m No. 1 and No. 3 were made on irradiated target of molybdenum trioxide containing natural molybdenum and target was irradiated with thermal neutrons flux density is ¦ = 1.1·10 14 n/cm 2 ·sec, FA is IRT-3M 36% enrichment of U-235, nominal power of the WWR-SM nuclear reactor is 10 MW. Generator No. 2 was made on target molybdenum trioxide enriched in molybdenum-98, which also was irradiated by thermal neutrons flux density - ¦ = 7.2×10 13 n/cm 2 ×sec, FA is IRT-3M 36% enrichment of U-235, nominal power of the WWR-SM nuclear reactor was 10 MW.
Each generator column contained 100g of alumina, on which Mo-99 was adsorbed in dynamic mode (sorption rate 2 ml/min) from an isopolymolybdate solution with a molybdenum concentration of 25 mg/ml. Washing of generator columns was carried out with acidified water (рН = 3.0). The elution of technetium-99m was carried out with a 0.9% NaCl solution with pH = 2.0. Technical characteristics of generators are given in the Table 4.
In the Table 5, the results of the chemical analysis of sodium pertechnetate solutions ( 99m Tc) on the first day of elution from experimental stationary generators are shown.
Description of the Manufacturing Technology of the Stationary Generator Technetium-99m No 3
For the manufacture of stationary generator No. 3, a molybdenum trioxide target irradiated with neutrons with a natural content of Mo-98 weighing 6g was used. In the vertical channel of reactor WWR-SM the thermal neutron flux density was ¦ = 7.2×10 13 n/cm 2 ×sec. The target irradiation time was 296 hours.
After irradiation, the target was dissolved in 85 ml of 1.0N NaOH solution, the resulting alkaline solution was acidified with 1.0N hydrochloric acid solution (70 ml), and 5 ml of bromine water was added to prevent reduction of molybdenum (VI) to molybdenum blue (Mo 2 O 5 ·MoO 3 ·6H 2 O) [3]. From the resulting solution of isopolymolybdate with an activity of 3.2 Ci in 99 Mo, with a concentration of molybdenum in a solution of 25 mg/ml, molybdenum was adsorbed on a column in a dynamic mode. The weight of the sorbent (Al 2 O 3 ) in the column was 100g, the flow rate of the isopolymolybdate solution was 2 ml/min. The average loading of molybdenum on alumina was 40 mg/g. Then, the generator column was washed with 150 ml of acidified water with pH = 3.0, and then 0.9% NaCl solution with pH = 3.0 was injected into the column. Analysis of the wash water showed that 99 Mo is absent in the wash water, which indicates a sorption value of more than 99%. A day later, Tc-99m was eluted from the generator column with a 0.9% NaCl solution pH = 3.0.
Samples of eluates were taken, 10.0 ml each, and chemical analysis was carried out according to the Russia State Pharmacopoeia 13 (XIII) [4]. The following characteristics of the eluate were determined: Identity (by spectrometric analysis), volumetric activity of technetium-99m (by radiometry), content of radionuclide impurities, radiochemical purity (by paper chromatography), content of sodium chloride and inactive impurities. The distribution of 99m Ts over fractions of the sodium pertechnetate eluate ( 99m Ts) of generator No. 3 is shown in Table 4.
Samples of eluates were taken, 10.0 ml each, and each was subjected to chemical analysis according to the Russia State Pharmacopoeia 13 (XIII) [4]. The following characteristics of the eluate were determined: Identity (by spectrometric analysis), volumetric activity of technetium-99m (by radiometry), content of radionuclide impurities, radiochemical purity (by paper chromatography), content of sodium chloride and inactive impurities. The distribution of 99m Tc over fractions of the sodium pertechnetate eluate ( 99m Tc) of generator No. 3 is shown in Table 6.
The results of the chemical analysis of the eluates showed that the eluates from the generators meet the requirements of Pharmacopoeia Monograph 42-2837-92. 0083-2002 [5].
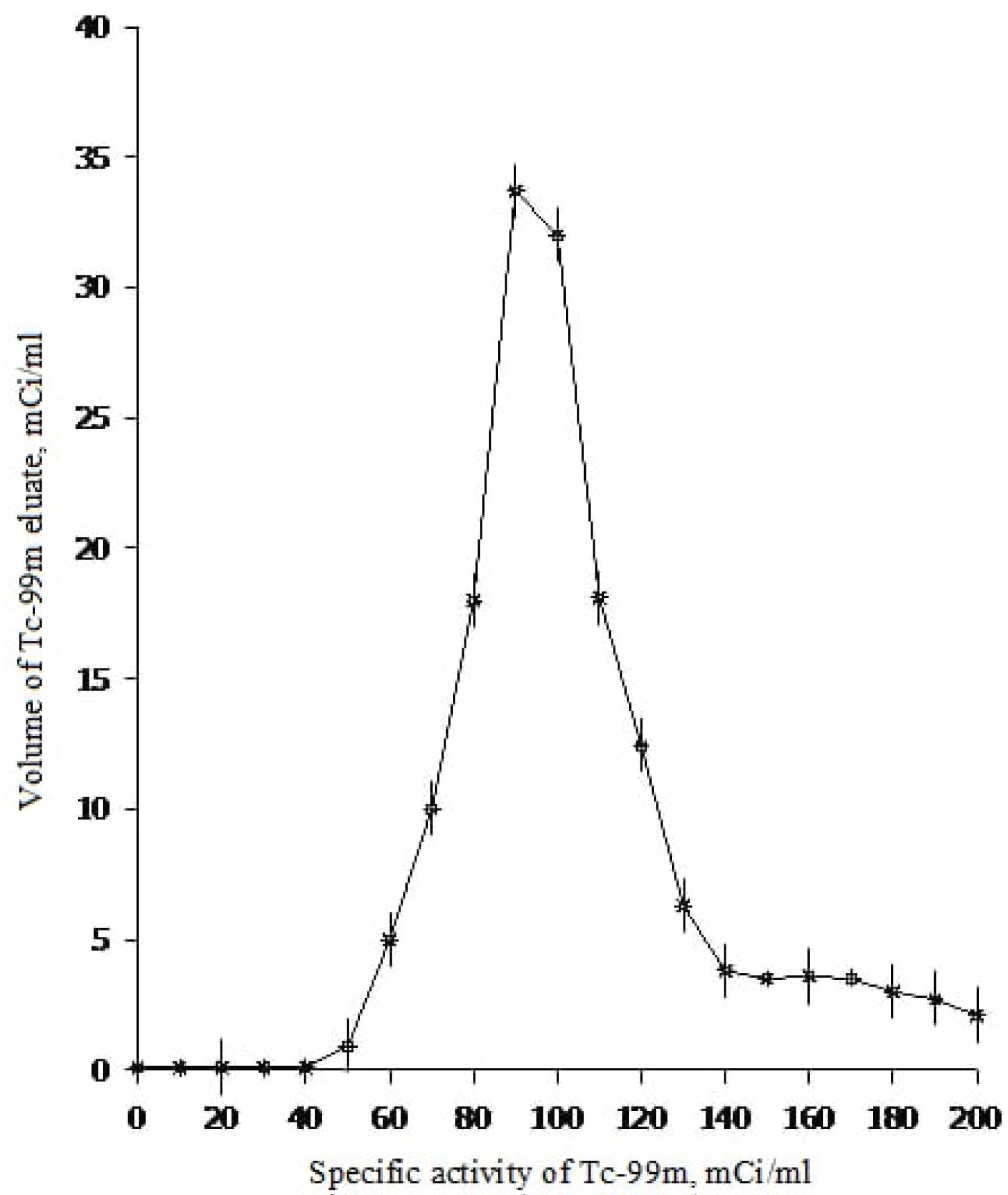
In Figure 6 the elution curve of 99m Tc from experimental generator No. 3 is shown.
Specifications of sodium pertechnetate solution ( 99m Tс) from experimental stationary generator Tc-99m No. 2 are shown in the Table 7.
The results of the analysis of the distribution of the elution of Tc-99m by fractions on the first day of the test and the results obtained on the 8 th day of the test are shown in Table 8.
The results of the table show that over time, the activity of Tc-99m in the main fractions decreases from 96.2% to 90%, and the proportion of Tc-99m in the first fraction slightly increases (from 0.15% to 6.78%). Apparently, there is some movement of Mo-99 in the column, and this leads to an increase in the concentration of Tc-99m in the first fraction and an insignificant increase in the content of impurity Mo-99 in all fractions.
Conclusion
The patterns of molybdenum sorption on aluminum oxide, its distribution between the solid and liquid phases of the generator system and in the chromatographic column of the generator have been established. The optimal conditions for the sorption of molybdenum on aluminum oxide, which provide at least 99% of its sorption, are determined:- The chemical form of molybdenum in solutions is isopolymolybdate, with a molybdenum concentration of 20-25 mg/ml, pH = 2-3; - the rate of passing the solution through the generator column 1-4 ml/min; - optimal load of Mo on the sorbent is 30-40 mg/g.
A technological scheme for the manufacture of a stationary technetium-99m chromatographic generator, prototypes of stationary technetium-99m generators were manufactured and tested, and eluents of sodium pertechnetate solution fully meeting the requirements of the international standards [6,7] were obtained.
References
- Angelides G, Jamouzis G, Karagiannis G, et al. (2017) SPECT and PET with ischemic heart failure. Heart Fail Rev 22: 243-261.
- Steigman J (1981) The chemistry of the technetium generator. Trans Amer Nucl Soc 38: 55-56.
- Alikina EN (2019) Analytical chemistry. Qualitative analysis. Perm state national research university. Perm 202.
- Narkevich IA (2018) State Pharmacopoeia of the Russian Federation in modern pharmaceutical analysis practice. 35-37.
- Sodium pertechnetate solution 99mTc from the generator. 99mTc Pharmacopoeia Monograph of Republic of Uzbekistan No. 42-2837-92. 0083-2002. 8.
- Sodium pertechnetate (99mTc) injection (fission). EUROPEAN PHARMACOPOEIA 7.0. 01/2008:0124 corrected 7.0.
- US PHARMACOPOEIA. Sodium pertechnetate 99mTc injection. Pertechnetic acid (H99mTcO4), sodium salt. Sodium pertechnetate (Na99mTcO4).
Corresponding Author
Ulugbek T Ashrapov, Department of Nuclear Energy and Nuclear Technologies of Institute of Nuclear Physics of Academy Sciences, 100214, poselok Ulugbek, Republic Uzbekistan, Tel: +998-99-490-27-71; Fax: +998-71-289-31-18.
Copyright
© 2024 Ashrapov UT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.