Trophinin Expression and Regulation in the Porcine Uterus and Embryo during the Time of Embryo Implantation
Abstract
Trophinin is an intrinsic membrane-bound protein that mediates homophilic cell adhesion by interacting with two cytoplasmic proteins, tastin and bystin. Trophinin is expressed in the uterus and embryos of primates, humans, and mice. The goals of our study were; 1) To determine whether the porcine uterus and embryo express trophinin transcripts and protein and 2) To determine whether ovarian steroids regulate uterine trophinin expression. Uterine tissue from pregnant and non-pregnant animals from various days of the reproductive cycle, liver, spleen, and heart were isolated from sexually mature gilts (n = 46) and analyzed for expression of trophinin mRNA transcripts. Ovariectomized gilts, given hormone replacement treatments, were examined for the presence of trophinin mRNA. Embryos were collected from bred pigs on days 7, 11, and 14 post-breeding. The embryos were tested for the presence of trophinin transcripts by RT-PCR analysis. Reverse Transcriptase (RT) PCR analysis demonstrated that trophinin mRNA was present in pregnant and non-pregnant porcine uteri. Over 80% of the DNA sequence of the porcine trophinin gene is homologous to human trophinin. Trophinin was expressed in all endometrial cells throughout the uterus, and the presence of an embryo did not affect trophinin expression by the uterus. Trophinin mRNA transcripts were detected at day 14 of the estrous cycle, and expression was maintained until day 24 in animals that established pregnancy. Porcine embryos showed high levels of trophinin expression around day 14 of development, which is during the window of implantation in the pig. Furthermore, this timeframe is when the filamentous embryo achieves superficial adhesion to the uterus. Progesterone induced the expression of trophinin in ovariectomized gilts given hormone replacement. Our results show that trophinin is expressed in the porcine uterus and blastocyst at the time of implantation, suggesting that it may be involved in the regulation of implantation in the pig.
Keywords
Porcine, Progesterone, Conceptus, Implantation, Pregnancy, Trophinin
Introduction
Implantation is a coordinated series of events that include the development of the embryo and the differentiation of the uterus and epithelial cells to an adhesive state [1-4]. This process involves cell-to-cell contact via their respective apical cell membranes. It is regulated through the production of cytokines, growth factors, and hormones by the uterine epithelium and the embryo [5]. Implantation occurs only during a restricted time period during the uterine estrous cycle, known as the "window of receptivity" [6].
Implantation in domestic species (i.e., pigs, cattle, sheep, and horses) differs in several ways from implantation in rodents and primates [7]. In primates and rodents, the embryo immediately attaches to the uterine epithelium upon entering the uterus. In domestic livestock, there is a so-called "pre-receptive period" for implantation [8]. During this period, there is migration of the embryo, spacing of the embryos (seen in pigs), conceptus signaling for maternal recognition of pregnancy, and endometrial glandular secretion. There is no displacement or invasion of the luminal epithelia by trophoblast cells; instead, the embryos adhere superficially to the uterine epithelium [9].
Adhesion events in the pig uterus during implantation involve a highly coordinated set of sequential interactions that include the activation of adhesion molecules with distinct but interrelated functions. Conceptus attachment to the uterine wall does not occur until hormonally controlled events change the non-adhesive pre-receptive uterine epithelium to an adhesive receptive epithelial surface [10].
In 1995, Fukuda [11] and colleagues used two human carcinoma cell lines HT-H [12,13] and SNG-M [14], to mimic the mechanisms that are involved in implantation. HT-H cells were established from a testicular teratocarcinoma tumor and could differentiate into trophoblastic cells [15]. The SNG-M cell line was established from the metastatic region of an endometrial tumor and can respond to estrogen and progesterone as well as exhibit characteristics similar to those of uterine epithelial cells [14]. This model allowed the investigators to identify three novel proteins: trophinin [11,16], tastin [11,16] and bystin [16]. Trophinin, tastin, and bystin comprise a complex mediating a unique cell adhesion between trophoblast and endometrial cells at their respective apical cell surfaces [11,17,18].
Expression of trophinin has been reported in both the trophoblast and the human placenta [17] However, Nadano, et al., demonstrated that trophnin does not play a significant role in mouse implantation, which is evident by the fact that trophinin gene knockout mice are fertile [19]. Furthermore, the expression of trophinin in mice is independent of the presence of embryos [20], whereas in the human trophinin is only expressed in the presence of a blastocyst [21-24]. Recently, it has been reported that trophnin is up-regulated by hCG in the endometrium [25].
Nakano, et al. 2004 [26] has previously reported trophinin expression during estrous cycle days 8 and 20 in the pig. They reported that trophinin immunostaining was not different among the different days of the estrous cycle, although, trophinin gene expression was higher in the luteal phase similar to what is seen in humans [26]. The present report expands upon their observation, but in addition reports that trophinin mRNA transcripts are present in the uterus and embryos of the pig during specific times of the estrous cycle in both pregnant and non-pregnant swine and that this expression is regulated by progesterone.
Materials and Methods
Tissue collection from pregnant and non-pregnant animals
Experiments involving the use of animals were conducted in accordance with protocols approved by the University of Illinois Animal Care and Use Committee (IACUC #99367). Cycling gilts were checked for estrus once a day, with the onset of estrus, denoted as day 0. Endometrial tissue was collected from normal cycling (non-mated) animals on day 3 (n = 2), day 7 (n = 3), day 9 (n = 3), day 11 (n = 3), day 14 (n = 3) and day 16 (n = 3). The non-mated animals were classified as non-pregnant. Endometrial tissue was also collected from mated animals on day 7 (n = 2), day 11 (n = 3), day 14 (n = 3), day 16 (n = 4), day 24 (n = 2) and day 40 (n = 3) after estrus. The bred animals were considered pregnant if embryos or fetuses were present in the reproductive tract at the time of tissue collection. Liver, spleen, and heart were collected from day 16 animals from both groups. Tissue for RNA isolation was placed in RNALater™ (Sigma chemical Co. St. Louis, MO) and stored at-80 ℃ until use.
Embryos were collected from mated animals on days 7, 9, 11, and 14 of the estrous cycle.
Tissue collection from hormone replacement animals
Progesterone (P4, 6α-methyl-17αβhydroxy-progesterone acetate), estrogen (E2, 17β-estradiol), and cottonseed oil were purchased from Sigma Chemical Company (St. Louis, MO). Twelve ovariectomized (OVX) crossbred (Yorkshire X Duroc) gilts were used in this experiment. Gilts were observed for behavioral estrus and were OVX on either day 4 or 5 of the estrus cycle. Ovaries were removed through a mid-ventral incision while the animals were under halothane gas anesthesia. Animals were allowed to recover for one day and were then subjected to steroid hormone replacement. Subcutaneous injections of either, E2 (100 μg/day) (n = 3), P4 (200 mg/day) (n = 3), or cottonseed oil vehicle (2 ml/day) (n = 3) were administered for 10 days as described previously [27]. Animals classified as sham (n = 3) controls were subjected to mid-ventral laparotomy. The uterine tract was observed to confirm that the animal was in estrus but did not receive any hormones. Pig uterine tracts were harvested on days 15-18. Portions of the uterus were placed in RNALater™ and stored in -80 °C until use.
RNA isolation and reverse transcriptase-polymerase chain reaction analysis
RNA was isolated from various tissues and uteri using TRIzol (Life Technologies, Gaithersburg, MD). Five micrograms of total RNA were used to synthesize first-strand cDNA (cDNA Cycle kit for RT-PCR, Invitrogen, San Diego, CA). The sequences for the trophinin primers were: (Forward) 5'-TAGCTTTGGTGGCATGCCTTGT-3', (Reverse) 5'-AAAGCCAGGGTTGGTGATTGGT-3'. This primer set amplifies a 553 bp product from both genomic DNA and cDNA. PCR reaction was performed by heating at 94 ℃ for 5 min, followed by 30 cycles of 94 ℃ 1 min, 58 ℃ 1 min, 72 ℃ 1 min, with a final extension step at 72 ℃ for 5 min and then stored at 20 ℃. Since our trophinin primers amplify both genomic and cDNA, our actin primers were designed to distinguish between genomic and cDNA. Actin primers were designed from the porcine beta-actin gene sequence with the following primer sequences: (forward) 5'-ATGGTGGGTATGGG-3', (Reverse) 5'-GCCATCTCGTTCTCGAAGTC-3', this primer set amplifies a 550 bp product from cDNA and a 790 bp fragment from genomic DNA. The authenticity of the PCR products was confirmed by sending purified samples to the W. M. Keck Center for Comparative and Functional Genomics University of Illinois, Urbana, IL, for sequencing. Sequence comparisons were made with databases in the BLAST network program (National Center for Biotechnology Information, NIH Bethesda, MD). Our pig sequences were 75% homologous to humans and 65% homologous to mouse, confirming previous reported results [26].
Results
Trophinin mRNA is expressed in the porcine uterus during the secretory phase
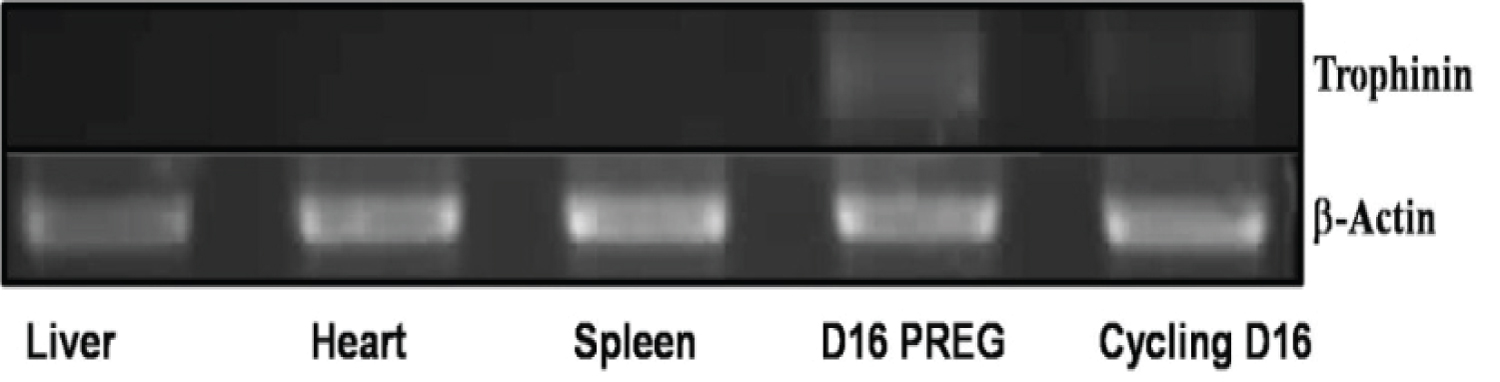
To determine whether trophinin is expressed in the porcine uterus, around the time of embryo implantation, we analyzed RNA isolated from the porcine uterus (day 16) as well as the liver, heart, and spleen. We monitored for the presence of the trophinin mRNA transcript by RT-PCR. The samples were reverse-transcribed and amplified by PCR using homologous regions in exon 12 of trophinin from both the mouse and human sequence. The results depicted in Figure 1 demonstrate that there were no trophinin transcripts detected in the liver, heart, or spleen samples. The band that represents trophinin mRNA is seen in both pregnant and non-pregnant animals (Day 16). This time point is during the window of receptivity in pigs, which ranges from days 11-18. The present data are consistent with the trophinin mRNA expression reported in mice and humans, where trophinin transcripts are also only found in the uterus of these species.
The presence of an embryo did not affect the expression of trophinin in the porcine uterus. Unlike the human, where the presence of an embryo controls the expression of trophinin, mice and swine express trophinin as part of the reproductive cycle during the window of receptivity.
Trophinin mRNA present on specific days of the reproductive cycle in pigs
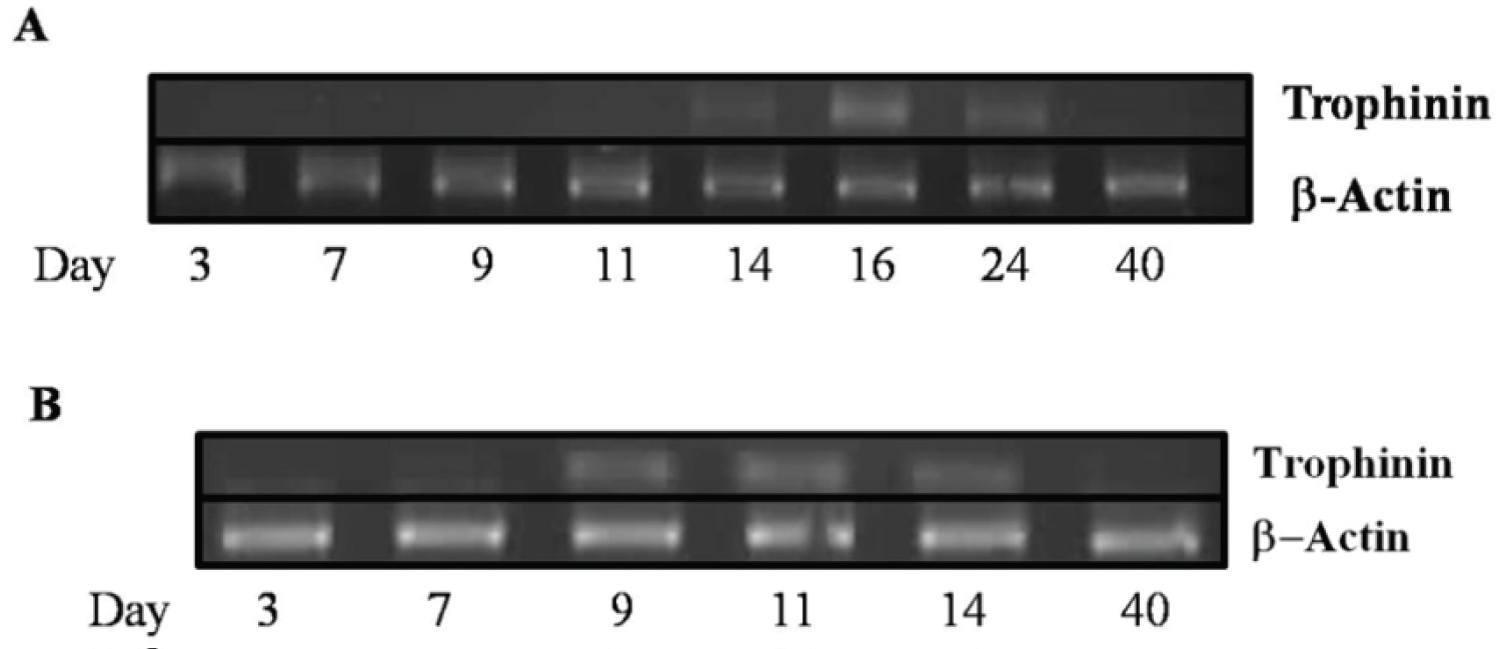
It had been previously reported that trophinin was transiently expressed during implantation in mice [20,26]. In humans, trophinin expression occurs in the maternal epithelial cells at the implantation site. Expression is dependent on the secretion of hCG secreted by the blastocysts [22] and continues to be expressed for up to ten weeks of pregnancy at the utero-placenta interface [11,16,17,23,24,28]. In the present study, using reverse transcriptase PCR, we show that trophinin transcripts are expressed during the window of receptivity around day 14 in the porcine uterus (Figure 2A). In the pregnant animals, trophinin transcripts were present at day 24 of pregnancy but were undetectable at day 40. To determine if transcripts were present earlier in the cycle in the porcine uterus, we performed nested-PCR. Trophinin transcripts were present as early as day 9, which would indicate that trophinin may be present but at very low levels and increase in subsequent days (Figure 2B).
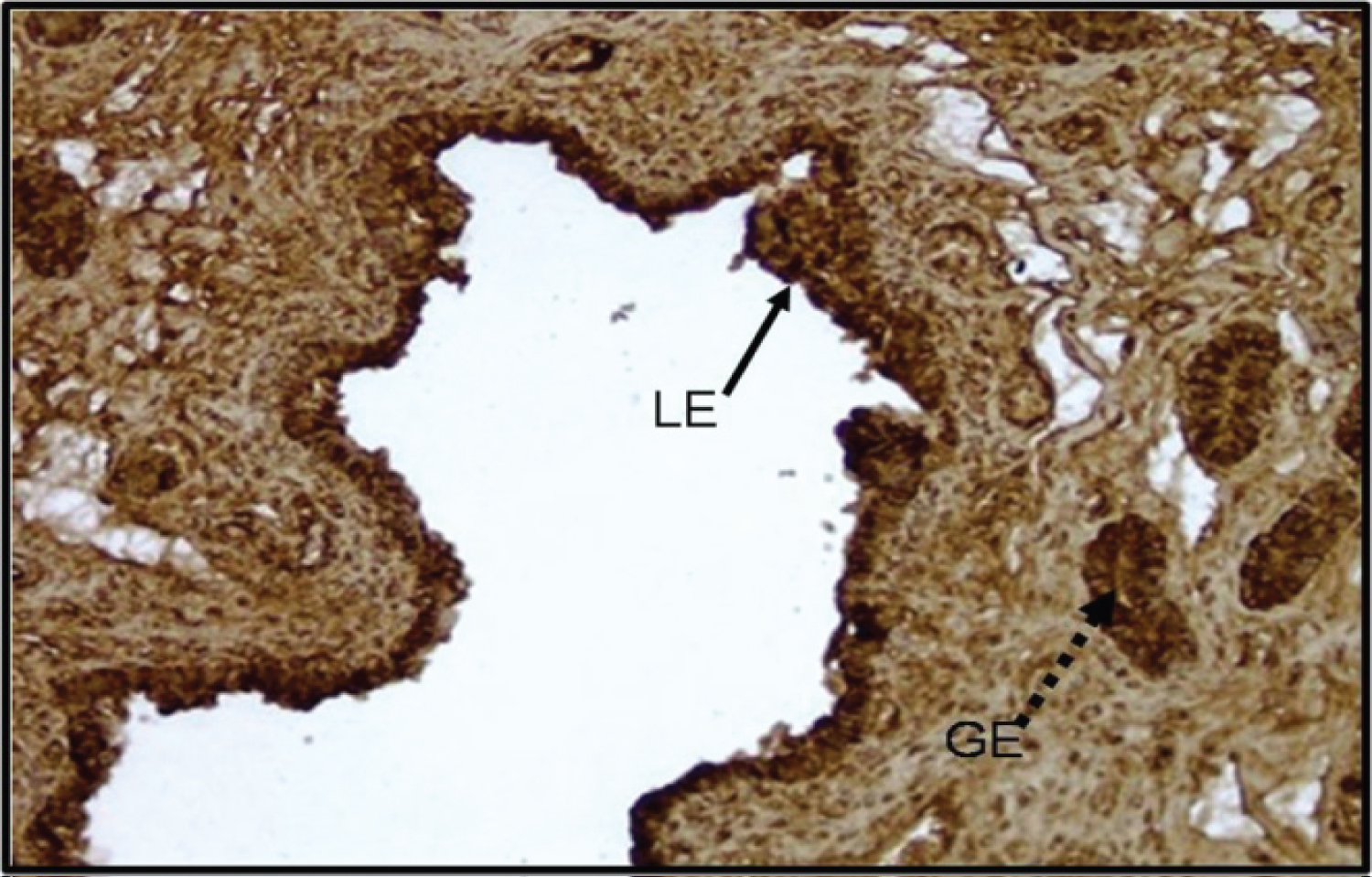
Immunohistochemistry of the pig uterus at day 14 of the porcine estrous cycle shows trophinin is present in the luminal and glandular epithelium (Figure 3). These results indicate that trophinin is present in the porcine uterus throughout the estrous cycle and the expression of this protein is embryo independent.
Trophinin expression in porcine embryos
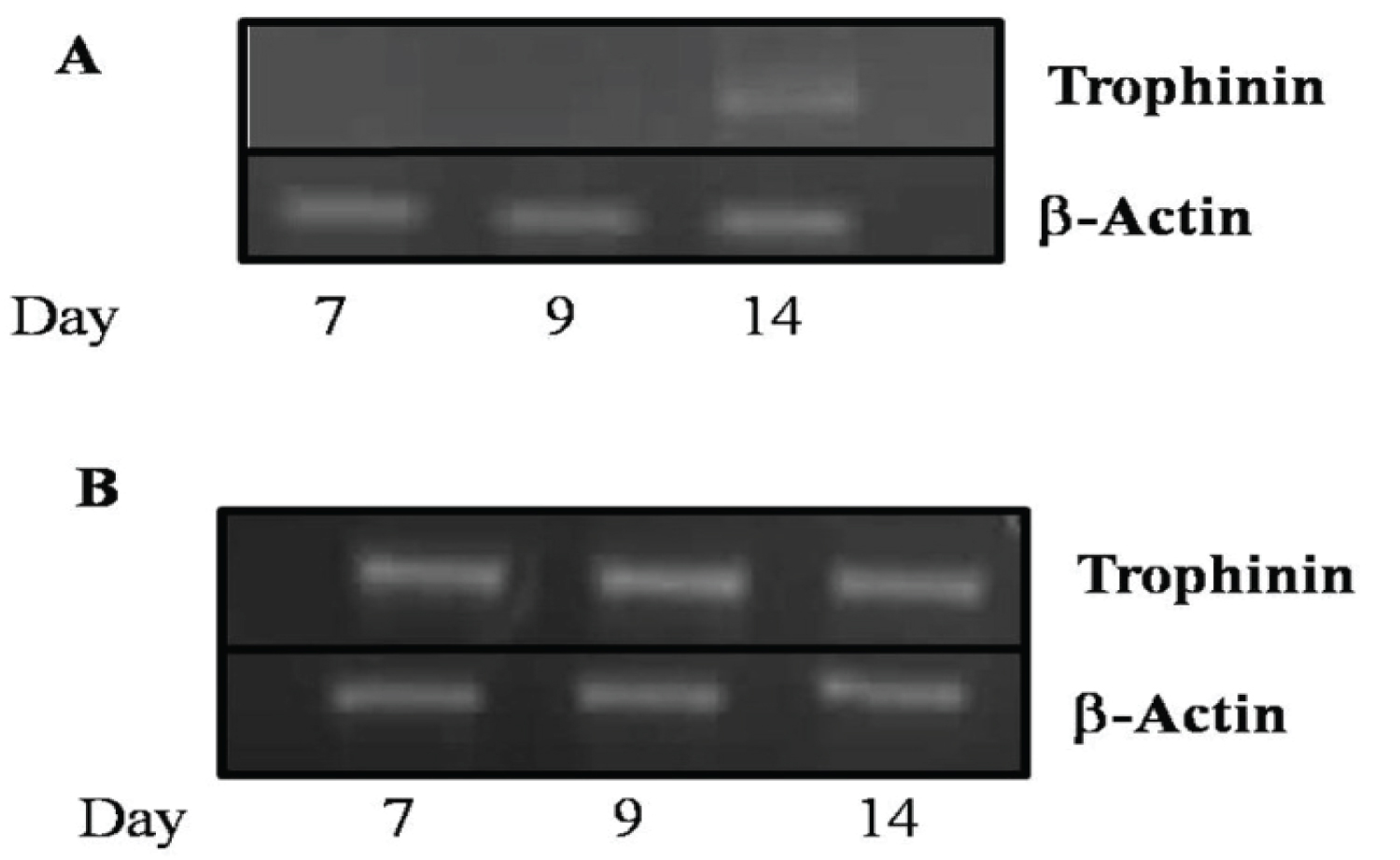
In non-human primates, it has been reported that there is expression of trophinin in blastocyst stage embryos [11,17]. In other species, the blastocyst stage embryo usually implants, but in the pig, implantation usually occurs with filamentous stage embryos [29,30]. The blastocyst stage embryo in the porcine usually happens around days 7-8 of the cycle. We examined pig embryos from various days of the reproductive cycle for the presence of trophinin mRNA transcripts by RT-PCR analysis. The presence of the trophinin mRNA transcript was only observed in embryos collected on day 14 (Figure 4A) of pregnancy. This timing coincides with the results observed in the endometrium examined earlier. However, the second round of nested PCR shows the transcripts are present in blastocyst stage embryos as early as day 7 of the cycle (Figure 4B).
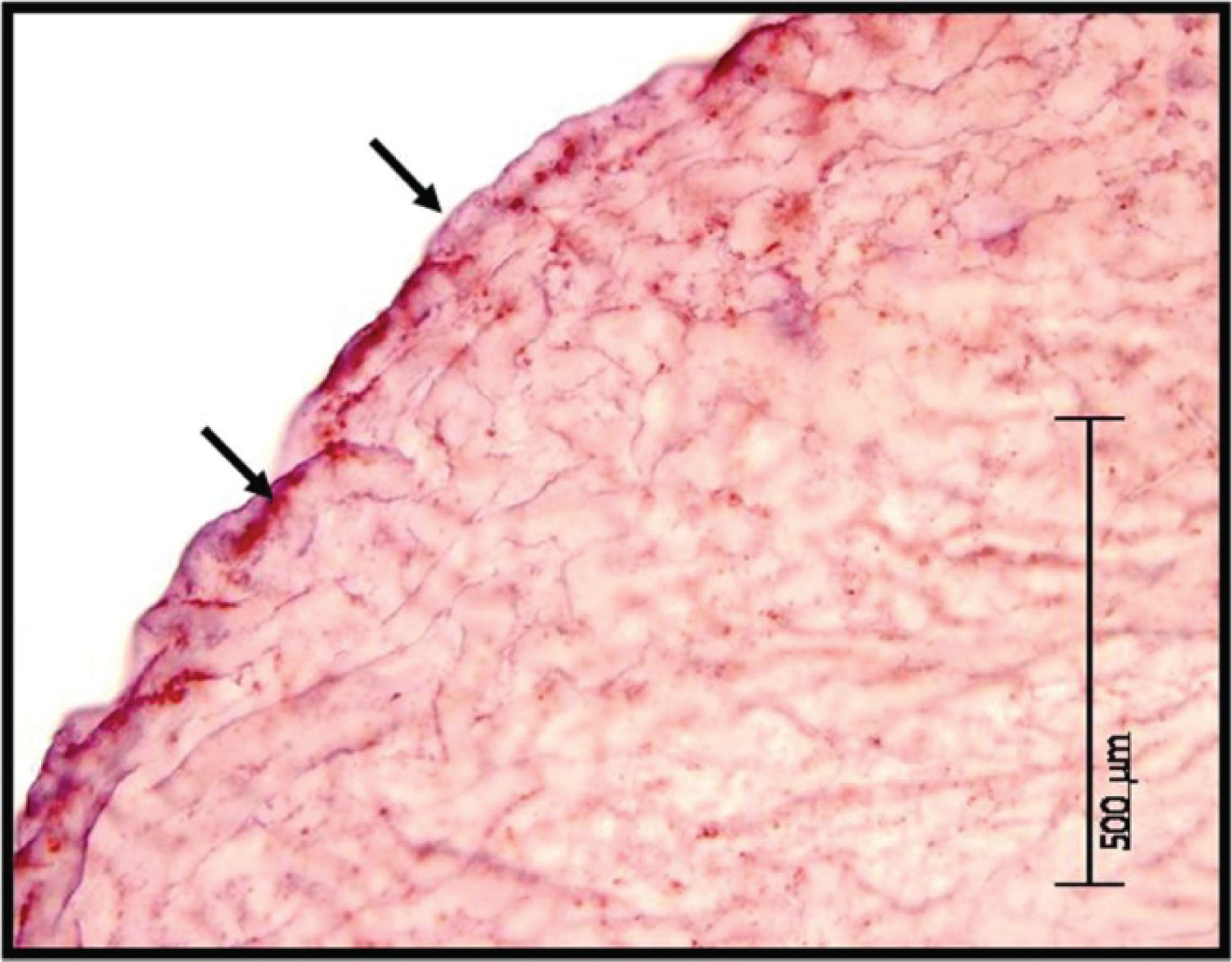
Immunohistochemistry of a Day 14 porcine embryo demonstrates intense punctate staining for trophinin protein at the apical portion of the porcine embryo (Figure 5).
Trophinin expression in the porcine uterus is regulated by progesterone
Regulation of trophinin varies between species. In the mouse, expression is correlated with increased estrogen production in the uterus [20]. In the human, expression is linked to hCG production by the embryo [21]. To determine which steroid hormon-regulates trophinin in the porcine uterus, we used an ovariectomized model. Normal cycling animals were ovariectomized on day 4 (estrus denotes day = 0). The ovariectomized animals received exogenous estrogen (n = 3), progesterone (n = 3), or corn oil (n = 3), for 10 days. Non-ovariectomized control animals did not receive any hormone treatments [27]. Endometrial tissue was isolated and analyzed for trophinin mRNA transcripts on day 16 of the estrous cycle (Figure 4). Trophinin transcripts were present in endometrial tissue from control animals (sham) and animals treated with progesterone (Figure 6). In contrast, animals that were administered corn oil or estrogen showed no trophinin mRNA transcripts. These results demonstrate that trophinin is a progesterone-regulated protein, which shows a similar expression pattern as other progesterone-regulated proteins (i.e., uteroferrin).
Discussion
Swine offers unique opportunities for reproductive studies, especially concerning implantation. The non-invasive nature of placentation in this species allows researchers to study mechanisms of adhesion that are not complicated by the erosion of the maternal endometrium [31]. Other domestic species, such as sheep with syndesmochorial placentation, and horses with chorionic girdle formation followed by diffuse epitheliochorial placentation, also offer distinctive features to understand the embryo-maternal communication leading to successful implantation [31-33].
Previous studies have concluded that trophinin has divergent roles regarding implantation in humans and mice [11,17,20,26,34,35]. Our data agree with those of Nakano (2004) and colleagues [26], in that trophinin is expressed in the porcine endometrium independent of the presence of an embryo. We report for the first time that trophinin expression in the uterus is progesterone regulated and confirm the presence of trophinin mRNA transcripts and protein in porcine embryos.
In the pig, it is well known that steroid hormones regulate uterine receptivity for embryo attachment [29-32,36]. Progesterone does not reach peak levels until ~day 12 in the porcine uterus [30,33,37-39]. During that time, there is a decrease in MUC-1 and an increase in specific integrins and uteroferrin [32,40-44], allowing implantation to occur. Trophinin expression in pigs follows this same pattern, contradicting previous work concerning trophinin expression in the porcine uterus [26]. These investigators reported expression of trophinin transcripts throughout the entire cycle with no cyclic changes between days 15 and 20.
In contrast, we show the presence of trophinin mRNA as early as day 8 with a more robust expression around days 11-12 of the cycle. This increased expression in the uterine epithelium coincides with increased progesterone levels between days 8-12 of the cycle. Our ovariectomized pig model also shows that only animals treated with progesterone expressed trophinin transcripts, whereas animals treated with estrogen or vehicle corn oil did not express trophinin.
The regulation of trophinin in the pig uterus highlights that the hormones involved are species-specific. Human trophinin is regulated by the production of hCG produced by the embryo during implantation, and this regulates its expression specifically at the site of implantation [18,21] reviews [32,33,38,44]. In the mouse, estrogen controls the expression of trophinin, and it is ubiquitously expressed throughout the entire uterus. However, mouse embryos can still successfully implant in the absence of trophinin [20,26]. Our data demonstrate that trophinin expression in the pig uterus is e regulated by progesterone.
The porcine embryo offers a unique pre-implantation model to investigate expression of trophinin. Trophinin protein was detected in non-human primate and human blastocyst stage embryos at the embryonic pole [11] and in human chorionic villi trophoblast at the feto-maternal interphase [16-18,20,21,35]. The porcine embryo is not spherical but filamentous and does not invade the uterine epithelium as some other species [30]. At the blastocyst stage, we observed the presence of trophinin mRNA by nested PCR (Figures 4 and Figure 5). Analysis of day 14 filamentous embryos demonstrated that there was more trophinin mRNA transcript being expressed at this time than at days 7 or 9. Others have also reported that trophinin transcripts are present in the placentas of human and non-human primates throughout early pregnancy [17,35]. Expression of trophinin by the embryo may serve other roles in addition to facilitating attachment at implantation and this merits further investigation.
Generalizations regarding the mechanisms of embryo implantation in different species are restricted by the wide variation in uterine shape, blastocyst size and morphology, timing of implantation, and invasive vs. non-invasive methods of implantation used by different mammalian species [32]. Generalizations regarding the role of trophinin during implantation need to consider such species variation. Trophinin protein has been implicated to be an essential implantation factor in humans and non-human primates [17,21]. In the mouse and pig, it seems that trophinin expression does coincide with the timing of implantation but may not be essential for implantation [18-20,26]. It would be interesting to determine if other domestic species (e.g., sheep and cow) express trophinin around the time of embryo implantation. Are there differences between the pig and mouse which are polytocous species, and the human, horse, and cow which are usually single-bearing species? The likelihood that trophinin is being utilized by other species during embryo implantation and its potential functions warrants more extensive studies.
In summary, our findings demonstrate that trophinin is expressed in the uterus of pregnant and not pregnant pigs and also in pig embryos. Trophinin expression in the uterus of the pig is regulated by progesterone and peaks at days 16-24 of pregnancy when porcine embryos would be implanting. The specific roles of trophinin in porcine implantation remain to be determined. Expression patterns of trophinin throughout the cycle suggest that porcine trophinin expression is estrous cycle-dependent, similar to what is observed in the mouse. Reports focusing on implantation in humans have shown that this adhesion molecule plays an important role in embryo implantation [18]. Studies in domestic animal species will likely yield additional insights into its functions during implantation.
Support
KSJ was supported by: Illinois Minority Graduate Incentive program (IMGIP) and USDA Multistate Research Project W-1171 (ILLU-538-393).
References
- Psychyoyos A (1963) Endocrine control of egg implantation. In: Greep R, Astwood E, Geiger S, Handbook of Physiology II. Washington, DC: American Physiological Society, 187-215.
- Schlafke S, Enders AC (1975) Cellular basis of interaction between trophoblast and uterus at implantation. Biol Reprod 12: 41-65.
- Denker H-W (1990) Trophoblast - Endometrial Interactions at Embryo Implantation: A cell biological paradox. In: Denker H-W, Aplin JD, Trophoblast Invasion and Endometrial Receptivity: Novel Aspects of the Cell Biology of Embryo Implantation. Boston, MA: Springer US, 3-29.
- Dey S (1996) Implantation. In: Adashi E, Rock J, Rosenwaks Z, Reproductive endocrinology, surgery and technology, New York: Lippincott-Raven, 421-434.
- Carson DD, Bagchi I, Dey SK, et al. (2000) Embryo implantation. Developmental Biology 223: 217-237.
- Psychoyos A (1986) Uterine receptivity for nidation. Ann N Y Acad Sci 476: 36-42.
- Denker HW (1993) Implantation: A cell biological paradox. J Exp Zool 266: 541-558.
- Bowen JA, Burghardt RC (2000) Cellular mechanisms of implantation in domestic farm animals. Semin Cell Dev Biol 11: 93-104.
- King RJB, Townsend PT, Siddle N, et al. (1982) Regulation of estrogen and progesterone receptor levels in epithelium and stroma from pre-and postmenopausal endometria. Journal of Steroid Biochemistry 16: 21-29.
- Bowen JA, Bazer FW, Burghardt RC (1996) Spatial and temporal analyses of integrin and Muc-1 expression in porcine uterine epithelium and trophectoderm in vivo. Biol Reprod 55: 1098-1106.
- Fukuda MN, Sato T, Nakayama J, et al. (1995) Trophinin and tastin, a novel cell adhesion molecule complex with potential involvement in embryo implantation. Genes Dev 9: 1199-1210.
- Ducibella T, Anderson D, Aalberg J, et al. (1982) Cell surface polarization, tight junctions, and eccentric inner cells characterize human teratocarcinoma embryoid bodies. Dev Biol 94: 197-205.
- Izhar M, Siebert PD, Oshima RG, et al. (1986) Trophoblastic differentiation of human teratocarcinoma cell line HT-H1. Dev Biol 116: 510-518.
- Ishiwata I, Nozawa S, Inoue T, et al. (1977) Development and characterization of established cell lines from primary and metastatic regions of human endometrial adenocarcinoma. Cancer Res 37: 1777-1785.
- Andrews RG, Torok-Storb B, Bernstein ID (1983) Myeloid-associated differentiation antigens on stem cells and their progeny identified by monoclonal antibodies. Blood 62: 124-132.
- Fukuda MN, Nozawa S (1999) Trophinin, tastin, and bystin: A complex mediating unique attachment between trophoblastic and endometrial epithelial cells at their respective apical cell membranes. Semin Reprod Endocrinol 17: 229-234.
- Suzuki N, Nakayama J, Shih IM, et al. (1999) Expression of trophinin, tastin, and bystin by trophoblast and endometrial cells in human placenta. Biol Reprod 60: 621-627.
- Fukuda MN, Sugihara K, Nakayama J (2008) Trophinin: What embryo implantation teaches us about human cancer. Cancer Biol Ther 7: 1165.
- Nadano D, Sugihara K, Paria BC, et al. (2002) Significant differences between mouse and human trophinins are revealed by their expression patterns and targeted disruption of mouse trophinin gene. Biol Reprod 66: 313-321.
- Suzuki N, Nadano D, Paria BC, et al. (2000) Trophinin expression in the mouse uterus coincides with implantation and is hormonally regulated but not induced by implanting blastocysts. Endocrinology 141: 4247-4254.
- Nakayama J, Aoki D, Suga T, et al. (2003) Implantation-dependent expression of trophinin by maternal fallopian tube epithelia during tubal pregnancies: Possible role of human chorionic gonadotrophin on ectopic pregnancy. Am J Pathol 163: 2211-2219.
- Fukuda MN, Sugihara K (2008) An integrated view of L-selectin and trophinin function in human embryo implantation. J Obstet Gynaecol Res 34: 129-136.
- Fukuda MN, Sugihara K (2012) Cell adhesion molecules in human embryo implantation. Sheng Li Xue Bao 64: 247-258.
- Fukuda MN, Sugihara K (2012) Trophinin in cell adhesion and signal transduction. Front Biosci (Elite Ed) 4: 342-350.
- Hajipour H, Farzadi L, Roshangar L, et al. (2021) A human chorionic gonadotropin (hCG) delivery platform using engineered uterine exosomes to improve endometrial receptivity. Life Sci 275: 119351.
- Nakano S, Kishi H, Ogawa H, et al. (2003) Trophinin is expressed in the porcine endometrium during the estrous cycle. J Reprod Dev 49: 127-134.
- Knight JW, Bazer FW, Wallace HD, et al. (1974) Dose-response relationships between exogenous progesterone and estradiol and porcine uterine protein secretions. J Anim Sci 39: 747-751.
- Aoki R, Fukuda MN (2000) Recent molecular approaches to elucidate the mechanism of embryo implantation: Trophinin, bystin, and tastin as molecules involved in the initial attachment of blastocysts to the uterus in humans. Semin Reprod Med 18: 265-271.
- Geisert RD, Yelich JV (1997) Regulation of conceptus development and attachment in pigs. J Reprod Fertil Suppl 52: 133-149.
- Geisert R, Whyte J, Meyer AE, et al. (2017) Rapid conceptus elongation in the pig: An interleukin 1 beta 2 and estrogeé regulated phenomenon. Mol Reprod Dev 84: 760-774.
- Bazer FW, Spencer TE, Johnson GA, et al. (2009) Comparative aspects of implantation. Reproduction 138: 195-209.
- Bazer FW, Wu G, Spencer TE, et al. (2009) Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Molecular Human Reproduction 16: 135-152.
- Bazer FW, Song G, Kim J, et al. (2012) Uterine biology in pigs and sheep. J Anim Sci Biotechnol 3: 23.
- Suzuki N, Zara J, Sato T, et al. (1998) A cytoplasmic protein, bystin, interacts with trophinin, tastin, and cytokeratin and may be involved in trophinin-mediated cell adhesion between trophoblast and endometrial epithelial cells. Proc Natl Acad Sci USA 95: 5027-5032.
- Fukuda MN, Nadano D, Suzuki N, et al. (1999) Potential involvement of trophinin, bystin, and tastin in embryo implantation. In: Carson DD, Embryo Implantation: Molecular, Cellular and Clinical Aspects. New York, NY: Springer New York, 132-140.
- Geisert RD, Zavy MT, Moffatt RJ, et al. (1990) Embryonic steroids and the establishment of pregnancy in pigs. J Reprod Fertil Suppl 40: 293-305.
- Moeljono MP, Bazer FW, Thatcher WW (1976) A study of prostaglandin F2alpha as the luteolysin in swine: I. Effect of prostaglandin F2alpha in hysterectomized gilts. Prostaglandins 11: 737-743.
- Bazer FW, Roberts RM (1938) Biochemical aspects of conceptus--endometrial interactions. J Exp Zool 228: 373-383.
- Gadsby JE, Balapure AK, Britt JH, et al. (1990) Prostaglandin F2 alpha receptors on enzyme-dissociated pig luteal cells throughout the estrous cycle. Endocrinology 126: 787-795.
- Baumbach GA, Bartley NG, Kattesh HG, et al. (1990) Immunolocalization and endocytosis of the uterine secretory protein, uteroferrin, in pre-implantation pig trophectoderm on day 11 of pregnancy. Anat Embryol (Berl) 182: 563-568.
- Genbacev OD, Prakobphol A, Foulk RA, et al. (2003) Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science 299: 405-408.
- Dharmaraj N, Gendler SJ, Carson DD (2009) Expression of human MUC1 during early pregnancy in the human MUC1 transgenic mouse model. Biology of reproduction 81: 1182-1188.
- Ren Q, Guan S, Fu J, et al. (2010) Temporal and spatial expression of Muc1 during implantation in sows. Int J Mol sci 11: 2322-2335.
- Waclawik A, Kaczmarek M, Blitek A, et al. (2017) Embryè maternal dialogue during pregnancy establishment and implantation in the pig. Mol Reprod Dev 84: 842-855.
Corresponding Author
Dr. Matthew B Wheeler, Biotechnology and Development Biology, Institute for Genomic Biology, Bioengineering, Biomedical and Translational Sciences, Veterinary Clinical Medicine; Department of Animal Sciences, University of Illinois, 1207 West Gregory Drive, Urbana, IL 61801, USA, Tel: (217)-333-2239, Fax: (312)-333-8286.
Copyright
© 2022 Jackson KS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.