Role of Extracellular Matrix Stiffness Inhibitors in Alzheimer's Disease
Abstract
Alzheimer's disease is closely related with ageing and age-related extracellular matrix stiffness increase. The association between stiffness and NF-kB activation was demonstrated and link between tissue stiffness and inflammation was suggested. The inhibitors of extracellular matrix stiffening have been reviewed as potential drug components for Alzheimer's disease retardation, prevention and treatment.
Keywords
Ageing, Alzheimer's disease, Stiffness, Inhibitors, NF-κB, Nrf2, Curcumin, Chitosan oligosaccharides
Introduction
Cardiovascular disease (CVD) and Alzheimer's disease (AD) are age-related deceases [1-3]. Mechanobiology of the brain in ageing and Alzheimer's disease have been recently reviewed [2].
Close association can be suggested between biological tissues stiffness and oxidative stress and inflammation. The objective of this brief review is to demonstrate that ageing is directly related with biological tissue stiffening and that tissue stiffness plays an important role in various age-related pathologies especially in AD.
In order to retard ageing and to prevent development of age-related diseases it is important to find destiffening methods and substances, especially natural without side effects.
Ageing, Stiffness and Cytoskeleton
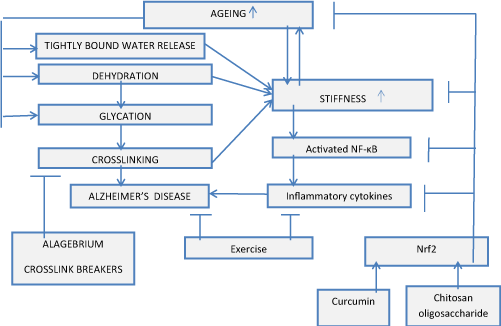
Advanced glycation end products (AGEs) contribute to amyloidosis in Alzheimer disease. AGEs may act to promote accumulation of additional amyloid [4]. So AGE crosslink breakers, for example alagebrium (ALT-711), can retard AD development. Alagebrium also reduced carotid artery stiffness by 37% [5]. AGEs in cardiovascular disease, diabetic complications, and Alzheimer's disease must be taken into account. Alagebrium can decrease amyloidosis in AD, figure 1.
Ageing is associated with increased tissue stiffness and cytoskeleton actin polymerization.Related with ageingdecrease in bound water content leads to an increase in biological tissue stiffness, and increased tissue stiffness results in NF-kB activation and triggerers actin polymerization [6]. Cytoskeleton controls activation, proliferation and apoptosis of T cells. During aging there is a decline in activation of CD4T cells [7]. T-cell activation depends on 3D mechanical microenvironment. Responsiveness of T cells to stimuli is higher on stiffer substrates [7]. T-cells are activated in AD patients [8]. So increased tissue stiffness with advanced age can be associated with AD and activated T cells.
Stiffness and Autophagy
It has been shown that rapamycin improves learning and memory and reduces Aβ and Tau pathologyby increasing autophagy [9]. The researchers at Free University of Berlin consider that influence and mechanism of ECM stiffness on autophagy in cancer cells still remains unclear. The increasing matrix stiffness results in elevated autophagy.Rho-ROCK-ERK signal pathway and actin cytoskeleton were essential for the regulation of autophagy by matrix stiffness [10]. The mechanobiology of autophagy has been recently reviewed [11]. Impaired autophagy could lead to Aβ accumulation.
Yes-associated protein (YAP) and the transcriptional co-activator with PDZ-binding Motif (TAZ) regulate gene expression depending on mechanical forces. YAP/TAZ cytosolic localization and nuclear translocation depends on mechanical properties of microenvironment and mechanotransduction [12]. YAP activity depends on extracellular matrix stiffness. YAP and TAZ are active and localize in the nucleus of cells on a stiff matrix, where they promote the expression of several growth-related genes and induce cell proliferation.YAP/TAZ is inactive and localized in the cytoplasm on soft matrix.
Trehalose has been reported as autophagy activator and enhancer [13,14] and trehalose also prevents transformation of tightly bound water into loosely bound and free water. [14,15]. Trehalose also inhibits inflammatory cytokine production [16].
Stiffness and Ad
Extracellular to intracellular water ratio increases with advanced age and can be related with driven by entropy increase in transformation of tightly bound water into loosely bound water. This transformation increases matrix stiffness. Dehydration also leads to increase of stiffness due to crosslinking related with glycation processes. Water plays an important role in amyloid formation and hydration water mobility is increased around tau amyloid fibers [14,15,17 ] .
Arterial stiffness, as measured by pulse wave velocity, was related with the extent of Aβ deposition [18]. It has been recently concluded that carotid artery stiffening, may contribute to AD pathology in patients with amnestic mild cognitive impairment [19].
AD can be associated with Aβ accumulation in brain. Overproduction of Aβ leads to the formation of plaque and AD development. Exercise leads to inhibition of Aβ expression [20,21].
Stiffness Inhibitors
Life style modifications have been proposed to improve arterial stiffness. Long-term ω-3 fatty acids (fish oil) supplementation in diet was also recommended to decrease arterial stiffness in the population with hypertension. Renin-angiotensin-aldosterone system antagonists, metformin, and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors, antibodies against TNF- have been also suggested [22]. For weight loss-induced decreases in arterial stiffness in overweight and obese men positive correlation between changes in arterial stiffness and circulating interleukin-6 (IL-6) levels was reported [23].
It has been found that low-dose aspirin consumption during 2 weeks decreases aortic stiffness in hypertensive patients [24]. And reduced risk of AD among 2 or more years users of aspirin was reported [25]. Long duration regular use of aspirin was also associated with reduced risk of prostate cancer [26] and breast cancer [27]. So it can be suggested that decreased stiffness leads to reduced risk of Alzheimer's disease.
The effect of curcumin on Alzheimer's disease has been reviewed [28]. Epidemiological studies demonstrated that incidence of AD among adults aged 70-79 years in India, where the consumption of curry is higher than in western countries, is 4.4 times less than that of adults aged 70-79 years in the United States. Curcumin suppresses production of pro-inflammatory cytokines IL-1, IL-6 and TNF-α [29]. So curcumin can show beneficial effects also in the treatment of COVID-19 by mitigating cytokine storm [30]. Curcumin inhibits cellular gene expression regulated by transcription factors NF-kB, AP-1, and Egr-1 [31]. Curcumin also suppresses Aβ and tau accumulation in animal models and improves mitochondria function [32].Curcumin blocks the aggregation and formation of fibrils in vitro and in vivo [33]. Curcumin upregulates Nrf2 nuclear translocation [34,35]. (-)-epigallocatechin-3-gallate (EGCG) is a flavonoid with the inhibition of Aβ fibrillogenesis [36].
Curcumin intake decreases arterial stiffness [37]. Decreased stiffness can be suggested as a main beneficial factor in the prevention and treatment of AD by curcumin. It was reported that ibuprofen inhibits plaque pathology in a mouse model for AD [38]. Increases in cell contractility, cell-cell junction size, and stiffness-dependent increases in permeability, can be decreased with simvastatin treatment [39].
The potential of chitosan oligosaccharides (COS) in AD prevention and treatment has been much less investigated, [40] but it was reported that chitosan astrocytoma cells were activated by amyloid β peptide and interleukin-1β [41]. COS with molecular weights (MW) from 5,000 Da to 14,000 Da induced AMP-activated protein kinase (AMPK) activation in intestinal epithelial cells and inhibited NF-κB transcriptional activity and NF-κB-mediated inflammatory response [42]. NF-κB suppression upregulates Nrf2 nuclear translocation [14,35] and similarly to effect of curcumin has potential to be beneficial for AD treatment. Chitosan oligosaccharides promote Nrf2 nuclear translocation, figure 1 [43,44].
COS decrease the release of pro-inflammatory cytokinesinterleukin-1β and tumor necrosis factor TNF-α inan amyloid-β1-42-induced rat model of Alzheimer's disease [45].
Chitosan oligosaccharide graft citronellol derivatives (COS-g-Cit1-3) reduced the expression levels of TNF-α and COS-g-Cit1-3 inactivated the NF-κB signaling pathway via inhibiting the phosphorylation of p65, IKBα and IKKβ [46].
The above mentioned cellular processes depend on ECM stiffness as presented in figure 1.
Concluding Remarks
The aged tissue has higher stiffness and is prone to various pathologies. High tissue stiffness initiates and drives age-related diseases. So it is very important to develop destiffening strategy and to find effective stiffness suppressors. Aspirin and non- steroidal anti-inflammatory drugs have demonstrated destiffening effect and reducing risk of Alzheimer's disease, prostate and breast cancers. Chitosan oligosaccharides reduced the expression levels of TNF-α and inactivated the NF-κB signaling. NF-κB suppression upregulates Nrf2 nuclear translocation and could be beneficial in AD prevention and treatment. Curcumin intake decreases arterial stiffness. Curcumin upregulates Nrf2 nuclear translocation, suppresses production of pro-inflammatory cytokines IL-1, IL-6 and TNF-α, inhibits cellular gene expression regulated by transcription factors NF-kB, and suppresses Aβ and tau accumulation in brain of AD animal models. Exercise leads to inhibition of Aβ expression.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public commercial or nonprofit sectors.
Conflicts of Interest
The author declares no conflict of interest.
Research in Context
Systematic review
There is an urgent need with world population aging and initiation and increase in a number of age-related diseases including Alzheimer's disease (AD) in order to better understand basic mechanisms leading to the pathologies developing with advanced age and to find the treatment strategies that are able to retard and prevent AD and related diseases. The goal was to identify biological changes associated with aging and to determine how these changes can be related with AD.
Interpretation
Cellular processes strongly depend on extracellular matrix mechanical stiffness that regulates through cytoskeleton such processes as transcription factors translocation from cytoplasm to nuclear and changes in gene expression. Dehydration driven by entrophy growth, transformation of tightly bound water into loosely bound and free water and glycation crosslinking reactions increase stiffness of extracellular microenvironment with aging. In context of clinical application this article provides a discussion on the future research directions.
Future Directions
The effective potential tissue stiffness inhibitors could be developed with the goal to retard aging and to prevent initiation of AD and other age related diseases. The links between inflammation, oxidative stress andtissue stiffness will be further investigated.
References
- Garcia GG and Miller RA (2011) Age-related defects in the cytoskeleton signaling pathways of CD4 T cells. Ageing Res Rev 10: 26-34.
- Hall CM, Moeendarbary E, Sheridan (2021) Mechanobiology of the brain in ageing and Alzheimer's disease. Eur J Neurosci 53: 3851-3878.
- Kerch G (2015) The potential of chitosan and its derivatives in prevention and treatment of age-related diseases. Marine Drugs 13: 2158-2182.
- Vitek MP, K Bhattacharya K, J M Glendening JM, et al. (1994) Advanced glycation end products contribute to amyloidosis in Alzheimer disease. PNAS 91: 4766-4770.
- Zieman SJ, Melenovsky V, Clattenburg L, et al. (2007) Advanced glycation endproduct crosslink breaker (alagebrium) improves endothelial function in patients with isolated systolic hypertension. J Hypertens 25: 577-583.
- Kerch G (2020) Role of changes in state of bound water and tissue stiffness in development of age-related diseases. Polymers (Basel) 12: 1362.
- Majedi FS, Hasani-Sadrabadi MM, Thauland TJ, et al. (2020) T-cell activation is modulated by the 3D mechanical microenvironment. Biomaterials 252: 120058.
- Town T, Tan J, Flavell RA, et al. (2005) T-cells in Alzheimer's disease. Neuromolecular Med. 7: 255-264.
- Caccamo A, Majumder S, Richardson A, et al. (2010) Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem 285: 13107-13120.
- Li Y, Randriantsilefisoa R, Chen J, et al. (2020) Matrix stiffness regulates chemosensitivity, stemness characteristics, and autophagy in breast cancer cells. ACS Appl Bio Mater 3: 4474- 4485.
- Hernández-Cáceres MP, Munoz L, Pradenas JM, et al. (2021) Mechanobiology of autophagy: The unexplored side of cancer. Front Oncol. 632956.
- Dupont S, Morsut L, Aragona M, et al. (2011) Role of YAP/TAZ in mechanotransduction. Nature 474: 179-183.
- Sarkar S, Davies JE, Huang Z, et al (2007) Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and a-synuclein.J Biol Chem 282: 5641-5652.
- Kerch G. (2021) Tissue Integrity and COVID-19. Encyclopedia 1: 206-219.
- Kerch G. (2018) Distribution of tightly and loosely bound water in biological macromolecules and age-related diseases. IntJ Biol Macromol 118: 1310-1318.
- Taya K, Hirose K, Hamada S, et al. (2009) Trehalose inhibits inflammatory cytokine production by protecting IkB-areduction in mouse peritoneal macrophages. Arch Oral Biol 54: 749-756.
- Fichou Y, Schirò G, Gallat FX, et al. (2015) Hydration water mobility is enhanced around tau amyloid fibers. Proc Natl Acad Sci USA 112: 6365-6370.
- Hughes TM, Kuller LH, Barinas-Mitchell EJM, et al. (2014) Arterial stiffness and ß-amyloid progression in nondemented elderly adults. JAMA Neurol 71: 562-568.
- Pasha EP, Rutjes E, Tomoto T, et al. (2020) Carotid stiffness is associated with brain amyloid- burden in amnestic mild cognitive impairment. J Alzheimer's Dis 74: 925-935.
- Kim SH, Ko YJ, Kim JY, et al. (2021) Treadmill running improves spatial learning memory through inactivation of nuclear factor kappa B/mitogen-activated protein kinase signaling pathway in amyloid-ß-Induced alzheimer disease rats. Int Neurourol J 25: S35-S43.
- Ko YJ and Ko IG (2020) Voluntary wheel running improves spatial learning memory by suppressing inflammation and apoptosis via inactivation of nuclear factor kappa B in brain inflammation rats. Int Neurourol J 24: 96-103.
- Wu CF, Liu PY, Wu TJ, et al. (2015) Therapeutic modification of arterial stiffness: An update and comprehensive review. World J Cardiol 7: 742-753.
- Kumagai H, Zempo-Miyaki A, Yoshikawa T, et al. (2018) Which cytokine is the most related to weight loss-induced decrease in arterial stiffness in overweight and obese men? Endocr J 65: 53-61.
- Pietri P, Vlachopoulos C, Terentes-Printzios D, et al. (2014) Beneficial effects of low-dose aspirin on aortic stiffness in hypertensive patients. Vasc Med 19: 452-457.
- Stewart WF, Kawas C, Corrada M, et al. (1997) Risk of Alzheimer's disease and duration of NSAID use. Neurology 48: 626-632.
- Jacobs EJ, Rodriguez C, Mondul AM, et al. (2005) A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J Natl Cancer Inst 97: 975-980.
- Johnson TW, Anderson KE, Lazovich D et al. (2002) Association of aspirin and nonsteroidal anti-inflammatory drug use with breast cancer. Cancer Epidemiol Biomarkers Prev 11: 1586-1591.
- Mishra S and Palanivelu K (2008) The effect of curcumin (turmeric) on Alzheimer's disease: An overview. Ann Indian Acad Neurol 11: 13-19.
- Kim GY, Kim KH, Lee SH, et al. (2005) Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J Immunol 174: 8116-8124.
- Rattis BAC, Ramos SG, Celes MRN (2021) Curcumin as a potentialtreatment for COVID-19. Front Pharm 12: 1068.
- Pendurthi UR, Williams JT, Rao LV, et al. (1997) Inhibition of tissue factor gene activation in cultured endothelial cells by curcumin. Suppression of activation of transcription factors Egr-1, AP-1, and NF-kappa B. Arterioscler Thromb Vasc Biol 17: 3406-3413.
- Kim Y and Clifton P. (2018) Curcumin, Cardiometabolic Health and Dementia. Int J Environ Res Public Health 15: 2093.
- Hamaguchi T, Ono K, Yamada M, et al. (2010) Curcumin and Alzheimer's disease. CNS Neurosci. Ther. 16: 285-297.
- Ashrafizadeh M, Ahmadi Z, Mohammadinejad R, et al. (2020) Curcumin activates the Nrf2 pathway and induces cellular protection against oxidative injury. Curr Mol Med 20: 116-133.
- Liu Z, Dou W, Zheng Y, et al. (2016) Curcumin upregulates Nrf2 nuclear translocation and protects rat hepatic stellate cells against oxidative stress. Mol Med Rep 13: 1717-1724.
- Bieschke J, Russ J, Friedrich RP, et al. (2010) EGCG remodels mature a-synuclein and amyloid-ß fibrils and reduces cellular toxicity. Proc Natl Acad Sci USA 107: 7710-7715.
- Akazawa N, Choi Y, Miyaki A, et al. (2013) Effects of curcumin intake and aerobic exercise training on arterial compliance in postmenopausal women. Artery Res 7: 67 - 72.
- Lim GP, Yang F, Chu T, et al. (2000) Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J Neurosci. 20:5709-5714.
- Lampi MC, Faber CJ, Huynh J, et al. (2016) Simvastatin ameliorates matrix stiffness-mediated endothelial monolayer disruption. PLoS One 11: 0147033.
- Naveed M, Phil L, Sohai M, et al. (2019) Chitosan oligosaccharide (COS): An overview, Int J Biol Macromol 129: 827-843.
- Kim MS, Sung MJ, Seo SB, et al. (2002) Water-soluble chitosan inhibits the production of pro-inflammatory cytokine in human astrocytoma cells activated by amyloid ß peptide and interleukin-1ß. Neurosci Lett 321: 105-109.
- Muanprasat C, Wongkrasant P, Satitsri S, et al. (2015) Activation of AMPK by chitosan oligosaccharide in intestinal epithelial cells: Mechanism of action and potential applications in intestinal disorders, Biochem Pharmacol 96: 225-236.
- Wang Y, Xiong Y, Zhang A, et al. (2020) Oligosaccharide attenuates aging-related liver dysfunction by activating Nrf2 antioxidant signaling. Food Sci Nutr 8: 3872-3881.
- Zhang X, Liang S, Gao X, et al. (2021) Protective effect of chitosan oligosaccharide against hydrogen peroxide-mediated oxidative damage and cell apoptosis via activating Nrf2/ARE signaling pathway. Neurotox Res 39: 1708-1720.
- Jia S, Lu Z, Gao Z, et al. (2016) Chitosan oligosaccharides alleviate cognitive deficits in an amyloid-ß1-42-induced rat model of Alzheimer's disease. Int J Biol Macromol 3: 416-425.
- Mao S, Wang B, Yue L, et al. (2021) Effects of citronellol grafted chitosan oligosaccharide derivatives on regulating anti-inflammatory activity. Carbohydr Polym 262: 117972.
Corresponding Author
Garry Kerch, Faculty of Materials Science and Applied Chemistry, Riga Technical University, P. Valdena 3, Riga, Latvia.
Copyright
© 2022 Kerch G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.