Architectural Arrangement of Neurons as Part of the Functional Unit of the Central Nervous System
Abstract
An understanding of the cellular structural relationships in human brain tissue will allow for improved appreciation of the functional importance. A model of the functional unit brings together the important cell types with improved visualization of the relationships. While much research evaluates the metabolic relationships of the brain cells a visualization of structural relationships improves our understanding in additional ways that will lead to future breakthroughs in Alzheimer's and other neurodegenerative diseases (https://www.dementiapreventioncenter.com/).
Introduction
Since Ramon y Cajal proposed the neuron as "Objective evidence of the anatomical unit of the Central Nervous System" there has been significant progress in the understanding of functional, histological, histochemical, and biochemical interactions [1,2]. Understanding the six structural elements: neurons, astroglia, oligodendroglia, microglia, vascular network, and interstitial fluid are now paramount in advancing future research in brain degenerative disorders. The interactions of these six elements are involved in the regulation of the cerebral blood flow [3-5]. The critical and complex interaction of all components are responsible for the normal functioning of the brain tissue [6,7]. Altering of any element, separately or in association with other elements, will result in an alteration of the performance of a functional unit. These cellular components of the functional units are organized into networks as supported by ultrastructural data [8]. We propose these six cellular, vascular, and interstitial fluid components build the functional unit. This "Crystalline Tissular Network of the Cerebral Cortex" as the functional unit of the central nervous system will provide access to new research concepts and models that deal with the histological, functional, or pathological changes in all central nervous system diseases including those classified as Alzheimer's disease and other types of dementia.
Material and Methods
Together we combined our long experience in neuropathology working with morphological methods, histochemical and vascular perfusion strategies as presented here. We have observed the five mentioned tissue elements of the brain in mice, rats, other animals, as well as in a large number of human brains. Innumerable histological serial sections, parallel and perpendicular to the cortical surface were made to confirm the cellular structural relationships [9]. The work is based on the classic works of Ramón y Cajal [10], Golgi [11], De Felipe and Jones [12], Río-Hortega [13], Iglesias-Rozas and Garrosa [14], Duvernoy [15], and Špacek [16]. With more than 40 years as neuropathologist and neuro histologists we used classical silver impregnation methods, selective histochemical stains, electron microscopic images, and post-mortem perfusion with radiological contrast and India ink methods. In all cases, for histological and histochemical study brain sections of adults between 30 and 100 years of age were used. The brains were fixed in 10% formaldehyde 24-48 hours after death. The data presented here includes histological and histochemical procedures that have been well described as referenced and in addition outlined here with notations in difference in techniques when appropriate.
Monoclonal anit-MAP2 staining
Selected sections of tissue were removed from paraffin cuts and placed in Target Retrieval Solution with pH 9.0 (DACO 2367) for 30 minutes at 95.5 ℃, cooled at room temperature for 20 minutes, then placed for 2 minutes into 1x wash buffer (DACO S 3006). Next, we used 10 minutes of peroxidase blocking reagent (DAKO S 2001) before a brief rinse in distilled water. Samples were then rinsed in 1x wash buffer (DACO S 3006) for 5 minutes. Tissue was then incubated at room temperature for 30 minutes with MAP2 (Clone HM-2) (SIGMA M9942) diluted 1:250-500 with DAKO dilution reagent S 2022. Tissue samples were then washed for 5 minutes with 1x wash buffer (DACO S 3006) followed by a 10-minute use of Dual-Link Reagent (DACO K4061 ready to use). The samples were then washed with 1x washing buffer for 5 minutes. AEC (DAC K3469) paint substrate was used for 10 minutes at room temperature followed by a brief rinsing in 1x wash buffer (DACO S 3006) and then briefly rinsed by distilled water. The slides were stained for 10 seconds with Mayer's Hematoxylin solution followed with distilled water rinse and then process with 0.05% NH3. Tissue samples were covered with aqueous mounting medium just after a 2x distilled water rinse.
Silver staining
Following the methods of Río-Hortega as referenced above, selected slide sections from human biopsies were stained with silver ammionocarbonate. Sections, 10-20 microns thick were floated on the silver solution for 60-120 minutes at 50 ℃. Staining was facilitated with pyridine, followed by reduction in 10% formalin.
Histochemical staining
Selected slide sections from human biopsies were air dried or baked on slide warmer overnight. Alcohol/chloroform 1:1 was used overnight followed by 100% and 95% alcohol to distilled water. Cresyl violet solution 0.1% was used for 5 to 10 minutes followed by rinse in distilled water. Samples were treated with 95% ethyl alcohol for up to 30 minutes as per variety of results microscopically. Dehydration in 100% alcohol and clearing with xylene was followed by permanent mounting.
Post-mortem perfusion with India ink
For this approach brains used were from adults between 30 and 100 years of age. Briefly, the brain is removed without separating the dura mater. The carotid arteries and one of the vertebral arteries are closed. The cerebral vascular system is perfused through the basilar artery with a saline solution and a few drops of detergent liquid. The detergent is used to break up erythrocytes and leukocytes. After about 15 to 30 minutes, the cerebral vascular system is perfused with black colloidal Chinese ink (pelican) until undiluted ink comes out of the cerebral veins. The brain is then fixed in 10% formalin for about 15 days. From then on, histological and histochemical cuts were made as necessary. In cases, for vascular studies, brain slices were included in methacrylate, after making the tissue transparent we sectioned into 5 mm slices. A similar method was used in the radiological resection, with X-ray opaque liquid. An obvious advantage to using this method of perfusion it does not interfere with other histological or histochemical methods reagents for glial cells and for vessels.
Glial fibrillary acidic protein staining: For astrocyte-specific molecule glial fibrillary acidic protein (GFAP) we used GFAP from Dako-Agilent, Madrid, Spain. The GFAP resulted in excellent visualization of astrocytes and all astrocytic processes. We took advantage of having parallel and perpendicular cuts in the same tissue sample thus allowing positioning from the brain surface.
Illustrations were developed in the free and open source 3D creation suite Blender. Creating 3D meshes and then using a vast variety of tools for manipulation to display revelant images.
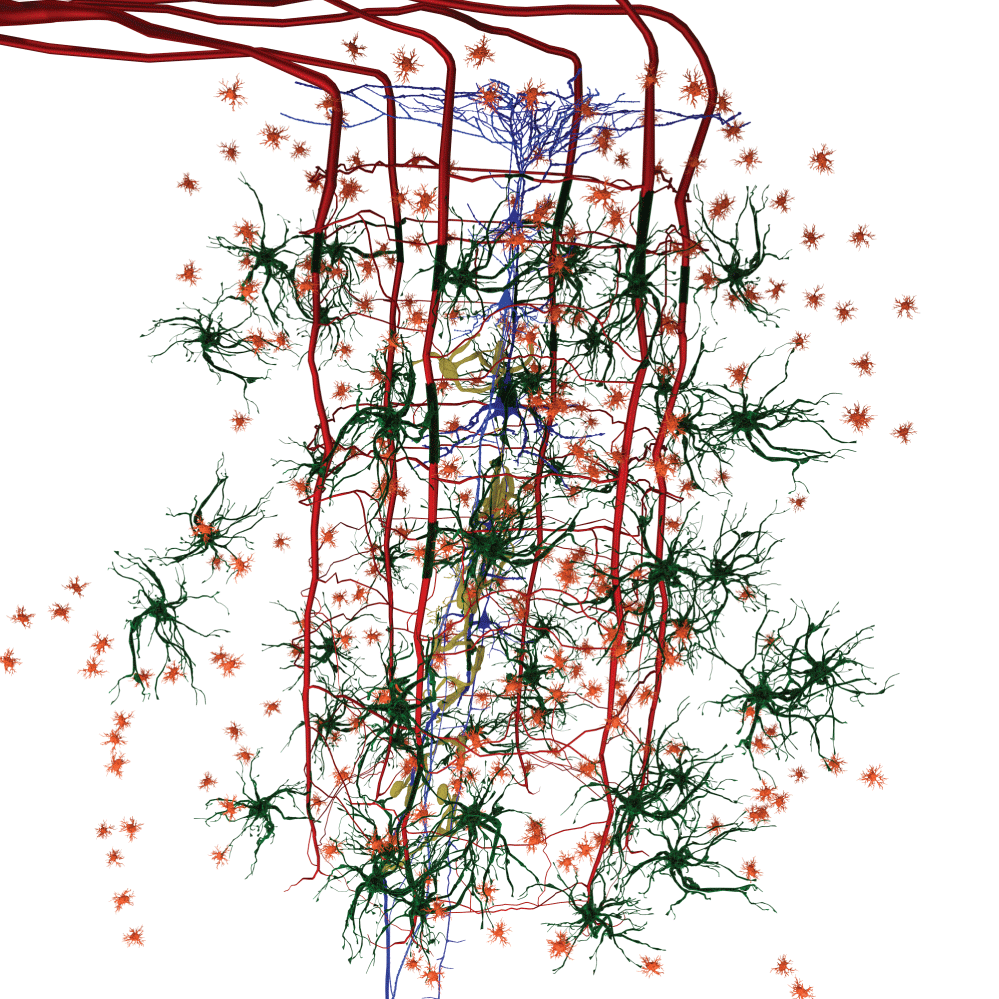
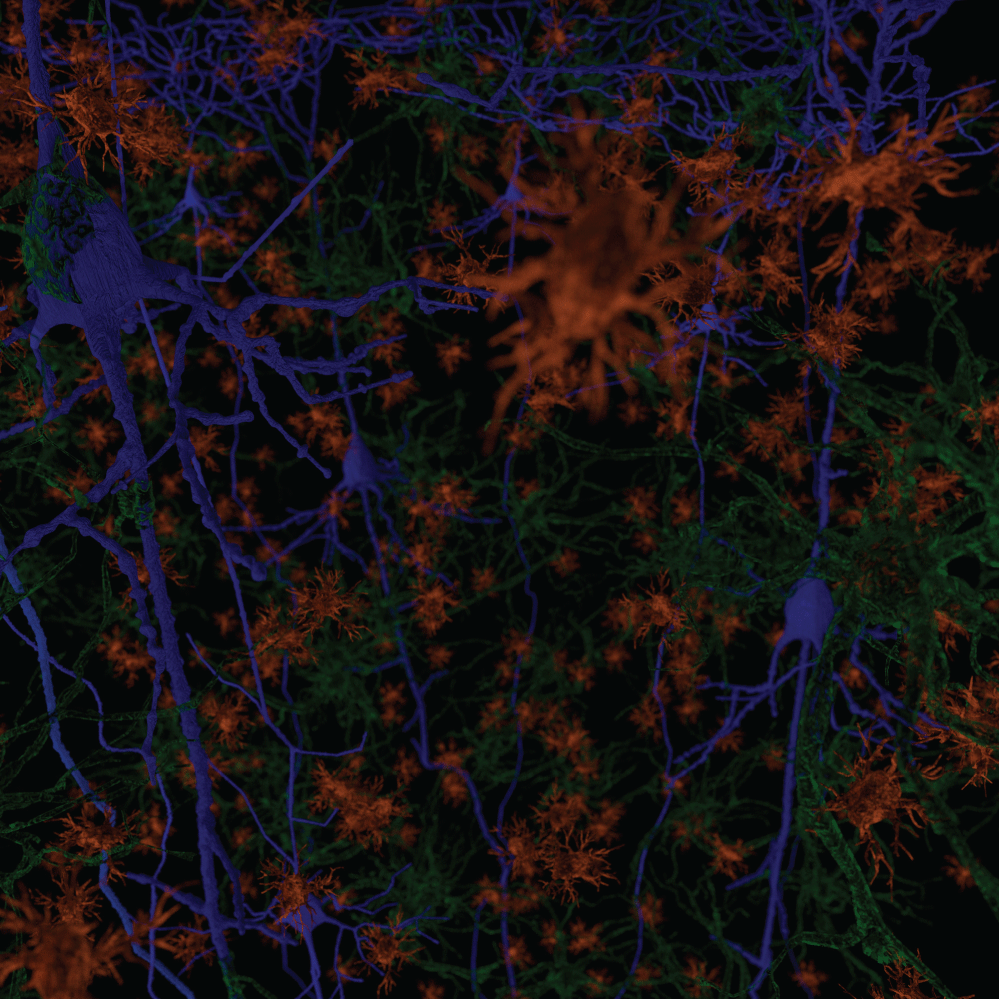
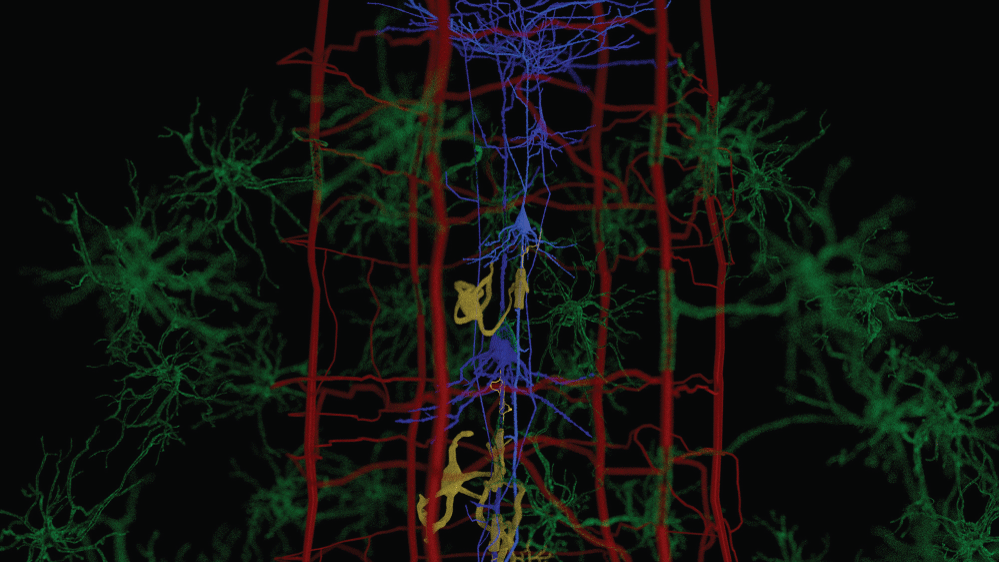
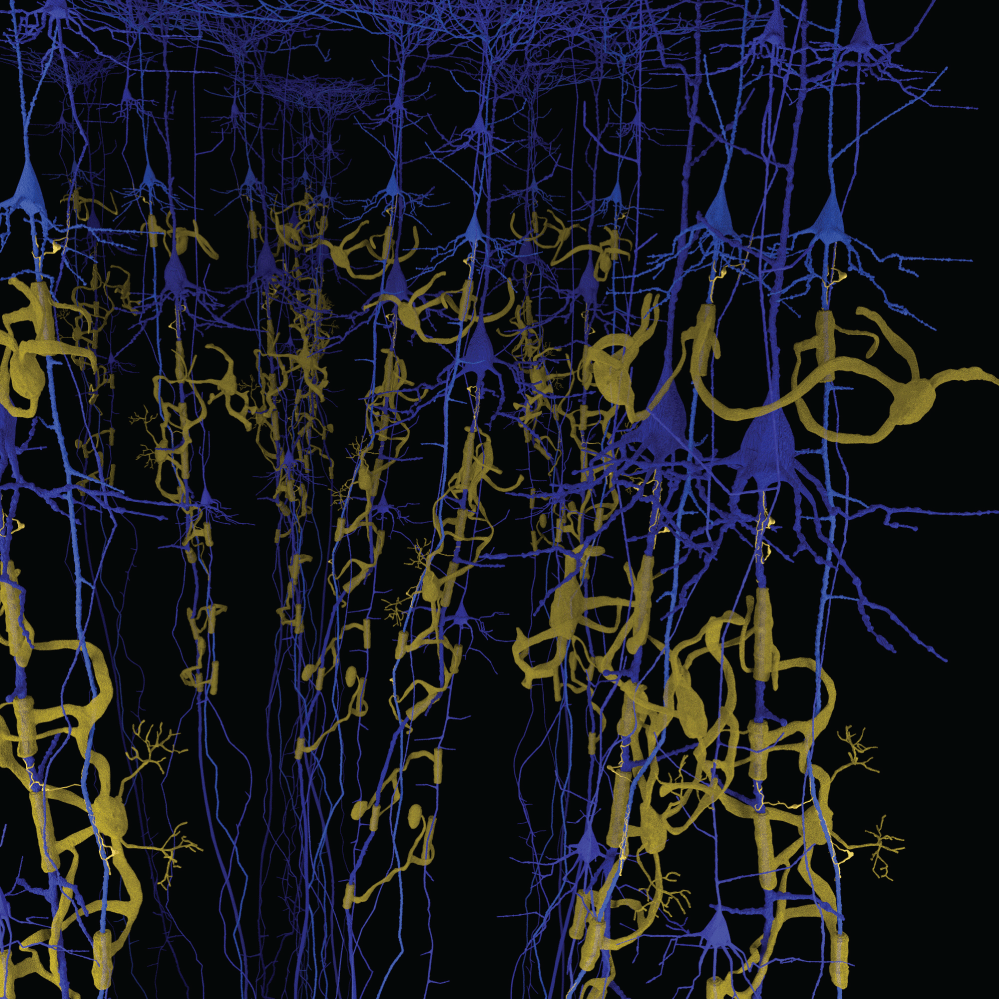
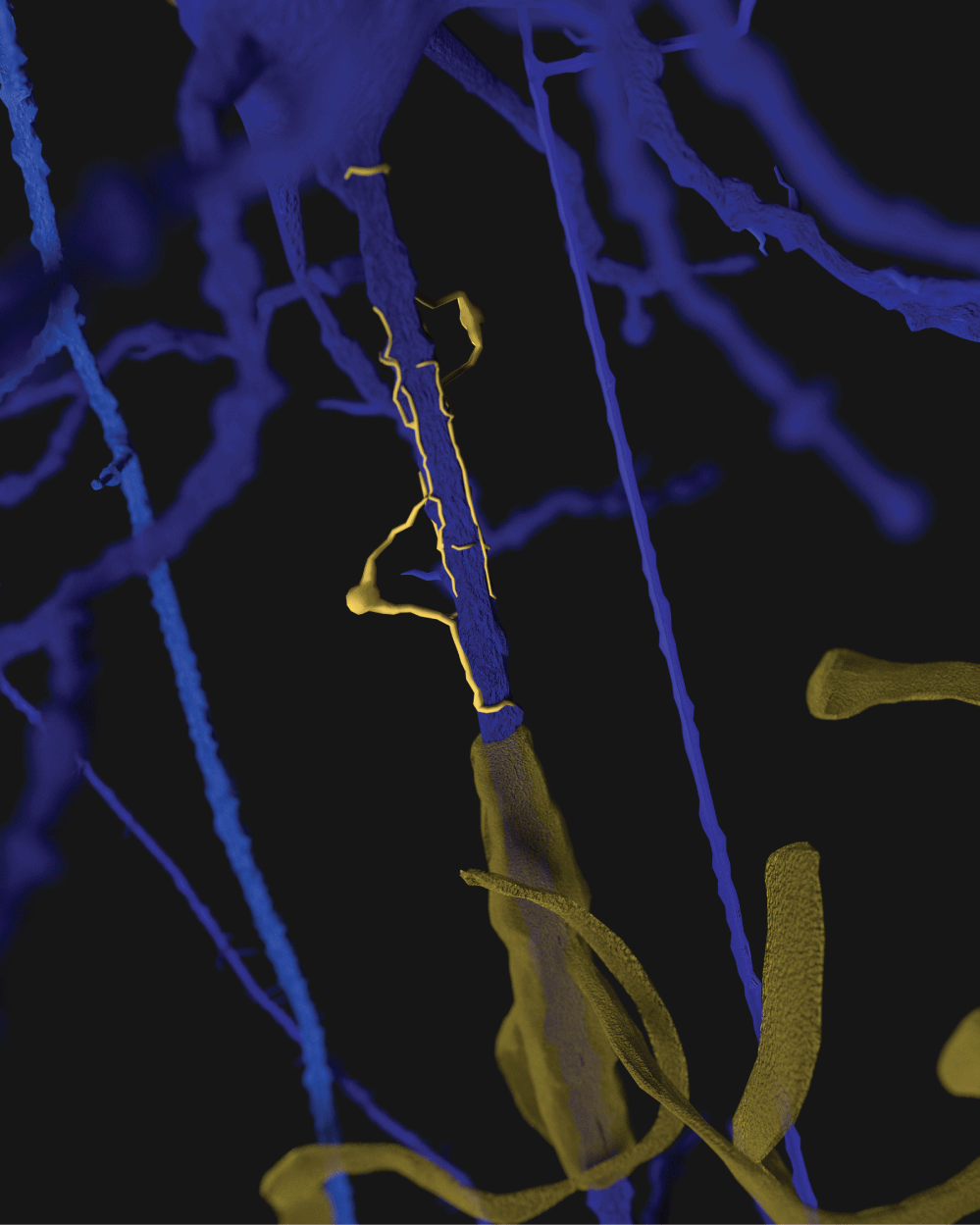
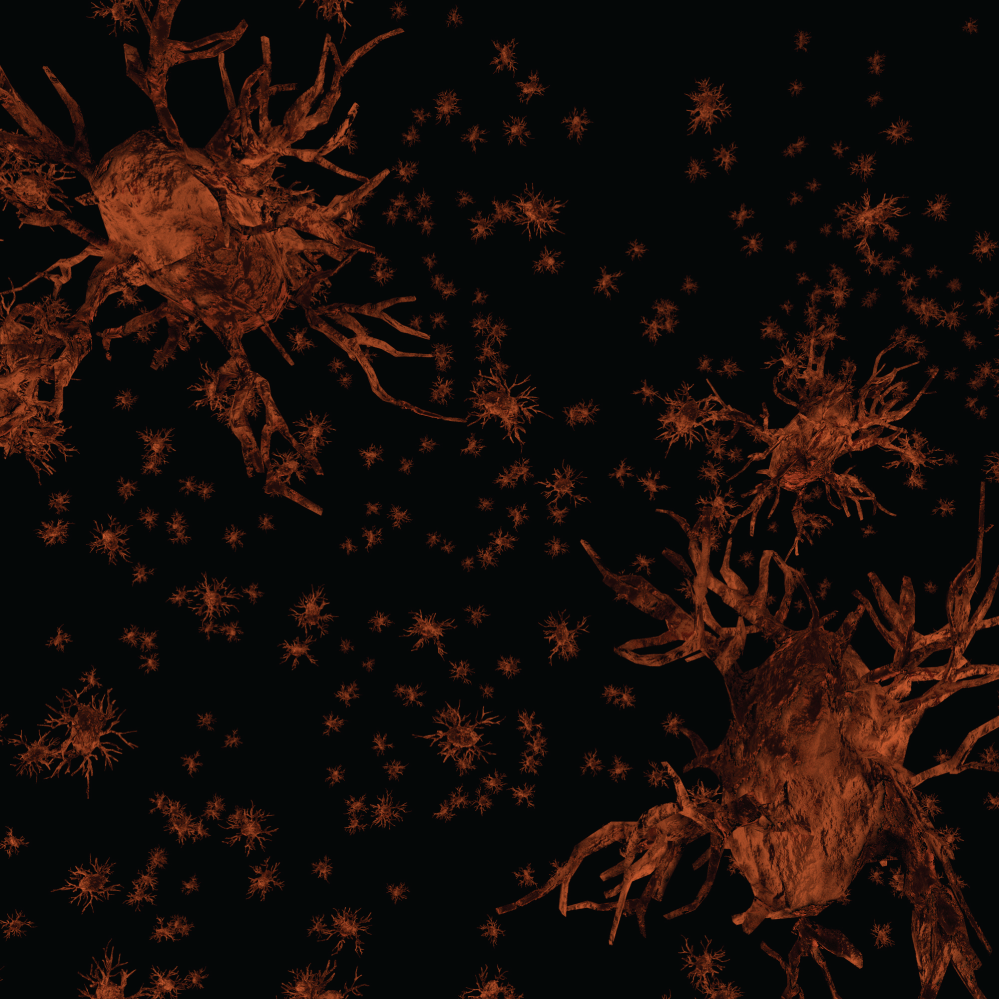
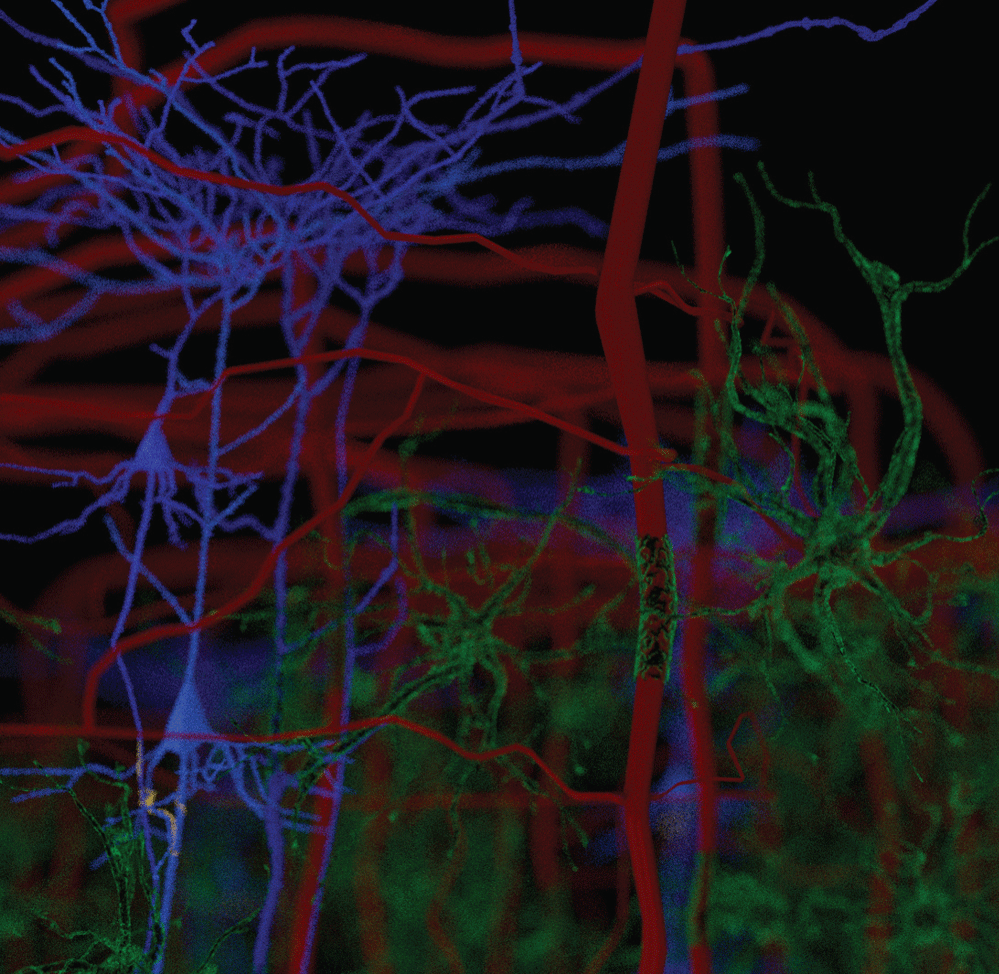
We have observed the five morphological elements generally viewed separately forming architectural, morphological, and dimensional structures which resemble three dimensional structures found in nature. The structures formed in the functional unit resemble mineralogical crystals with specific arrangements of cells at predictable locations in three-demensional space. The visualization of the biological structures that manifest as they interpenetrate each other to form a complex architectural network are part of a critical step in understanding diseases that cause progressive damage to the brain. An improved understanding of the neuropathological findings after damage to functional unit is significantly improved. These three-dimensional functional units are presented separately and together. We present some of the elements of a functional unit as combined with other elements to accentuate the important relationships without additional confusion from presenting all six elements at the same time. Figure 1 illustrates without any subtly the very complex nature of working with all six components at the same time.
Figure 1 and Figure 2 are illustrations of the architectural arrangements of cells making up the functional unit of the central nervous system. Detailed in the illustration are the vital components of capillaries (red), neurons (blue), oligodendrocytes (gold), astrocytes (green), microglia (orange), and interstitial fluid (background). The neuronal column as part of the functional unit is supported by all of the associated cell types and interstitial fluid.
Neurons
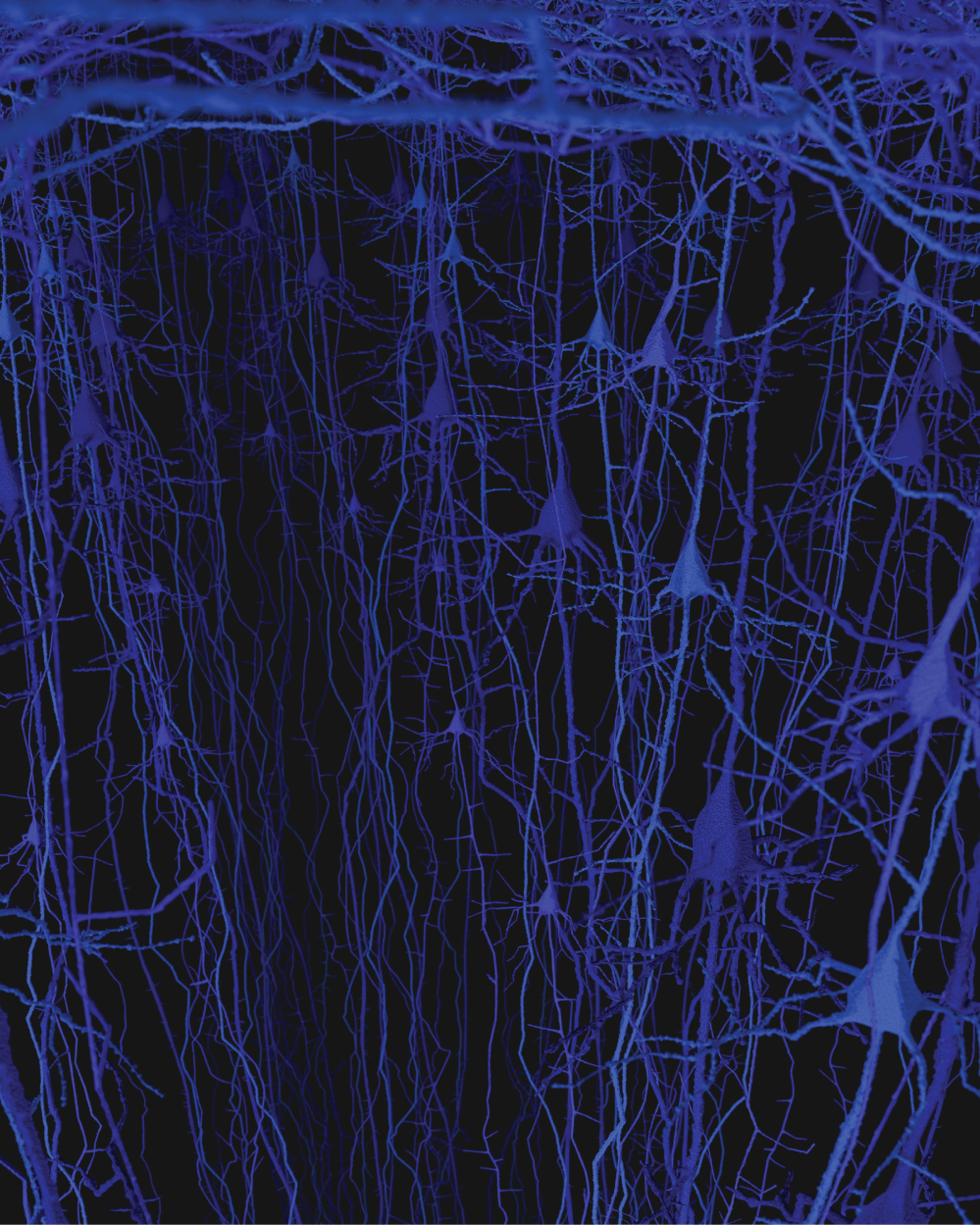
The brain is believed to have one hundred billion neurons [17]. Two to three million neurons are related to the five senses and a few hundred thousand neurons are responsible for all motor function. Each functional unit or neuron column is estimated to contain fifty to one hundred neurons; however additional accurate studies in humans are needed to study the different brain regions [18]. The fine projections at the top of the neuron are the dendrites and they connect to other neurons (Figure 3). The body of the neuron is the swelling in the middle and remains and area of great dedicated research understanding the physiologic events occurring. The axons project off the bottom of the image and can be up to 1 meter long as they form connections with other neurons in the brain or spinal cord.
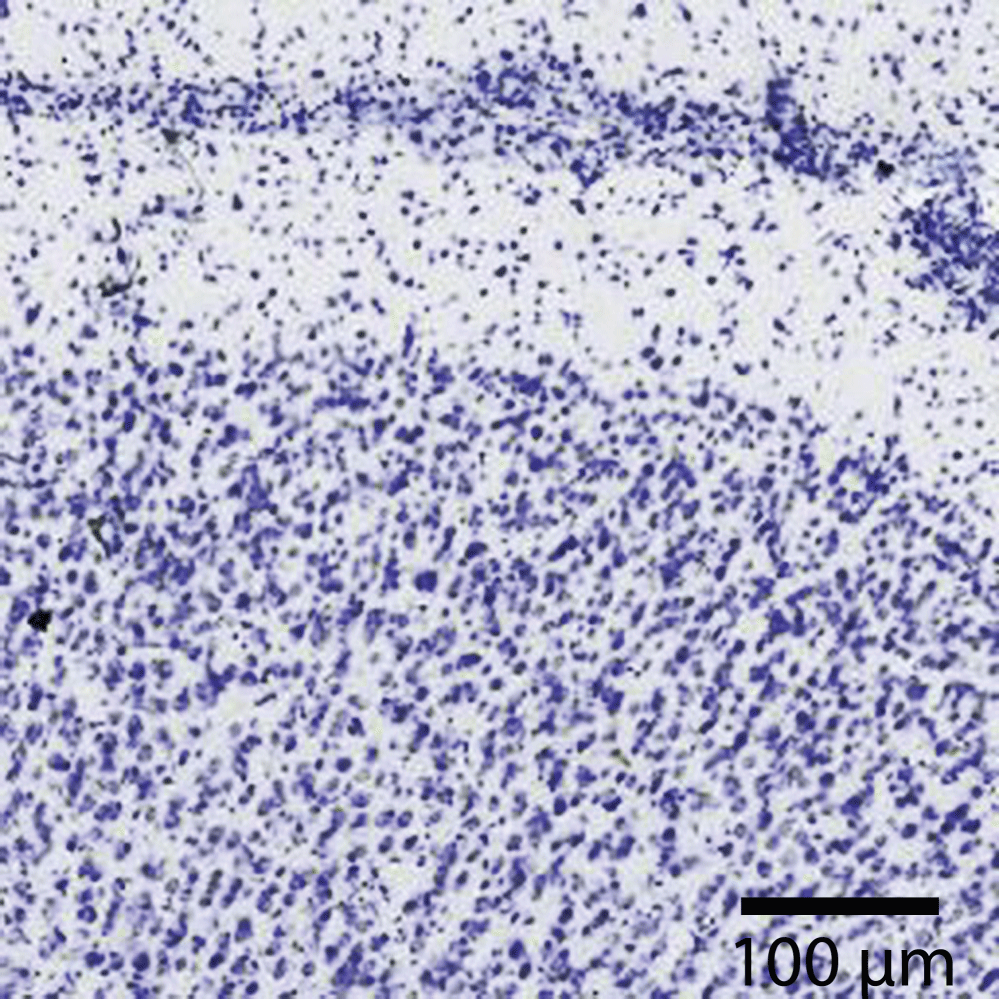
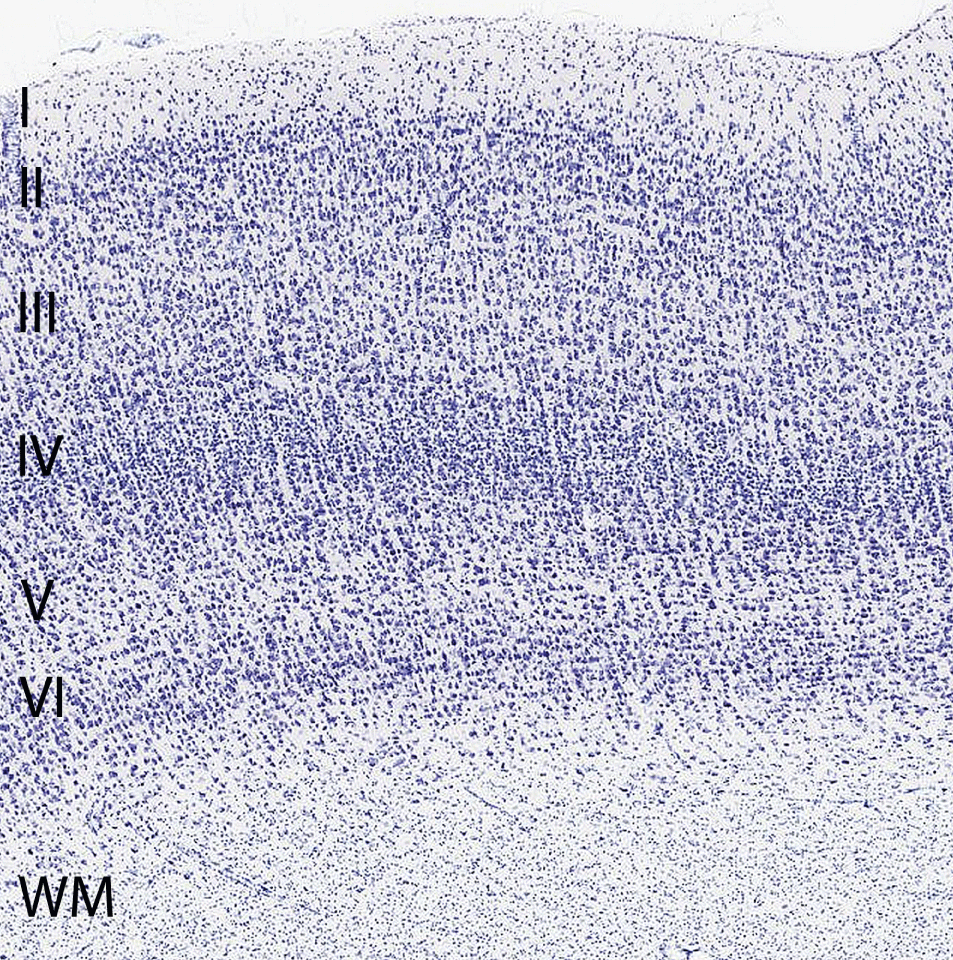
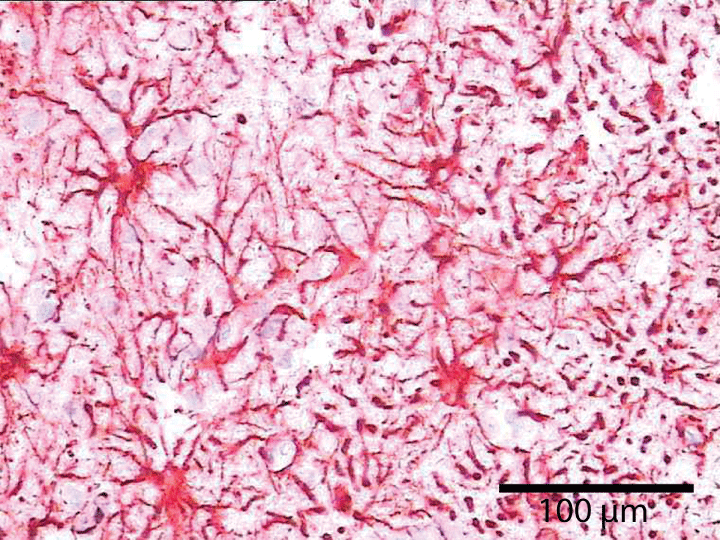
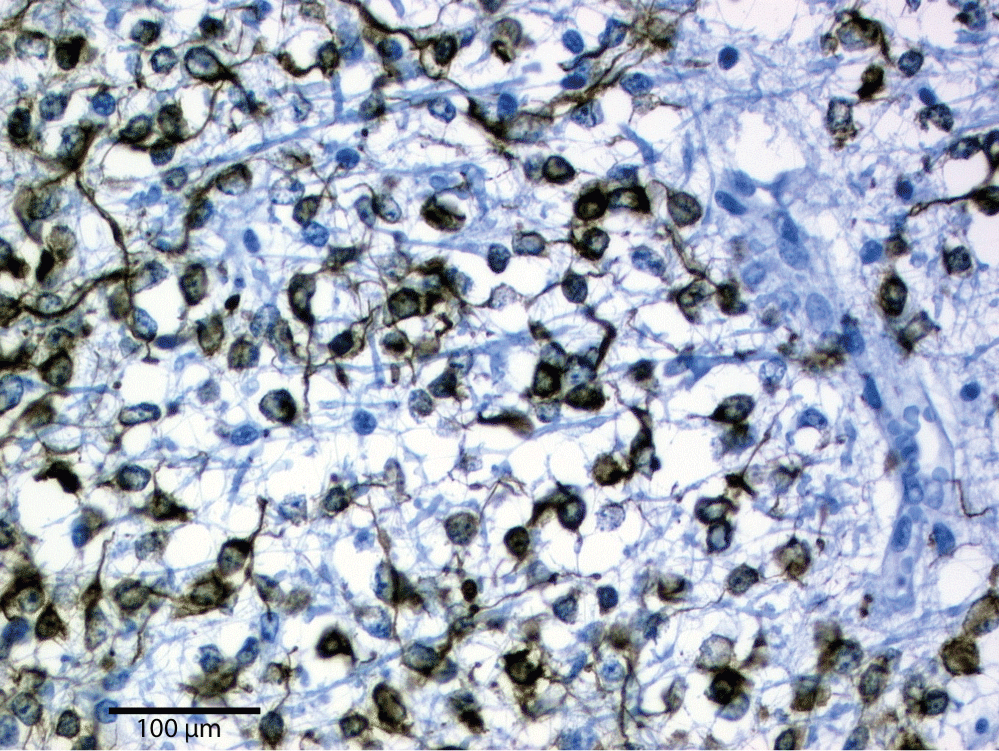
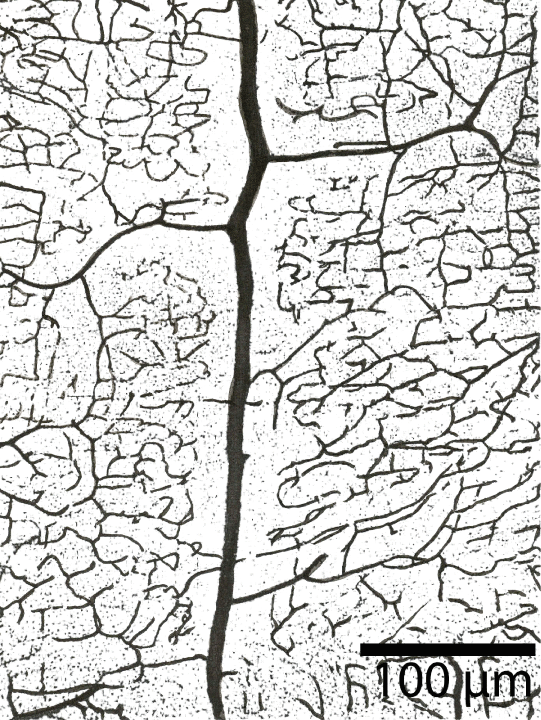
Individual neurons are rarely more than 8-20 µm from a brain capillary [5]. Neurons of the cerebral cortex form narrow vertical columns as shown especially by Ramón y Cajal using silver impregnation, Golgi with the traditional histological methods of haematoxylin and eosin, and Nissl with other silver impregnation stains (Figure 4). In the human neocortex we find between 5 and 165 neurons per 0.001 mm3. On average there are 57 cells per 0.001 mm3 (Figure 5). The columns contain 25-80 neurons with numbers of neurons being higher the visual cortex [19]. The columns are not strictly separated from each other and can be found at times intertwined with adjacent functional units. The complex interactions between microglia, astrocytes, and neurons have also been observed in culture [20].
Astrocyte
The astrocytes are specialized brain cells arranged in a specific geometric pattern (Figure 6). The location of astrocytes forming icosahedrons adds to their functional importance. By connecting the capillaries with the neurons, they transport nutrients and energy helping the neurons function properly. Without the astrocytes the neuron would die. The astrocytes functionally create the blood brain barrier as they regulate the contact between blood and the neuron. The astrocytes add to interstitial fluid, part of the glymphatic system [21]. The interstitial fluid works to provide nutrition and allows the brain to clear waste materials in a manner that increases with proper sleep. There exists a general flow of interstitial fluid through the brain tissue as discussed below.
The defects in blood brain barrier function in some neuropathological diseases, especially those involving glia, suggest that continuing induction during adult life is necessary for normal function of the brain tissue. We have seen astrocytes occupy a strategic position in their location in the functional unit. The astrocytes are situated between capillaries and neurons with gap junctions formed between astrocyte processes allowing them to communicate with each other (Figure 7). The astrocytes are also able to communicate with other astrocyte processes that are in contact with the endothelial cells of the capillaries forming the blood brain barrier [22].
There are at least eleven different types of distinct astrocytes phenotypes and eight involve specific interactions with blood vessels (Figure 8). As an astrocyte is able to secrete a range of biological and chemical agents they can directly affect the local surroundings of each functional unit [23]. Evidence from cell culture studies show that the astrocyte is able to up regulate many blood brain barrier functions, strengthening the tight junction's physical barrier [24]. Cultured brain endothelial cells and astrocytes express functional receptors for a high proportion of the agents that act as neurotransmitters and modulators in the brain [25]. In situ, groups of astrocytes are coupled by gap junctions, forming clusters that define microdomains of neural or glial function, with associated capillaries forming the boundaries [6].
Astrocytes secrete transforming growth factor-ß (TGFß), which downregulates brain capillary endothelial expression of fibrinolytic enzyme tissue plasminogen activator (tPA) and anticoagulant thrombomodulin (TM) [26]. Glutamate is the major excitatory transmitter of the brain, and astrocyte processes surrounding synapses can take up glutamate through transport proteins (particularly EAAT 1 and 2). The astrocytes transport Na+ contributing to water clearance at the blood brain barrier as part of their function. Glutamate is converted to glutamine within the astrocyte and recycled to the neurons [27]. In a number of pathologies, the function of the blood brain barrier is altered, and several disorders appear to involve disturbances of endothelial–glial interaction like Alzheimer's disease, glioblastomas, and epilepsy [7].
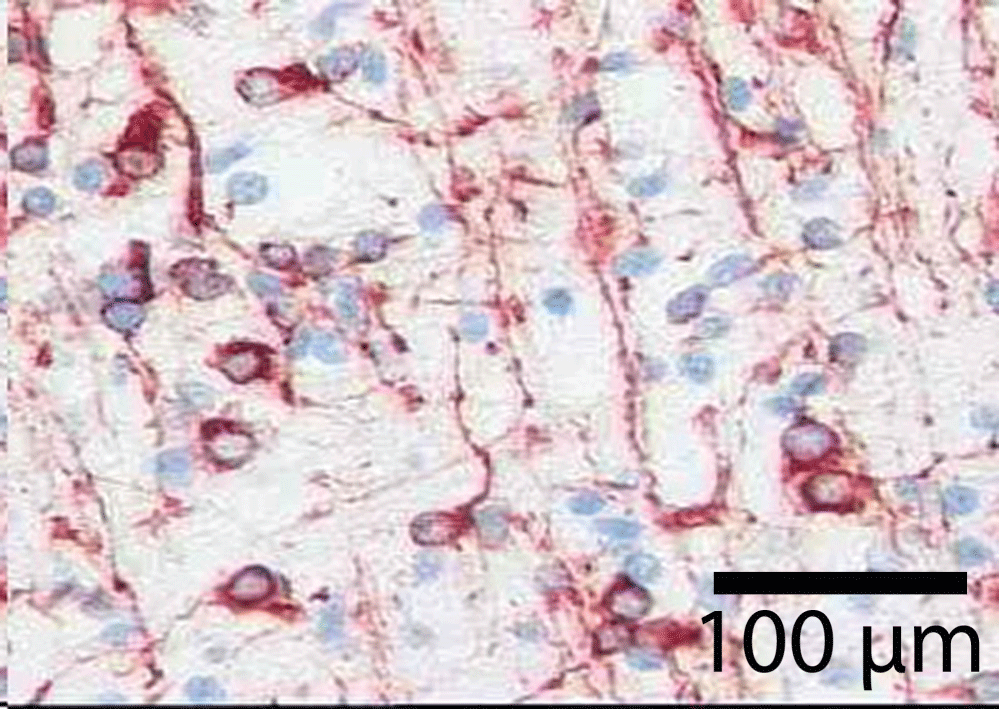
Astrocytes contribute to ionic, amino acid, neurotransmitter, and water homeostasis of the brain in several ways. Astrocytes forming perivascular end feet as part of the blood brain barrier have a particular role in these processes with signalling of microglia within the neurovascular unit [28]. Using silver impregnation and immunohistological methods like Cajal's gold sublimate and particularly GFAP, we find in the cortex, as Rio Hortega and Penfield noted, little or no variety in the phenotypes of protoplasmic forms of astrocytes [29]. In vertical and horizontal cuts of the brain cortex, the astrocytes are arranged in hexagonal nettings in both parallel and perpendicular cuts. The location of the astrocytes in the functional unit is predictable and follow an expected geometric pattern. They have contacts with neurons, capillaries, arterioles, venules and with other astrocytes (Figure 9). The architectural arrangement of the astrocytes forms a three-dimensional network of icosahedra. The branches of astrocytes build a three-dimensional dense network that is repeated across multiple functional units of cortex.
The pericytes wrap around vessels and are also going to be found in direct contact with the endothelial cells associated with gap junctions. The pericytes are found in close association with astrocytes where they contact the blood vessels. Pericytes contain contractile fibers that are consistent with contractile smooth muscle actin [30].
Oligodendroglia
Oligodendroglia wrap and line the axon associated with neurons adding structural support. Oligodendrocytes have a special ability to form insulating wraps around neurons. The oligodendrocytes come in a variety of shapes and sizes, predictably described by special staining. By wrapping projections around the neurons, the oligodendrocyte supports and protects sometimes multiple neurons (Figure 10). Loaded with microtubules and tau proteins when damaged they add to scar [31] (Example: neurofibrillary tangle).
Some authors have shown that in addition to the oligodendrocyte providing protection to the axon there is a production of lactate supplied to the associated neuron axons [32].
The modern immunostaining with butyrylcholinesterase (BchE) was most commonly used in the microscopic evaluation of the oligodendroglia. The morphology and distribution of oligodendroglia cells or oligodendrocytes into four different phenotypes was masterfully described by Rio Hortega: Type I perineuronal (and occasionally perivascular), Type II and III as periaxonic in the cortex and Type IV periaxonic in the white matter [33] (Figure 11). The periaxonic (Type IV) are similar to peripheral Shawn cells of the nerves [34].
The oligodendrocytes in the brain cortex (Types II and III) are disposed along the length of axonal processes, scattered within the neuropil and concentrated immediately around the neuronal cell bodies (Type I). Modern studies with histochemical methods, like myelinic basic protein (MBP), testify to the histological results. The oligodendrocyte is involved with forming myelin around neurons, at times coming in contact with multiple neurons at the same time (Figure 12 and Figure 13). The oligodendrocyte poplulation in the adult human remains relativly constant in a healthy environment [35]. Much is still to be discovered in the axon and oligodendrocyte relationship when placed under stress or insult [36].
Capillaries
The capillaries are the smallest blood vessels in the body. In the functional unit, to the center of the hexagonal cage of arterioles and capillaries are the neurons. This part of the functional unit created from capillaries and arterioles is in direct contact with the astrocytes. The astrocytes have direct access to the blood vessels where red blood cells and blood plasma provide oxygen and nutrients (Figure 14). The width of a single red blood cell is about 8 microns, and red blood cells pass through capillaries not much larger. The estimated length of just the capillaries is about four hundred miles or the distance from Washington, DC, to Boston [37].
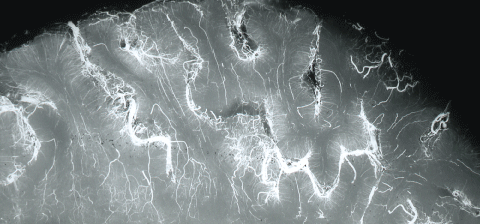
This capillary network provides one hundred ft2 of surface area for the interactions supplying nutrients. Vessels and capillaries predominantly are ordered in the brain cortex in vertical hexagonal columns with multiples anatomises. The geometric distribution of capillaries and arterioles are manifested excellently in postmortal infusions with India ink with cuts parallel to the brain surface [38] (Figure 15).
As the brain suffers damage over time the available functional units are reduced and there is a decrease the ratio of cerebral blood flow/cardiac output as the decades progress during human life [39]. Adjustments in medical management and diagnosis to mitigate the various etiologies that can affect the performance of the functional unit may improve cerebral blood flow/cardiac output ratios due to less tissue dysfunction.
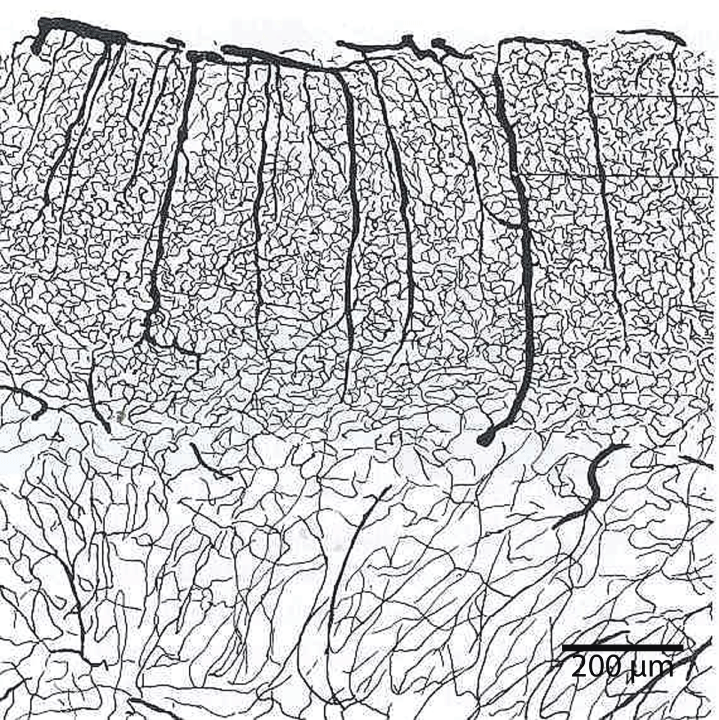
Brain capillaries are surrounded by, or closely associated with, basal membrane, pericytes, glial basal membrane, perivascular end feet of astrocytes, and microglia cells. In the larger vessels (arterioles, arteries and veins) smooth muscle cells form a continuous layer [3]. The brain endothelium is able to enhances the growth and differentiation of associated astrocytes [40]. Blood vascular networks constitute a major component of the structure of the brain in addition to neurons, glia, microglia and extracellular milieu. The hexagonal vascular network-unit is also surrounded completely by the end feet of astrocytes. The ramification of intracerebral vessels has been the object of numerous studies [15]. The branching pattern of the arterioles and capillaries was a focus of this study (Figure 16).
Terminal branches of the arterial surface of the brain penetrate the cortex at right angle and divided on course into other arteries and arterioles. Ruckes described the arterial network as being a polygon-like formed structures with many anastomoses around the column of neurons [41]. The density of vascular vessel in the cortex has been found to be proportional to the number of neurons [42]. Blood vessels in the brain are also surrounded by the end feet of astrocytes. The hemodynamic correlates of neuronal activity as measured by blood-oxygen-level dependent function magnetic resonance imaging provide this neuron and astrocyte relationship [43] (Figure 17).
Specific interactions between brain neurons, capillaries, astrocytes, oligodendroglia and microglia cells within neurovascular units can influence the both physiological and pathological conditions. Additional research is needed to improve imaging and monitoring of processes of the functional unit in the living brain to better validate the brain function and improve human treatments. The trans endothelial electrical resistance (TEER) is typically 2-20 ohm.cm2 in peripheral capillaries and can be > 1,000-ohm cm2 in brain endothelium [44].
Interstitial Fluid and Interstitial System
The cerebral interstitial fluid occupies up to 20% of the total brain volume and plays important roles across a variety of critical brain functions including neuronal communication [45]. The interstitial fluid works to provide nutrition and allows the brain to clear waste materials in a manner that increases with proper sleep. There exists a general flow of interstitial fluid through the brain tissue. Cerebral interstitial fluid originating from the capillary wall works its way through the rest of the brain tissue and then drains back into systemic circulation.
Brain interstitial fluid is in part absorbed via dural lymphatic vessels [46]. Interstitial fluid and cerebrospinal fluid may also drain into cervical lymph nodes by uncertain mechanisms [47]. Integrated and associated with a wide variety of additional functional proteins (Example: glycosaminoglycans, proteoglycans, laminin, fibronectin, and other glycoproteins) the extracellular matrix along with the interstitial fluid comprises the interstitial system [48].
Microglia
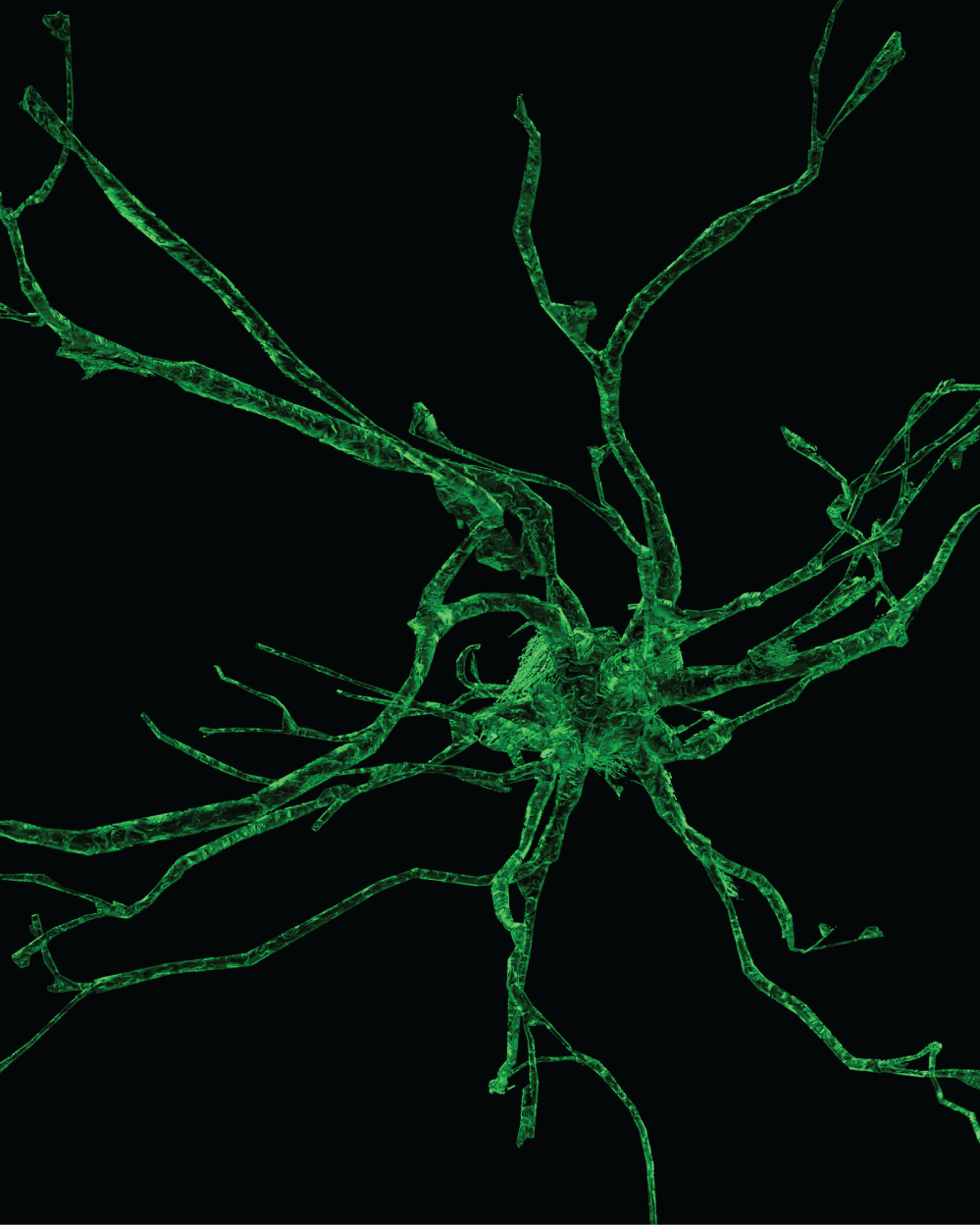
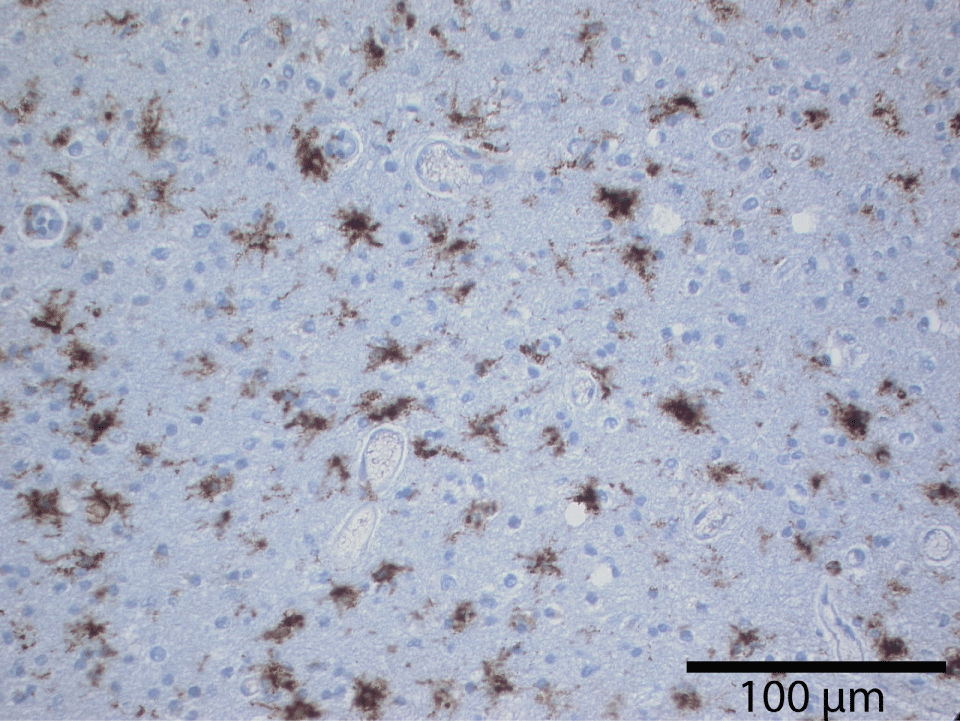
The microglia may be considered the scavengers in the brain tissue. These specialized brain cells are spread throughout the nervous system and act to target infections and foreign material. The microglia can change their role and function as needed. They are able to respond to any need to fight inflammation or infection rapidly. They produce many chemicals assisting in brain health including the production of amyloid precursor protein. Microglia have many phenotypes in adult human brain. The most common appearance on ramified microglia is of highly branched small glial cells within the neuropil. Branching occurs in all planes. Ramified microglia appear uniformly dispersed throughout the brain and form an extensive reticular array. This pattern differs from the hexagonal distribution of astrocytes and from predominant periaxonic distribution of oligodendrocytes (Figure 18).
Quantitative studies have demonstrated the basal ganglia and cerebellum have more microglia than cerebral cortex [49]. This fact may be significant to explain regional differences in CNS pathology. Nearly one third of the cortical neurons have microglial satellite cells [50]. Perivascular microglia are usually not highly ramified and seem more similar to macrophages in morphology but have a similar function [51]. Most evidence suggests the ramified form of microglia is linked to a function of the local tissue environment.
The microglial cells are also excellently described by del Rio Hortega with silver impregnation [52]. The immunohistological modern methods (MAB 1569, CD 60, 25F9, MHCII, Nonspecific esterase, and RCA-1), for microglia testify exactly the morphological descriptions and distribution in the cerebral cortex made by Rio Hortega. In the grey matter they are bipolar or multipolar and take up a homogeneous dense distribution between the other cell types, or as perineuronal and perivascular satellites. The nucleus and cytoplasm remain small and hypochromic and its shape in bipolar cells is elongated. In multipolar phenotypes the nucleus may be irregularly triangular or polyhedral. No matter the specific phenotype the microglia are diffusely and uniformly distributed in cerebral tissue (Figure 19).
Studies of microglia in the healthy brain has shown that the microglia are able to extend and retract their processes as they assist the immune system of the central nervous system [53].
Discussion
We propose this three-dimensional architectural arrangement of neurons as part of the functional unit of the central nervous system also considered "Crystalline Tissular Network of the Cerebral Cortex" is the crucial issue to properly interpret the normal function and pathological changes of central nervous tissue. This functional unit of the central nervous system demonstrates the complex interaction of a basic biological system. Not only the composition and pathological changes of the five separate structural elements and their interactions must be known, but also how the tissue works in a unified manner after the selective impairment of any component. Understanding how any defect in a component of the functional unit will be resolved by a variety of physiologic process will be vital to understanding how the manifestation of a variety of cerebral vascular diseases could be related to the same etiology (Figure 20).
Using the functional unit and the Crystalline Tissular Network as a tool to help explain cerebral minimum damage (example: micron stroke, where damage is too small to be detected by MRI and the individual may not have any symptoms) may open up new fields of study [54]. As described the micron stroke causes very small damage to the functional unit. When the damage happens to just a few astrocytes then one may not notice it clinically. However, over time, the effects of micron strokes are devastating. Along with larger strokes, these may contribute to reason for much brain atrophy, brain age, white matter disease, leukoaraiosis, age related white matter changes, white matter hyperintensities, lacunar infarcts, chronic microvascular changes, chronic microvascular ischemic changes, small vessel disease, periventricular white matter changes, and other radiology terms that describe damage to the brain identified on CT or MRI that may not be clinically evident to the patient. The concept of micron stroke differs significantly from the concept of silent cerebral ischemia. This is because micron stroke does not require anatomic-pathological criteria that can be seen on MRI. Studies evaluating silent cerebral ischemia and transient ischemic attack define focal hyperintense T2-weighted images to match defined criteria [55]. Evaluation of the complete functional unit and potential damage to any element allows for a more significant advancement of research in neurodegenerative diseases. Damage to adjacent functional units will result in an ever-increasing size of lesion as viewed by MRI of the brain.
Significant research needs to be directed to the smallest area of damage that could occur and the resulting neuropathologic changes that would then be found as the result of damage to a few oligodendrocyte or part of an astrocyte. In a similar way damage to an industrial building where insult was to the different bricks, stones, cementing, piping and more, of the cerebral building would leave the building with a functional or structural defect. We believe the importance of this architectural tissue association it is vital to properly interpret the normal function and the pathological changes in the central nervous tissue. Not only must we know the composition and pathological changes of the five structural elements separately and in local tissue interactions but as the building, the brain will adapt and work in conjunction with the selective degradation of different bricks, stones, other components and the pipes of the structure.
Considering damage of 10 microns to a portion of the functional unit will go unnoticed as a single event, yet the combined effect over time of thousands of micron strokes will leave the functional unit unable to participate properly as part of the mind. Damage from micron strokes and larger strokes will progress through a neuropathologic process resulting in synaptic loss. A three-minute animation was created for perspective of the integral location and cooperation of these cells as they all work to support the neuron [56]. Proper categorization and the specific etiology leading to micron stroke will facilitate a rapid identification and treatment to those who are affected. The manifestation of the damage to the neuron function leads to a variety of current descriptive diagnosis - resulting in a variety of diseases designated on factors that are not the etiology. When a diagnosis is made based upon manifestations of a mechanism but not the etiology; a significant inaccuracy remains. A variety of ever changing classification systems will not yield the same result as placing a diagnosis upon an etiology. This inaccuracy in designing a diagnosis based upon a clinical manifestation brings with it the missed opportunity to confirm the etiology in the patient and prevents accuracy in treatment and therapy. As an example, currently designated as amyotrophic lateral sclerosis, essential tremor, acephalgic migraine, movement disorder, frontotemporal dementia (dementia lacking distinct histopathologic features), vascular dementia, dementia with Lewy bodies, Parkinson's disease, Alzheimer's disease, optic neuritis, mixed dementia, normal pressure hydrocephalus, or multiple sclerosis; all remain a "diagnosis of exclusion". However, when all of the potential factors that can cause damage to neurons are not considered, then the diagnosis remains incomplete. A complete and accurate investigation including all of the potential causes of injury and damage to the functional unit will convert many of the "diagnosis of exclusion" into a diagnosis based upon the results of the investigation (Example: hypercoagulable state, prothrombin G20210A defect, Factor V, anticardiolipin). For comparison one can consider the treatment approach and strategy when dealing with disease of the brain based upon etiology, as examples: Vasculitis, sarcoidosis, Behcet's disease, space-occupying lesions, systemic lupus erythematosus, Lyme disease, Wilson's disease, hypoxia, lysosomal disorders, or CNS lymphoma. Once the etiology for damage to functional unit and larger geographic areas of the brain is identified treatment options improve.
Many of the details related to amyloid, alpha-synuclein, and Tau proteins found in neuropathology samples as deposited could be related to the extracellular matrix integrated with the interstitial system. Where cerebral interstitial fluid containing a vast variety of biological components that are involved with the proper function of neurons is yet to be explored. (Example: proteoglycans, hyaluronic acid, heparan sulfate proteoglycans, metallopeptidases, tissue plasminogen activators, chondroitinase and others). Making an accurate diagnosis of etiology of neurodegenerative diseases of the CNS becomes more direct once the view of amyloid as some neurotoxic agent changes to amyloid being the result of prior insult to the functional unit and a physiologic response to the stressor. Many of the proteins and other microscopic findings currently used to categorize different neurodegenerative diseases into a category are indigenous to the central nervous system. As we described above, the cellular associations with a variety of these biological components has been described. Alteration of the cerebral cortex after damage to the functional unit as currently investigated will reveal the findings of those biological components. Some examples include: amyloid precursor protein and then findings of amyloid, abnormal aggregates of protein including alpha-synuclein described as Lewy bodies, abnormal aggregates of hyperphosphorylated tau proteins described as neurofibrillary tangles. Etiology leading to formation of the microscopic findings may be advanced by improved understanding of the functional unit.
Understanding the healthy relationship of the functional unit and all of its associated cells allows a more directed evaluation of neuropathology. Amyloid as a response to damage may explain why the brain tissue will suffer atrophy as damage continues, rather than manifestation of increased swelling of the brain [57]. If amyloid deposition was the driving etiology behind most neurodegenerative disease, then as deposition increased the overall volume and structure of the brain would be one of swelling and displacement of structures. Brains of the most demented and most heavily affected by amyloid (along with other neuropathologic changes) are also those with the most profound global atrophy noted [58]. Understanding the physical and physiological relationships of cerebral interstitial fluid as part of the extracellular network in its complex interactions with the functional unit is important because of the direct interactions with the other cells of the brain.
Future challenges will be in detailed scientific investigation of the functional unit. Damage to a single component may result in different manifestations based upon yet to be identified factors. Cellular structures may be implicated as playing a primary role or we may discover that combinations of related cellular elements lead to many neurodegenerative disorders.
References
- Cajal SR, Purkiss U, Fox CA (1954) Neuron theory or reticular theory? Objective evidence of the anatomical unit of nerve cells. Instituto Ramón y Cajal, Consejo superior de investigaciones cientificas, Madrid.
- Cajal SR (1909) Histologie du système nerveux de l'homme & des vertébrés. Maloine, Paris.
- Iadecola C (2004) Neurovascular regulation in the normal brain and in Alzheimers disease. Nat Rev Neurosci 5: 347-360.
- Anderson CM, Nedergaard M (2003) Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci 26: 340-344.
- Schlageter KE, Molnar P, Lapin GD, et al. (1999) Microvessel Organization and Structure in Experimental Brain Tumors: Microvessel Populations with Distinctive Structural and Functional Properties. Microvasc Res 58: 312-328.
- Nedergaard M, Ransom B, Goldman SA (2003) New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci 26: 523-530.
- Abbott NJ, Rönnbäck L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7: 41-53.
- Kuffler Stephen W, John G.Nicholls (1977) From neuron to brain: a cellular approach to the function of the nervous system. Sinauer, MA, Sunderland,
- Hernández F, Iglesias JR, Martínez de Morentin J (1971) Histochemical study of ubiquinones in the central nervous system of mice. J Anat 110: 73-79.
- Cajal Santiago Ramón Y (1995) Histology of the Nervous System of Man and Vertebrates. Oxford University Press, New York.
- C Golgi (1908) "La Doctrine du Neurone, Theorie et Faits". In: KB Hasselberg, SO Pettersson, KAH Morner, CD Wirsen, MCG Santesson, Imprimerie Royale, Norstedt & Soner, Stockholm, 1-31.
- Cajal SR, DeFelipe J, Jones EG (1988) Cajal on the cerebral cortex: An annotated translation of the complete writings. Oxford University Press, New York.
- Río-Hortega PD (1990) Pío del Río Hortega. Fundación Banco Exterior, Madrid.
- Iglesias-Rozas JR, Garrosa M (2013) Río-Hortegas third contribution to the morphological knowledge and functional interpretation of the oligodendroglia. Elsevier, London.
- Duvernoy H, Delon S, Vannson J (1981) Cortical blood vessels of the human brain. Brain Res Bull 7: 519-579.
- Špaček J (1970) Three-dimensional reconstructions of astroglia and oligodendroglia cells. Zeitschrift Für Zellforschung Und Mikroskopische Anatomie 112: 430-442.
- Herculano-Houzel S, Jon H Kaas (2011) Gorilla and orangutan brains conform to the primate cellular scaling rules: implications for human evolution. Brain Behav Evol 77: 33-44.
- Field E (1970) Das zentralnervensystem in Zahlen und Tabellen. Journal of the Neurological Sciences 11: 95.
- Blinkov SM, Glezer II, Haigh B (1968) The human brain in figures and tables: A quantitative handbook. Basic Books, New York.
- Hansson E, Rönnbäck L (2003) Glial neuronal signaling in the central nervous system. FASEB J 17: 341-348.
- Yang L, Kress BT, Weber HJ, et al. (2013) Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Transl Med 11: 107.
- Kettenmann H, Ransom BR (2005) The concept of neuroglia: a historical perspective. In: Kettenmann H, Ransom BR, Oxford University Press, 1-16.
- Abbott NJ (2002) Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat 200: 629-638.
- Rubin LL (1991) A cell culture model of the blood-brain barrier. J Cell Biol 115: 1725-1735.
- Hansson E, Rönnbäck L (2003) Astrocytic receptors and second messenger systems. Advances in Molecular and Cell Biology.
- Tran ND, Correale J, Schreiber SS, et al. (1999) Transforming Growth Factor- Mediates Astrocyte-Specific Regulation of Brain Endothelial Anticoagulant Factors Editorial Comment. Stroke 30: 1671-1678.
- Simard M, Nedergaard M (2004) The neurobiology of glia in the context of water and ion homeostasis. Neuroscience 129: 877-896.
- Marroni M, Marchi N, Cucullo L, et al. (2003) Vascular and parenchymal mechanisms in multiple drug resistance: a lesson from human epilepsy. Curr Drug Targets 4: 297-304.
- Penfield W (1936) Epilepsy and surgical therapy. Arch Neur Psych 36: 449-484.
- Bergers G, Song S (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 7: 452-464.
- LoPresti P, Szuchet S, Papasozomenos SC, et al. (1995) Functional implications for the microtubule-associated protein tau: localization in oligodendrocytes. Proc Natl Acad Sci U S A 92: 10369-10373.
- Fünfschilling U, Supplie LM, Mahad D, et al. (2012) Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485: 517-521.
- Del Río Hortega P (1928) Tercera aportación al conocimiento morfológico e interpretación funcional de la oligodendroglía. Mem Real Soc Esp Hist Nat 14: 5-122.
- Iglesias-Rozas JR, Garrosa M (2013) Bibliographic Background. In: Iglesias-Rozas JR, Garrosa M, RíO-hortega'S Third Contribution to the Morphological Knowledge and Functional Interpretation of the Oligodendroglia. 5-21.
- Yeung M, Zdunek S, Bergmann O, et al. (2014) Dynamics of Oligodendrocyte Generation and Myelination in the Human Brain. Cell 159: 766-774.
- Piaton G, Gould RM, Lubetzki C (2010) Axon-oligodendrocyte interactions during developmental myelination, demyelination and repair. J Neurochem 114: 1243-1260.
- Wiedeman MP (1963) Dimensions of Blood Vessels from Distributing Artery to Collecting Vein. Circulation Research 12: 375-378.
- Iglesias-Rozas JR (1992) Histologische, immunologische und epidemiologische Untersuchungen von 113 Medulloblastomen. Aktuelle Neuropädiatrie 1991: 192-195.
- Xing C, Tarumi T, Liu J, et al. (2016) Distribution of cardiac output to the brain across the adult lifespan. J Cereb Blood Flow Metab 37: 2848-2856.
- Estrada C, Bready JV, Berliner JA, et al. (1990) Astrocyte Growth Stimulation by a Soluble Factor Produced by Cerebral Endothelial Cells in vitro. J Neuropathol Exp Neurol 49: 539-549.
- Ruckes J (1967) Die arterielle Vascularisation der Pia mater des neugeborenen. Frankfurt Z Pathol 76: 227-234.
- Richard L De CH Saunders, Bell MA (1971) X-ray microscopy and histochemistry of the human cerebral blood vessels. Journal of Neurosurgery 35: 128-140.
- Thompson JK (2003) Single-Neuron Activity and Tissue Oxygenation in the Cerebral Cortex. Science 299: 1070-1072.
- Gilles FH, Leviton A, Dooling EC (1983) The Developing human brain: Growth and epidemiologic neuropathology. J Wright-PSG, Boston.
- Lei Y, Han H, Yuan F, et al. (2017) The brain interstitial system: Anatomy, modeling, in vivo measurement, and applications. Progress in Neurobiology 157: 230-246.
- Aspelund A, Antila S, Proulx ST, et al. (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. The Journal of Experimental Medicine 212: 991-999.
- Koh L, Zakharov A, Johnston M (2005) Integration of the subarachnoid space and lymphatics: Is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Research 2: 6.
- Crocker SJ, Pagenstecher A, Campbell IL (2003) The TIMPs tango with MMPs and more in the central nervous system. Journal of Neuroscience Research 75: 1-11.
- Haymaker W (1982) Histology and histopathology of the nervous system. Thomas, Springfield, IL, USA.
- Nakajima K, Kohsaka S (1993) Functional roles of microglia in the brain. Neuroscience Research 17: 187-203.
- Hickey W, Kimura H (1988) Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science 239: 290-292.
- Tremblay MÈ, Lecours C, Samson L, et al. (2015) From the Cajal alumni Achúcarro and Río-Hortega to the rediscovery of never-resting microglia. Front Neuroanat 9: 45.
- Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308: 1314-1318.
- Orehek AJ (2012) The Micron Stroke Hypothesis of Alzheimer's Disease and Dementia. Med Hypotheses 78: 562-570.
- Gaita F, Corsinovi L, Anselmino M, et al. (2013) Prevalence of Silent Cerebral Ischemia in Paroxysmal and Persistent Atrial Fibrillation and Correlation With Cognitive Function. J Am Coll Cardiol 62: 1990-1997.
- https://youtu.be/MSEcOlKwmSs.
- Di Paola M, Macaluso E, Carlesimo GA, et al. (2007) Episodic memory impairment in patients with Alzheimer's disease is correlated with entorhinal cortex atrophy. A voxel-based morphometry study. J Neurol 254: 774-781.
- Pini L, Pievani M, Bocchetta M, et al. (2016) Brain atrophy in Alzheimer's Disease and aging. Ageing Res Rev 30: 25-48.
Corresponding Author
Allen J Orehek, Dementia Prevention Center, Scranton, Pennsylvania, USA.
Copyright
© 2018 Orehek AJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.