Rensheng Shouwu Protects against Neuronal Injury by Inhibiting the Apoptosis Induced by Hypoxia and Reoxygenation
Abstract
Rensheng Shouwu Capsule (RSSW) is an approved and patented drug of Traditional Chinese Medicine that has been used for years to treat vascular dementia and neural syndrome related to cerebral-vascular ischemia. However, the underlying mechanism of RSSW remains unclear.
Previous studies have indicated that RSSW could significantly reduce cerebral ischemic injury and improve vascular dementia. In the present study, cultured cortical neuron hypoxia/reoxygenation injury model was used to test the neuroprotection of RSSW and its mechanisms in vitro.
RSSW 0.2~5.0 μg/ml was demonstrated to increase intracellular endogenous superoxide dismutase, restore the level of mitochondrial membrane potential in hypoxia/anoxia-injured neurons in a dose-dependent manner. RSSW was also shown to inhibit neuronal apoptosis dose-dependently by suppressing the activity of caspase 3 and caspase 9 and by decreasing the level of reactive oxygen species, the leak of lactate dehydrogenase and the accumulation of malondialdehyde, in hypoxia/anoxia-injured neurons. These findings suggest that the neuroprotection of RSSW from hypoxia injuries may be related to the improvement of intracellular endogenous antioxidants, and the inhibition of the caspase 3 and caspase 9 expressions, which might represent the mechanisms underlying RSSW prevention and treatment of neurodegenerative disorders and neural disorders related to cerebral-vascular ischemia.
Keywords
Rensheng shouwu (RSSW), Neuroprotection, Primarily cultured cortical neuron, Hypoxia/Reoxygen, Apoptosis, Caspase
Introduction
Stroke and neurodegenerative disease account for the majority of public health problems in middle-aged and elderly people. These health problems have become a heavier burden over the years to families and society, as the average lifespan has continued to increase. However, combating stroke and neurodegenerative disease has proven to be an enormous challenge [1-3].
Oxidative stress has been implicated in the progression of stroke and neurodegenerative diseases, such as Alzheimer's disease (AD), Parkinson's disease (PD) and amyotrophic lateral sclerosis (ALS) [4-6]. Oxidative stress is the result of an imbalance in pro-oxidant/antioxidant homeostasis that leads to the generation of toxic reactive oxygen species (ROS). Reactive oxygen species react preferentially with certain atoms to modulate functions ranging from cell homeostasis to cell death [5].
Significant amounts of oxygen free radicals (oxidants) are generated during cerebral ischemia/reperfusion, and oxidative stress plays an important role in brain ischemic neuronal apoptosis and brain damage after stroke. Apoptosis is the main mechanism of cerebral ischemic neuronal damage. In addition to oxidizing macromolecules, leading to cell injury, oxidants are also involved in cell death/survival signal pathways and cause mitochondrial dysfunction [4].
Early signs of cell apoptosis include mitochondrial dysfunction, increased membrane permeability, and decreased membrane potential. Intracellular ROS increase dramatically and induce changes to mitochondrial membrane potential, damage to mitochondrial DNA, changes to the electron transfer chain, and disruption of mitochondrial function, eventually leading to neuronal degeneration and death [6].
Increasing endo-antioxidant function, restoring mitochondrial dysfunction, and reducing the apoptosis induced by oxidative stress are promising strategies to treat the cerebrovascular and neural disorders related to ischemic brain damage, such as ischemic stroke, vascular dementia, and neurodegenerative diseases, such as AD, PD, and others [4-10].
Chinese medicine plays an important role in the prevention and treatment of cerebral ischemia-reperfusion injury and neurodegenerative diseases, such as AD and PD among others [10-12]. Renshen Shouwu Capsule (RSSW) is a patented and Chinese Pharmacopoeia approved Traditional Chinese Medicine (TCM). It is widely used to treat several diseases, including neurodegenerative diseases, amnesia, insomnia, and mental decline [13]. RSSW is composed of Ginseng (Root of Ginseng-Panax ginseng C.A. Mey) and fleece-flower root (Radix Polygoni Multiflori-Polygonum multiflorum Thunb). RSSW had significant neuroprotective effects against middle cerebral artery occlusion (MCAO) and vessel occlusion caused by Ischemic reperfusion (4-VO I/R) injury and a therapeutic effect on cognitive disorders in vascular dementia (VD) rats [14]. Our previous studies have shown that RSSW administration significantly reduced the size of the lesion of the insulted brain hemisphere and improved the neurological behavior of MCAO rats. In addition, RSSW markedly reduced an increase in brain infarct volume from an I/R-induced MCAO and reduced the cerebral water content in a dose-dependent manner. Administration of RSSW also increased pyramidal neuronal density in the hippocampus of surviving rats after transient global brain ischemia and improved the learning and memory ability of rats with 4-VO induced vascular dementia in a dose-dependent manner [14]. Although RSSW has significant neuroprotective effects against MCAO and 4-VO I/R injury and a therapeutic effect on cognitive disorders in VD rats, the mechanism underlying its neuroprotective effects remains to be elucidated. Therefore, we aimed to investigate the neuroprotective properties of RSSW against oxidative stress neuronal damage induced by hypoxia/reoxygen in primary rat cortical neurons. Edaravone is an free radical scavenger of clinical use for ischemic stroke and other neurodegenerative disorders [15]. We used edaravone as the positive agent to explore the mechanism of RSSW neuroprotection.
Materials and Methods
Preparation of a standardized extract from Renshen Shouwu
Standardized extract from Renshen Shouwu (RSSW) was prepared as previously described [14]. The extract contains more than 16.7 mg/g of total ginsenoside (including 8.52 mg/g ginsenoside Rb1, 1.92 mg/g ginsenoside Rd, 3.33 mg/g ginsenoside Re, and 2.9 mg/g ginsenoside Rg1) and 27.1 mg/g of stilbene glucoside (SBG), as characterized by high performance liquid chromatography.
Primary cultures of rat cortical neuronal cells
Procedures involving animals were conducted in accordance with the Animal Ethics Committee of Guangdong Pharmaceutical University. Rats' cortical neuron culture and purification were conducted essentially as Wei, Bei and Luo reported [16,17] with minor modifications. Cerebral cortices were isolated from 1 postnatal-day Sprague-Dawley rats and triturated in ice PBS for 1 min (90% tissue dispersal). Tissue dispersal was incubated in sterile tube with 2 ml of cell dissociation solution (0.02% ethylene diamine tetraacetic acid (EDTA) solution and 0.25% trypsin solution) at 37 °C for 15 min. The dissociation was terminated with Dulbecco Minimum Essential Medium (DMEM)/F12 containing 10% fetal serum. The dissociated samples were filtered using 35 μm nylon mesh. The suspension was centrifuged at 1,000 rpm for 5 min, and then cells were resuspended in DMEM/F12 supplemented with glucose (0.6% wt./vol.), penicillin (100 U/ml), streptomycin (100 mg/ml), and 10% fetal calf serum. Cells were diluted with DMEM/F12 to approximately 1 × 109/L and plated into 6-well-plates (or 30 mm-plates), which were previously coated with 100 mg/L poly-L-lysine. Cells were incubated at 37 °C, 5% CO2, 95% humidity for 4 h. After neurons were attached to the plate, the medium was discarded, and neurons were maintained in Neurobasal medium supplemented with 2% B-27 and L-glutamine (0.5 mM). After the cells grow mature (approximately 10-12 days), immune fluorescence identification of neuronal cells and hypoxia/reoxygenation were conducted.
Hypoxia/reoxygenation model and cell culture treatment
The neurons were treated with Na2S2O4 at a concentration of 2 mM in the glucose-free Earle's balanced salt solution (EBSS, pH 7.4) medium for 4 h (hypoxia). The hypoxia procedure was terminated by replacing the anoxic medium with Neurobasal medium for an additional 2 h (reoxygenation). Various concentrations of RSSW l, m, h (0.2, 1.0, 5.0 μg/ml, for RSSW l, RSSW m, RSSW h, respectively) and Edaravone (2 mM) were added into the cultures 24 h before inducing hypoxia/reoxygenation, respectively. Control cultures were treated in an identical way without Na2S2O4 but EBSS. Next, the cultures were harvested for the detection of cell viability, apoptosis, mitochondrial membrane potential, ROS, SOD, MDA, Caspase 3, and Caspase 9.
Cell viability assay
Cell viability, an indication of the cytotoxicity or neuroprotection of RSSW and Na2S2O4, was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and the absorbance at 570 nm measured with a micro plate reader .
Neuronal injury assays
Lactate dehydrogenase (LDH) leakage in extracellular supernatant medium, an index of cell injury, was determined with an LDH assay commercial kit (Nanjing Jian Chen BioChem Co.) at 440 nm.
The protective effects of RSSW on cortical neurons induced by H/R were measured by determining the amount of apoptotic cells as described by Li and Chen [4,18].
Hoechst 33258 staining for morphological analysis of apoptosis
Neurons were seeded on sterile cover glasses placed in the 6-well plates. After all treatments, cells were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 10 min, and then incubated with 50 μM Hoechst 33258 staining solution for 10 min. Apoptotic morphological changes in the nuclear chromatin of neurons were detected and then counted under a fluorescence microscope(ZEISS Axio Observer A1).
Apoptosis assays
Neurons were plated in 12-well plates and treated with H/R and SRRW. The apoptosis ratio was analyzed after all treatments via using Annexin V/FITC Apoptosis Detection Kit (BD Biosciences, San Diego, CA) according to the manufacturer's instructions. Annexin V/FITC and propidium iodide double stain were used to evaluate the percentages of apoptosis. Annexin V- and PI- cells were used as controls. Annexin V+ and PI- cells were designated as apoptotic and Annexin V+ and PI+ cells displayed necrotic. Tests were repeated in triplicate.
Measurement of SOD and MDA in neuron cultures
To assess the antioxidants and lipid peroxide in cells after H/R insult, the cultures were harvested and washed with ice-cold PBS and then pooled in 0.1 M PBS/0.05 mM EDTA buffered solution and homogenized. The homogenate was centrifuged for 1 h at 10,000 × g at 4 °C. The supernatants were used in the assay [16]. We assessed the content of protein in cells by the Coomassie blue protein binding method with bovine serum albumin. The content of intracellular superoxide dismutase (SOD) and malondialdehyde (MDA) was determined colorimetryly with a commercial assay kit for SOD and MDA (Nanjing Jian Chen BioChem Co.) under manufacturer instruction.
Determination of ROS level
Intracellular ROS levels were determined by a dichlorofluorescein diacetate (DCFH-DA) assay with Reactive Oxygen Species Assay Kit (Beyotime Company, Haimen, China) as SUN described [18]. Briefly, cells (4 × 104 cells/ml) were seeded in 96-well plates, and 2 days later were pretreated with RSSW for 24 h prior to exposure to H/R. After the indicated treatments, neurons cultured in 96-well plates were incubated in the dark with 10 μM DCFH-DA for 30 min at 37 °C and were washed twice with PBS. Fluorescence emission at 525 nm (kem) from 488 nm excitation (kex) was measured on a fluorescence micro plate reader (BERT hold Technologies, Mithras LB 940) and expressed as a percentage of the DCF fluorescence generated in control cells under identical incubation conditions.
Mitochondrial membrane potential (Δψm) detection
The Δψm was determined by the Mitochondrial Membrane Potential Assay Kit using JC-1 (Beyotime Biotechnology) as Xu described [19]. After the indicated treatments, neurons were loaded with JC-1 for 30 min at 37 °C as directed by the manufacturer, and images were acquired using a ZEISS Axio Observer A1 inversed fluorescence microscope. Using fluorescence microscopy to pictures, the fluorescence intensity was determined. Monomeric JC-1 green fluorescence emission at 480 nm and aggregate JC-1 red fluorescence emission at 530 nm were measured on a micro plate reader. The Δψm of neurons in each treatment group was calculated as the ratio of red to green fluorescence.
Determination of Caspase-3, 9 Activity in neurons by ELISA assays
The activity of caspase-3 and caspase-9 was determined using the caspase-3/-9 activity kit (Beyotime Institute of Biotechnology, Haimen, China) based on colorimetric assay of the yellow formazan chromophore p-nitroaniline (pNA) after cleavage from the labeled substrate DEVD-pNA or LEHD-pNA [18,20]. To evaluate the activity of caspase-3/9, cells were homogenized in 80/100 μl reaction buffer (1% NP-40, 20 mM Tris-HCl (pH 7.5), 137 mM NaCl and 10% glycerol) containing 10 μl caspase-3 substrate (Ac-DEVD-pNA) (2 mM) or caspase-9 substrate (Ac-LEhD-pNA) (2 mM) after all treatments. Lysates were incubated at 37°C for 2 h. Samples were measured with an ELISA reader (Multiskan Ascent) at an absorbance of 405 nm. The detail analysis procedure is described in the manufacturer's protocol. The caspase activity, normalized for total proteins of cell lysates, was then expressed as fold of the baseline caspase activity of control neurons.
Statistical analysis
The data were processed from two to three independent experiments with five to six cultures per experiment, except for the flow cytometric assay with SPSS 13.0 and expressed as the mean ± SD. Raw data were analyzed with GraphPad Prism 5.0 software (GraphPad Software, Inc., San Diego, CA). Statistical significance was determined by using one-way analysis of variance (ANOVA), followed by post hoc test least significant difference (LSD). Differences were considered to be significant for p < 0.05.
Results
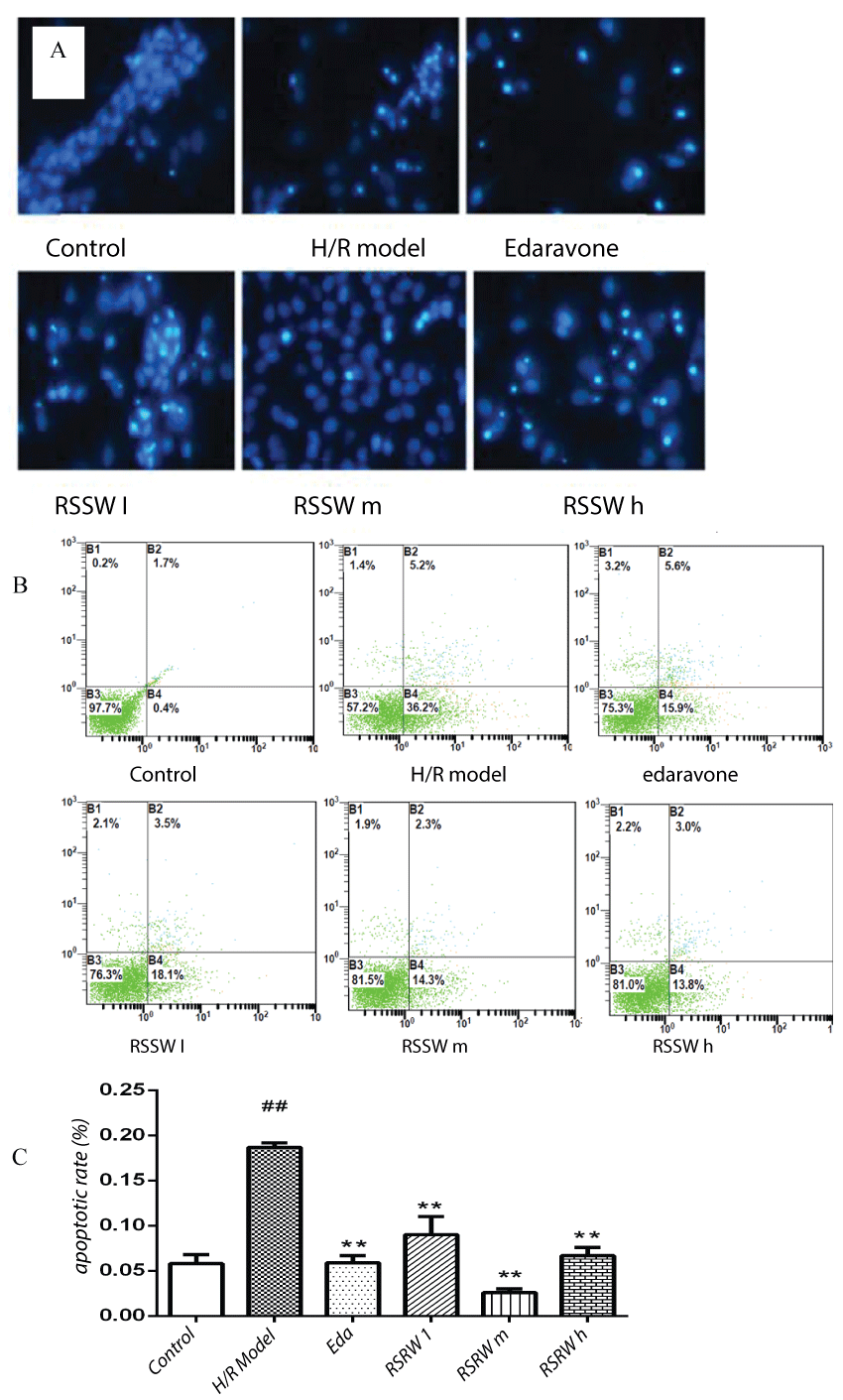
RSSW effect on H/R induced neuronal apoptosis
Cell viability of neurons exposed to hypoxia/reoxygenation (Na2S2O4 at concentration of 2 mM for 4 h) was significantly reduced compared with the control group. RSSW (0.1~25 μg/ml) or 2 mM edaravone (did not affected the cell viability of the cultured neurons.
Hoechst 33258 staining and Annexin V/FITC Apoptosis Detection showed that 20% of neurons exposed to hypoxia/reoxygenation suffered from apoptosis, indicating nuclear chromatin agglutination, condensation nuclear chromatin, or broken chromatin fragment with dense fluorescence. Pre-treatment with RSSW was observed to increase the cell viability and reduce apoptosis rate of neurons exposed to hypoxia/reoxygenation in a concentration-dependent manner. Edaravone also showed a similar effect to RSSW (Figure 1A, Figure 1B and Figure 1C). This indicated that RSSW had the ability to reduce the neuron damage and prevent neuron apoptosis induced by hypoxia/reoxygenation.
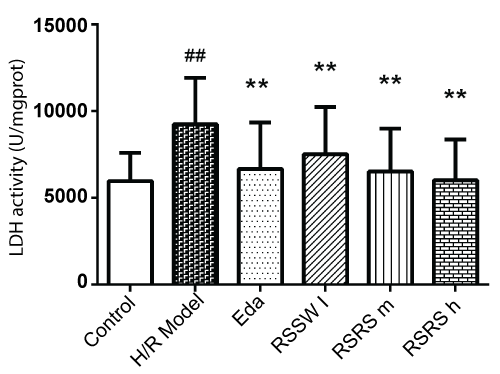
RSSW effect on neuron LDH leakage
Intracellular LDH will leak into the neurons liquid culture medium after hypoxia/reoxygenation injury. Determining LDH leakage quantity in the supernatant fluid of the cultures can measure the damage of cells. As shown in figure 2, H/R induced significant increase of the LDH levels by 53% in the neuron culture medium. Pre-treatment with RSSW significantly decreased the LDH levels in the neuron culture medium in a concentration-dependent manner. Edaravone also showed similar effects to RSSW on neuron LDH leakage.
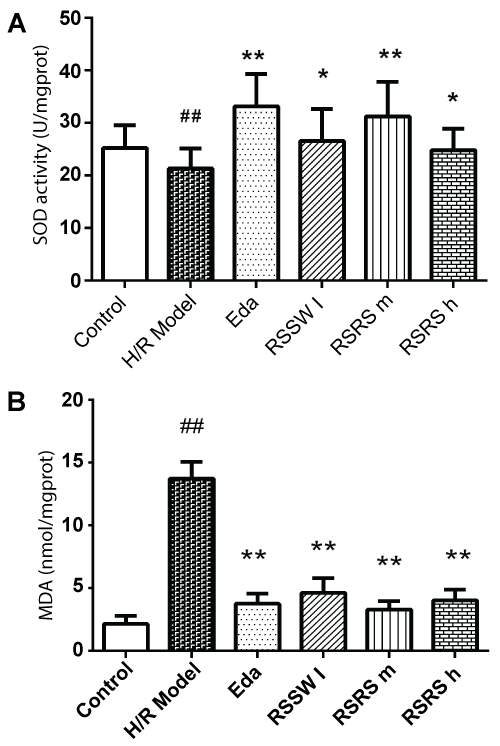
The effect of RSSW on SOD, MDA Level in neurons exposed to H/R
As shown in figure 3, H/R induced SOD reduction and almost 5 times increase of MDA in the neurons exposed to H/R. Pre-treated with RSSW significantly enhanced intracellular SOD and dramatically reduced the intracellular MDA content in the neurons exposed to H/R in a dose-dependent manner except high concentration of RSSW. A similar effect was observed with edaravone.
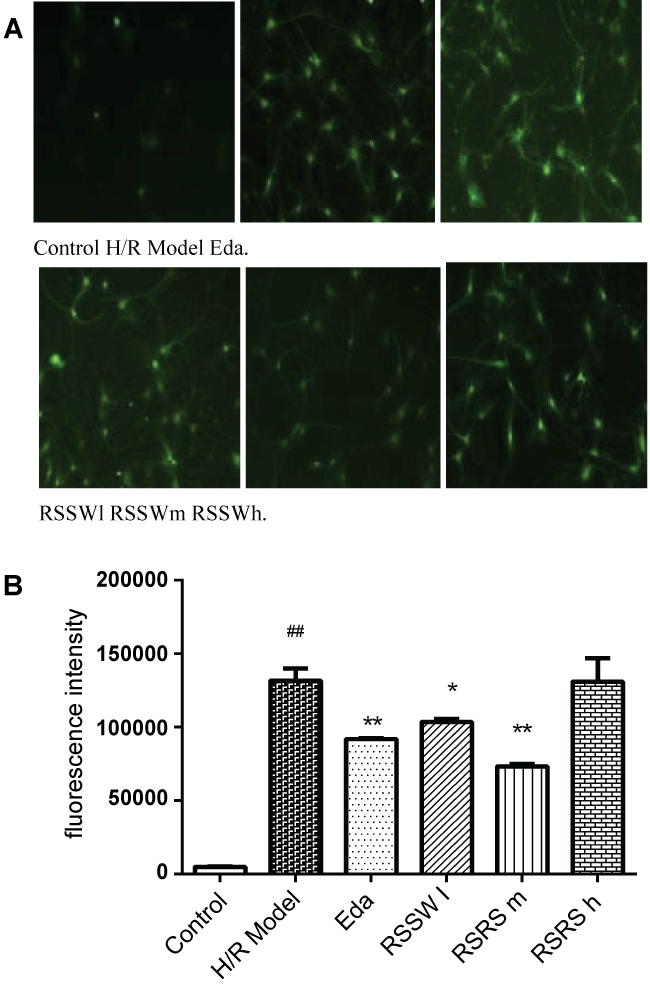
RSSW effect on neuron intracellular ROS
High concentration of reactive oxygen species can induce apoptosis and even lead to its necrosis by oxidative stress reaction, which can be used to measure the level of apoptosis. As shown in figure 4, H/R induced a significant increase of intracellular ROS in the neurons as indicated by the green fluorescence intensity. Pre-treatment with RSSW significantly decreased the intracellular ROS in a concentration-dependent manner except higher than 5 μg/ml. Edaravone also showed a similar effect of RSSW on neuron intracellular ROS.
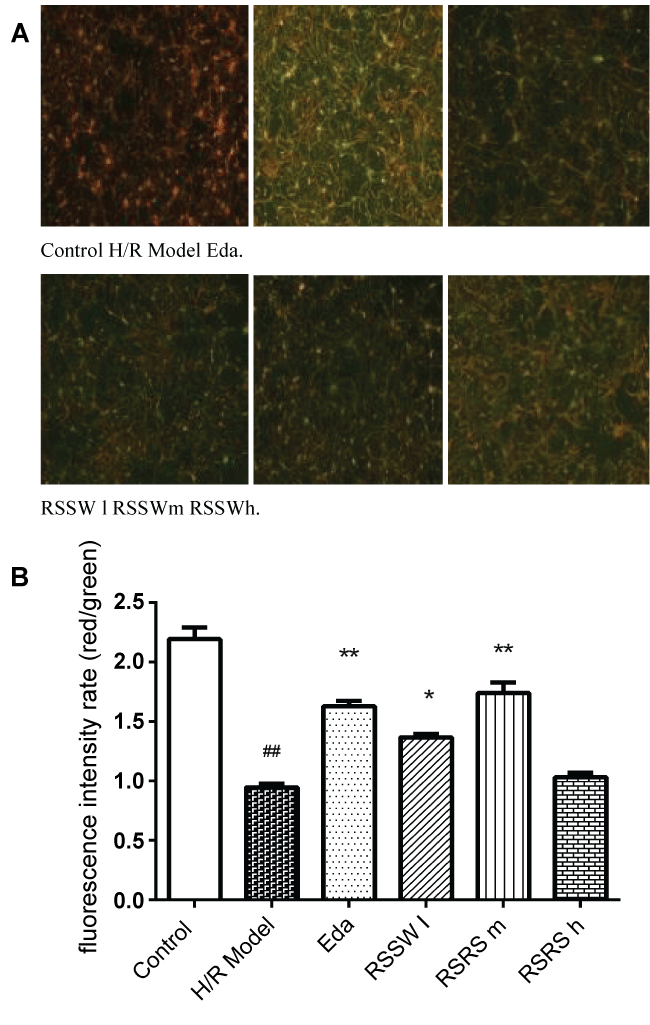
RSSW effect on neuron mitochondrial membrane potential (Δψm)
Mitochondria are the main sites of ATP in animal cells, and are the important cell devices to promote cell energy conversion and participate in cell apoptosis. Decreasing of membrane potential level indicates the degree of cell apoptosis. As shown in figure 5, H/R significantly reduced Δψm levels by 60% in the neuron, and RSSW significantly recovered Δψm to 80% of the control level in the neuron culture exposed to H/R in a concentration-dependent manner except higher than 5 μg/ml. Edaravone also showed a similar effect of RSSW on neuron Δψm.
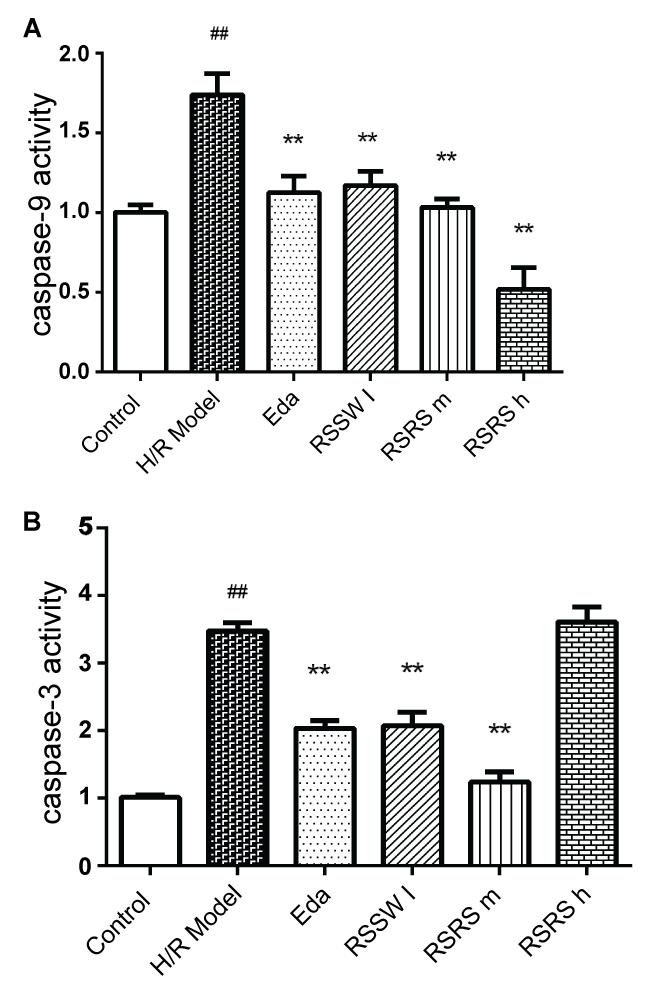
RSSW effect on Caspase-3,9 activity in the neurons exposed to H/R
H/R significantly increased Caspase 3, 9 activities in the neurons. Pretreatment with RSSW decreased caspase-3,9 activity in the neurons exposed to H/R, and the same was observed with Edaravone pre-treatment (Figure 6A). However, the high concentration of RSSW did not reduce the increased caspase-3 activity induced by H/R (Figure 6B).
Discussion
Edaravone, a free radical scavenger, has been used for the treatment of ischemic stroke and other neurodegenerative disorders. It showed a protective effect on oxidative damage of neurons, v is scavenging oxygen free radicals, inhibiting lipid peroxidation, which play a vital role in the treatment of cerebral ischemia [15]. We used edaravone as the positive agent to explore the mechanism of RSSW neuroprotection. Our study found that oxidative stress stimulation by Na2S2O4 induced apoptosis of the cultured rat cortical neurons. As like edaravone, RSSW protect neurons to alleviate the apoptosis and the leakage of LDH in the insulting neurons while increasing SOD activity and the mitochondrial membrane potential of the insulting neurons, and reducing the level of reactive oxygen species and the accumulation of MDA neuronal cells, which accompanied with the inhibition of the activity of caspase 3 and caspase 9 of the neurons. This indicates that RSSW might protect neurons from hypoxia/reoxygenation-induced injuries by reducing oxidative stress, which provided a scientifical data for the RSSW Clinical usage in the treatment of oxidative stress related disorder such as ischemic stroke and neurodegenerative diseases.
Oxidative stress is a major pathophysiologic mechanism in the progression of stroke and neurodegenerative diseases, such as AD, PD and ALS [4-6]. In stroke, AD, and PD, abnormal brain metabolism caused by decreased cerebral blood flow is the cause of early pathological changes in hypoxic-ischemic encephalopathy and appears in pathophysiological responses that occur mainly in neuronal apoptosis [2-5]. Several studies have indicated that cell apoptosis is closely associated with oxidative stress. Oxidative stress results in a sharp increase in intracellular ROS, which causes a change in mitochondrial decreased membrane potential, damages to mitochondrial membrane structure, damages to mitochondrial DNA and the electron transfer chain, and disruption of mitochondrial function. These effects of oxidative stress eventually lead to neuronal apoptosis, degeneration, and death [6-9,21,22]. Indeed, mitochondrial dysfunction, increased membrane permeability, and decreased membrane potential are early markers of cell apoptosis [8,9].
Ischemia and hypoxia in the brain disrupt neuronal oxidative function and produce large amounts of free radicals. SOD-level indirectly responds to the body's ability to scavenge oxygen free radicals [4-6,21,22] and thereafter free radical scavenging can alleviate neurological injury [23].
Neuron apoptosis is regulated by active genes, such as caspases, and is reversible under certain conditions by inhibiting or blocking the expression of caspases during treatment or intervention using certain special agents in the early apoptosis stage. This phenomenon may have a protective effect on insulted cells and may be beneficial to the functional restoration of the injured and damaged neuron [21,22].
The main pathway of apoptosis contains endogenous and exogenous processes, eventually brought together in a cascade of caspase-family effector proteins, which can be used during apoptosis to induce degradation of intracellular proteins [21-24]. Hypoxic injury caused by endogenous apoptosis mainly activates BH3-only family protein and, during a series of reactions that activate caspase-9, a cascade of downstream caspase-family effector proteins become activated, finally leading to cell apoptosis [21-24]. During this process, Caspase-3 is the key caspase cascade protein that serves as the common part of many downstream effector receptor-mediated apoptosis pathways [8,9,21]. Sometimes is Caspase in other caspase. Previous studies have suggested that hypoxia/reoxygenation could damage nerve cells and cause apoptosis by producing excessive reactive oxygen [21,24]. Furthermore, it was found that hypoxia/reoxygenation could activate caspase-3 activity, leading to apoptosis in cultured cortical neurons [24]. Therefore, inhibiting caspase-3 and caspase-9 activity can reduce the hypoxia/reoxygenation neuronal damage that produces apoptosis, thereby increasing neuronal survival and improving neurological function.
Our study showed that RSSW could decrease the activity of caspase-3, and caspase-9 and inhibit cell apoposis in a dose-dependent manner. Although at high doses caspase-3 vitality was increased, overall our data indicated that RSSW protected neurons from apoptosis via inhibiting the activity of caspase-3 and caspase-9 and improving mitochondrial function.
The present study shows for the first time that RSSW significantly inhibits neuronal apoptosis, suppresses oxidative stress through inhibition of caspase 3 and 9, and protects neurons against hypoxic neuronal injury.
We previous reported that administration of RSSW (50, 100, and 200 mg/kg) has significant neuroprotective effects against MCAO and 4-VOI/R injury and a therapeutic effect on cognitive disorders in VD rats. In this study, 0.2~1 μg/ml of RSSW contains approximately 3.34 ng/g of total ginsenoside (including 1.74 ng/g Rb1 and 0.58 ng/g Rg1) and 5.42 ng/g of stilbene glucoside. This concentration is consistent with the actual concentration in the rat brain after administration of 100 mg/kg RSSW (unpublished data). It was reported that ginsenoside (the active ingredient of Panax ginseng) and stilbene glucoside (the active ingredient of fleece-flower root) were effective at protecting neurons from oxidative stress-induced neurotoxicitye at the concentration of 1~5 μg/ml [14,17-21]. This might indicate that RSSW showed neuroprotection via improving hypoxia injury through its active ingredients of ginsenoside and stilbene glucoside, which have been shown to have antioxidant properties, such as scavenging oxygen free radicals. It was noted in our experiment that a higher concentration (more than 5 μg/ml) of RSSW did not lead to greater neuroprotection when studying the reduction of ROS and MDA, inhibition of caspase 3 activity, apoptosis ratio to lower level, or increase of mitochondrial membrane potential. Our finding is consistent with other reports on the neuroprotective effects of some agents with antioxidant properties in oxidative stress models, which might contribute to their ability to produce free radicals that induce apoptosis [25]. The underlying cause remains to be further elucidated. It might also suggest that the dosage of RSSW and other antioxidants should be controlled properly in the clinic use as not to give excessive dose to avoid the reverse effect and waste the herbs resources.
In conclusion, this study's findings suggest that the neuroprotection of RSSW from hypoxia injuries may be related to its ability to increase intracellular endogenous antioxidant SOD, reduce ROS, improve mitochondrial membrane potential, and decrease caspase 3 and caspase 9 activity in neurons exposed to oxidative stress. These processes might represent the mechanisms underlying the use of RSSW in the prevention and treatment of neural disorders related to cerebrovascular ischemia and some neurodegenerative diseases, such as PD and AD.
Acknowledgments
This study was supported by grants from the Natural Sciences Funds, Republic of China No.81473588,2014, the Guangdong Province Science and Technology New Drug R & D Key Project No. 2013A022100041 and the Natural Science Foundation of Guangdong Province NO.2014A030313585.
Conflict of Interest
We wish to confirm that there are no known conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome.
Highlights
• RSSW can inhibit neuronal cell apoptosis induced by hypoxia/reoxygenation.
• RSSW can increase the SOD activity, reduce ROS, and improve mitochondrial membrane potential in cortical neuron suffering from hypoxia/reoxygenation injury.
• RSSW protects neuron from apoptosis by reducing the activity of caspase 3 and caspase 9.
References
- AS Go, D Mozaffarian, VL Roger, et al. (2013) Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation 127: e6-e245.
- A Ward, HM Arrighi, S Michels, et al. (2012) Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement 8: 14-21.
- AM Crizzle, S Classen, EY Uc (2012) Parkinson disease and driving: an evidence-based review. Neurology 79: 2067-2074.
- H Chen, H Yoshioka, GS Kim, et al. (2011) Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal 14: 1505-1517.
- KJ Barnham, CL Masters, AI Bush (2004) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3: 205-214.
- S Orrenius, V Gogvadze, B Zhivotovsky (2007) Mitochondrial Oxidative Stress: Implications for Cell Death. Annu Rev Pharmacol Toxicol 47: 143-183.
- JA Klein, SL Ackerman (2003) Oxidative stress, cell cycle, and neurodegeneration. J Clin Invest 11: 785-793.
- JK Andersen (2004) Oxidative stress in neurodegeneration: cause or consequence?. Nat Med 10: S18-25.
- CB Park, NG Larsson (2011) Mitochondrial DNA mutations in disease and aging. J Cell Biol 193: 809-818.
- XH Shang, XY Xu (2013) Progress in the experimental study of the protective effect of Chinese herbal medicine and its extract on cerebral ischemia. China Journal of Chinese Materia Medica 38: 1109-1115.
- DS Liu, YH Zhou, ES Liang, et al. (2013) Neuroprotective effects of the Chinese Yi-Qi-Bu-Shen recipe extract on injury of rat hippocampal neurons induced by hypoxia/reoxygenation. J Ethnopharmacol 145: 168-174.
- J Zhao, Y Zhao, WP Zheng, et al. (2008) Neuroprotective effect of curcumin on transient focal cerebral ischemia in rats. Brain research 1229: 224-232.
- Pharmacopoeia of the People's Republic of China, Volume 1. (2015) Chemical Industry Press, Beijing, 468.
- L Wan, YF Cheng, ZY Luo, et al. (2015) Neuroprotection, learning and memory improvement of a standardized extract from Renshen Shouwu against neuronal injury and vascular dementia in rats with brain ischemia. J Ethnopharmacol 165: 118-128.
- BJ Lee, Y Egi, K Van Leyen, et al. (2010) Edaravone, a free radical scavenger, protects components of the neurovascular unit against oxidative stress in vitro. Brain research 1307: 22-27.
- WJ Bei, WL Peng, LQ Zang, et al. (2007) Neuroprotective effects of a standardized extract of Diospyros kaki leaves on MCAO transient focal cerebral ischemic rats and cultured neurons injured by glutamate or hypoxia. Planta Med 73: 636-643.
- J Wei, WR Fang, L Sha, et al. (2013) XQ-1H Suppresses Neutrophils Infiltration and Oxidative Stress Induced by Cerebral Ischemia Injury Both In Vivo and In Vitro. Neurochem Res.
- XB Li, Y Li, JZ Chen, et al. (2010) Tetrahydroxystilbene glucoside attenuates MPP+-induced apoptosis in PC12 cells by inhibiting ROS generation and modulating JNK activation. Neurosci Lett 483: 1-5.
- Shangcheng Xu, Mindi He, Min Zhong, et al. (2015) The neuroprotective effects of taurine against nickel by reducing oxidative stress and maintaining mitochondrial function in cortical neurons. Neurosci Lett 590: 52-57.
- FL Sun, L Zhang, RY Zhang, et al. (2011) Tetrahydroxystilbene glucoside protects human neuroblastoma SH-SY5Y cells against MPP+-induced cytotoxicity. Eur J Pharmacol 660: 283-290.
- DS Warner, H Sheng, IB Haberle (2004) Oxidants, antioxidants and the ischemic brain. J Exp Biol 207: 3221-3231.
- A Lewen, P Matz, PH Chan (2000) Free radical pathways in CNS injury. Neurotrauma 17: 871-890.
- D Martinvalet, P Zhu, J Lieberman (2005) Granzyme A induces caspase-independent mitochondrial damage, a required first step for apoptosis. Immunity 22: 355-370.
- H Ueda, R Fujita, A Yashida, et al. (2007) Identification of prothymosin-alpha1, the necrosis-apoptosis switch molecule under the in vitro ischemia condition. Neuroscience Research 176: 58.
- WJ Bei, WL Peng, Y Ma, et al. (2005) Flavonoids from the leaves of Diospyros kaki reduce hydrogen peroxide-induced injury of NG108-15 cells. Life Sciences 76: 1975-1988.
Corresponding Author
Wei-jian Bei, Professor and Scientific Office, The Institute of Chinese Medicinal Sciences, Key unit of Modulating Liver to Treat Hyperlipemia State Administration of Traditional Chinese Medicine, Guangdong Province Research Centre for Chinese Integrative Medicine Against Metabolic Disease, Guangdong TCM key laboratory against metabolic diseases, Guangdong Pharmaceutical University, Guangzhou Higher Education Mega Centre, Guangzhou, 510006, P.R.China, Tel: +86-20-39352607.
Copyright
© 2016 Zhu J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.