Antibody Response at 1, 4, and 6 Month after a Third Dose of Intradermal Vaccination of PFE-BNT in Adults Who Have Completed Two-Doses of Sinovac (Inactivated Vaccine)
Introduction
Studies reported waning of antibody level in people who have completed two doses of inactivated vaccine as time passes [1]. WHO with support of the Strategic Advisory Group of Experts (SAGE) on Immunization and its COVID-19 Vaccines Working Group has announced the recommendation guidance for booster doses vaccination [2]. To response to the concerns of COVID-19 pandemic, Thai government has launched a recommendation on June 2021 to administer a third vaccine dose in people who have completed 2 doses of inactivated vaccine for more than 3 months. However, due to the shortage of vaccine supply during that period, vaccine distribution was prioritized by risk for acquiring COVID-19 infection and risk of developing serious illness. Thus, we conducted an open label study of intradermal vaccination of PFE-BNT in adults who have completed two-doses of Sinovac (inactivated vaccine). The trial was registered at TCTR20211023002. Safety and tolerability of PFE-BNT ID injection has already reported in the previous study [3]. In this report, we intended to monitor the persistent of the antibody level after the ID PFE-BNT injection at month 4 and 6.
Methods
42 participants who completed 2 doses of inactivated vaccines for more than 3 months and had received 6 ug of the PFE-BNT vaccine at deltoid region via intradermal route in the PFE-BNT intradermal route trial were continued to monitor their antibody level at month 4 and 6. 4 ml of venous blood was recollected using gel and clot activator tubes. An automated AdviseDx SARS-CoV-2 IgG II assay, Architect was used to detect immunoglobulin class G (IgG) antibodies to the receptor binding domain (RBD) of the S1 component of the spike protein of SARS-CoV-2 [4]. The titer greater than 50 AU/mL is considered positive (detection range, 6.8-80,000 AU/ml). The Architect assay sensitivity is 92.7% (95% CI 90.2-94.8) and specificity is 99.9% (99.4-100%). The anti-spike antibody titer was shown to correlate with in vitro neutralization of SARSCoV-2. Wilcoxon-signed-rank test and semi-log dot plot of antibody graph was performed using R version 4.1.2.
Results
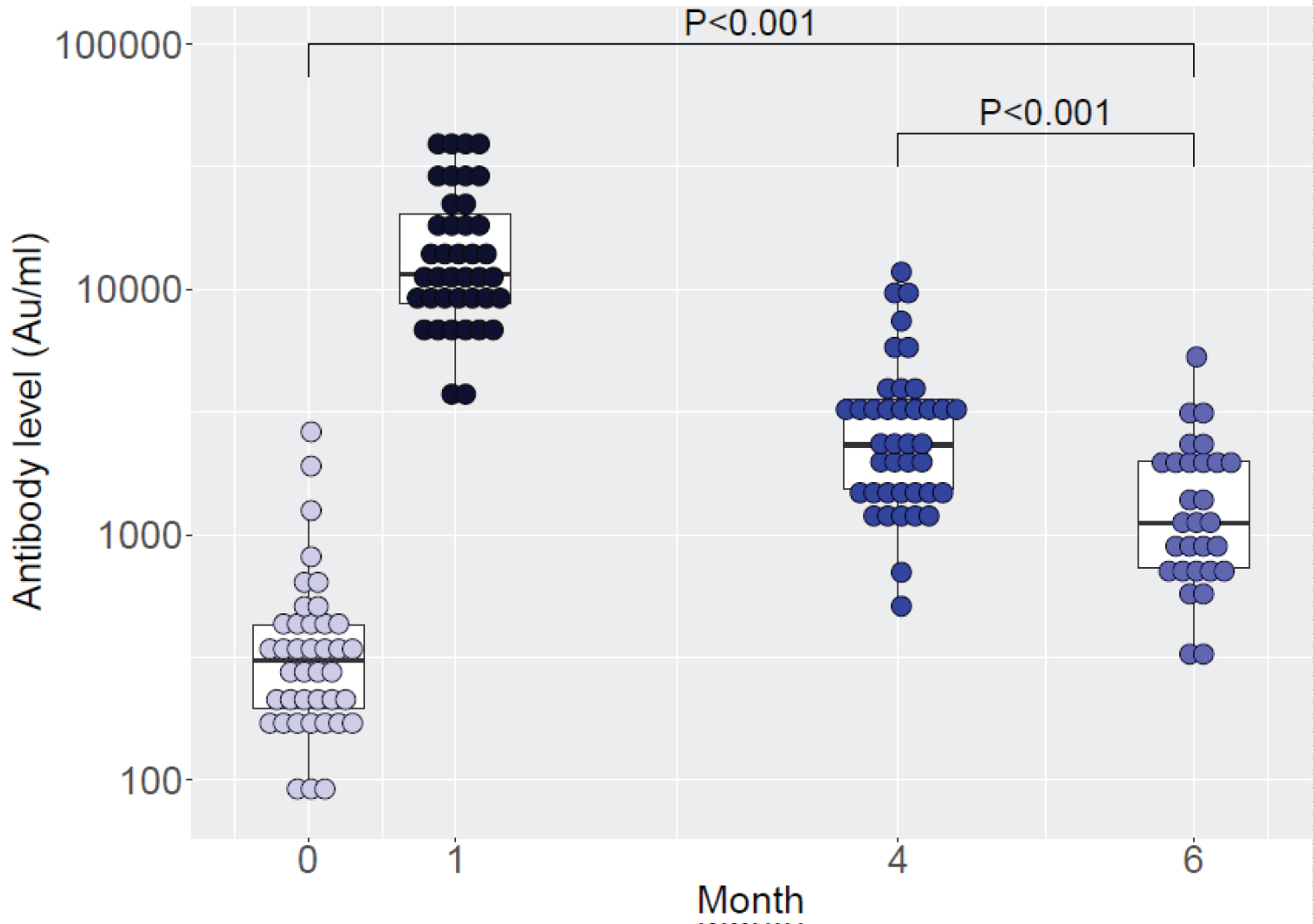
42 participants had their blood test for antibody level at before the ID injection, month 1, and 4 after injection. 29 participants had blood test at month 6. 13 were withdrawn from the study because they had received other Covid-19 vaccination prior to blood examination on month 6. There was no participant had Covid-19 infection during follow up. Patients' demographic data and antibody level were shown in the Table 1 and the antibody levels were shown on the Figure 1.
Discussion
This study found that the PFE-BNT vaccine ID route as a booster dose after completing 2 doses of inactivated vaccine (Sinovac) could significantly increase the antibody response at 1st month and the level of antibody remained high until month 6. The findings are in accordance with other study of an IM booster dose of PFE-BNT vaccine at first month [5] and a study form Siriraj Clinical Research Centre that a full dose and a half dose booster of PFE-BNT vaccine after 2 doses of Sinovac (inactivated vaccine) proved to be effective against both the Delta variant and the Omicron variant [6]. The strengths of our study are we are the leader in reporting the antibody level after PFE-BNT vaccine ID injection and we examined the antibody level until 6 months.
Limitations of this study include: Our samples are small, 13 out of 42 participants were removed from the study at month 6, the neutralizing antibody was not performed, and the correlation of antibody levels to protection is unknown.
Conclusion
The use of an intradermal route of PFE-BNT vaccine may be considered to use as a booster dose.
References
- Khoury DS, Cromer D, Reynaldi A, et al. (2021) Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 27: 1205-1211.
- World Health Organization: WHO (2021) Interim statement on COVID-19 vaccine booster doses [Internet].
- Chalermphanchai N, Arunothong W, Jettavan N, et al. (2022) Safety, tolerability, and antibody response after intradermal vaccination of PFE-BNT in adults who have completed two-doses of Verocell (inactivated vaccine). Vaccine X. 10: 100148.
- Abbot (2022) AdviseDx SARS-CoV-2 IgG II for use with architect [Internet].
- Lustig Y, Gonen T, Melzer L, et al. (2021) Superior immunogenicity and effectiveness of the 3rd BNT162b2 vaccine dose [Internet]. Infectious Diseases (except HIV/AIDS) Dec.
- Fronde N (2022) Study: Sinovac with a Pfizer booster more effective than AstraZeneca [Internet].
Corresponding Author
Wachiraporn Arunothong, Division of Research and Innovation, Lampang Hospital, Thailand
Copyright
© 2022 Arunothong W. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.