SARS-CoV-2 Vaccine: Reconnoitering the Prospects
Abstract
COVID-19 pandemic which has taken the whole world by a storm is the major concern of all the regulatory agencies working globally, across continents, countries, and states. Being the worst pandemic in a century, it has not only claimed numerous lives and overwhelmed the health facilities in the world's most developed nations but also brought the world economy to an unprecedented downfall. However, the pace at which the planetary scientific community responded to confront the situation also matched the incredible pace of the pandemic. In a very short span of less than a year, an overall landscape of 180 candidate vaccines using available vaccine platforms was developed. Many of these vaccines have reached various clinical trial phases, some clearing the phase 1, others even crossed the phase 2, and are currently being evaluated in the phase 3. Progress on nine such candidate vaccines currently being evaluated in the phase 3 trials including ChAdOx-1, Sputnik V, and Ad5-nCoV is very encouraging. If all the trials go fine than the world will have its first anti-COVID-19 vaccine early next year. But the flip side also necessities to be considered as vaccine reactogenicities might overcome the protection efficacy. Its actual test will be the phase 4. Hiking against all odds, this will be the first vaccine in the history of vaccine development to be made available in such a short time through public-private co-operation and active involvement of regulatory and financing agencies.
Keywords
COVID-19 vaccines, Phase III Clinical Trials, SARS-CoV-2, Immunity
Abbreviations
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; MERS: Middle East Respiratory Syndrome; VAERD: Vaccine Associated Enhanced Respiratory Disease; GMT: Geometric Mean Titer; COVID-19: Coronavirus Disease 2019; nAb: Neutralizing Antibody
Introduction
COVID-19 (Coronavirus disease 2019), a respiratory infectious disease, has emerged like a whirlwind that has infected 33,476,219 individuals and caused 1,004,669 deaths globally, as of September 28, 2020. Every month the COVID-19 pandemic is accompanied by the traumatization of tens of thousands of lives, healthcare systems, and the global economy. Moreover, "in the age of COVID-19, nobody is safe until everybody is safe" [1]. The current global landscape of SARS-CoV-2 vaccines include a total of 180 candidates. A number of these could reach various clinical trial phases, some clearing the phase 1, others even crossed the phase 2, and are currently being evaluated in the phase 3 as surveyed extensively in our previous review [2]. Progress on nine such candidate vaccines currently being evaluated in the phase 3 trials including ChAdOx-1, Sputnik V, and Ad5-nCoV is very encouraging. If all the trials go fine then the world will have its first anti-COVID-19 vaccine early next year. This report focuses on the further advancements in COVID-19 vaccines since our earlier publication "COVID-19 Vaccines: A Comprehensive Status Report" [2] and provide the readers with a summary of vaccines presently under phase 3 trials, how they are progressing in the phase 3 trials and the expected date of completion of each of these trials.
Where do we stand today?
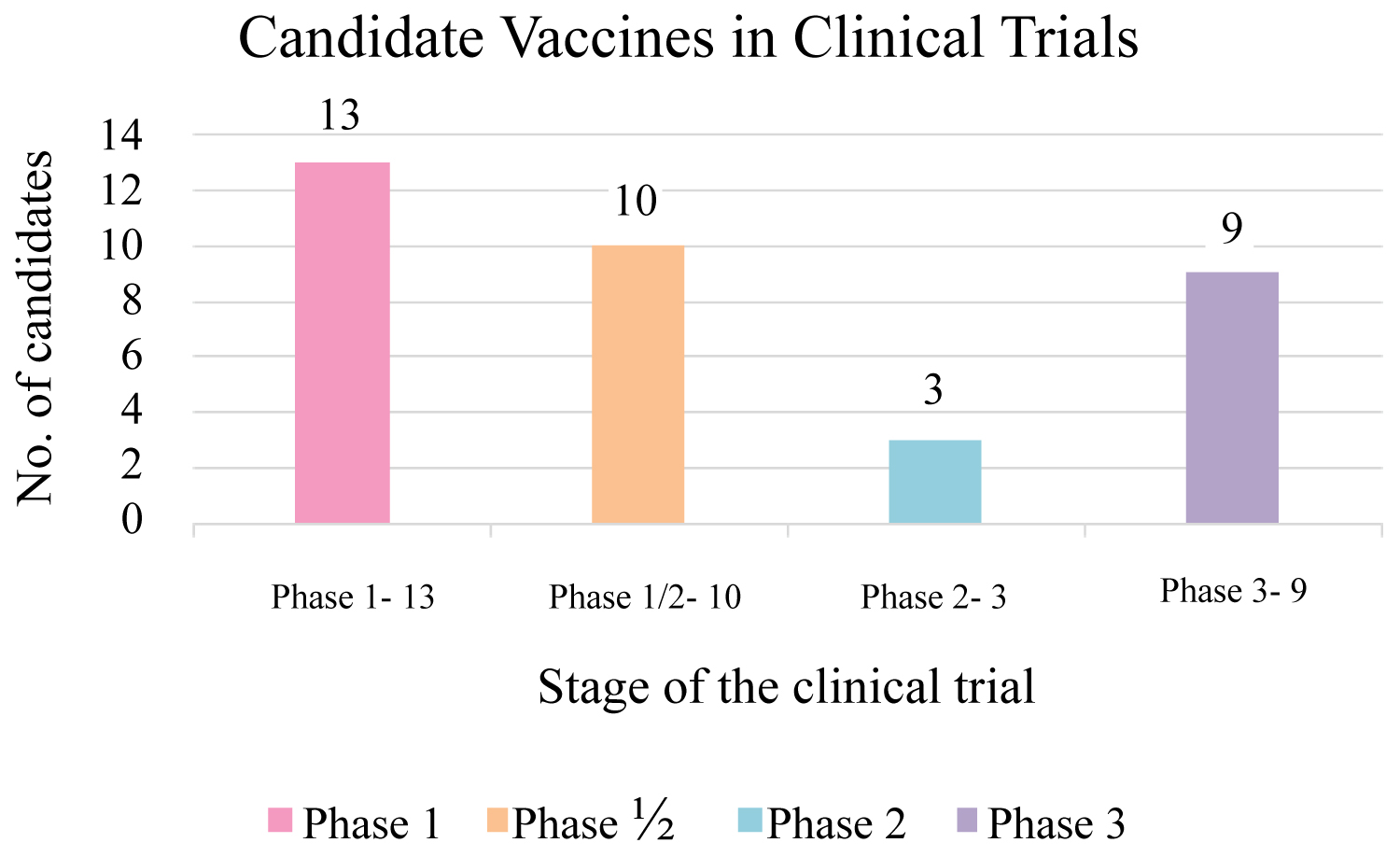
It's interesting to decipher that within 7 months since COVID-19 is declared a pandemic by WHO nearly 180 rapid and conceited vaccine development programs are operating to position an immunogenic and safe vaccine against SARS-CoV-2 at the earliest [3], to bring the acute phase of this pandemic to an end. A wide range of vaccine candidates based upon various platforms including protein subunit, virus-like particle, live attenuated, inactivated viral vectored, DNA, RNA, and nanoparticle-based are in various stages of clinical trials (Figure 1). Multiple vaccine candidates have reached the third stage of clinical trials (Table 1) but no vaccine is available for the public use in the current scenario.
Status of the vaccines currently in phase 3 trial
ChAdOx-1/Covishield/AZD1222 | University of Oxford/AstraZeneca: ChAdOx-1 is the front-runner in the race of COVID-19 vaccine development. Initial reports have established the safety and immunogenicity profile of the vaccine candidate [4]. It is currently under phase 3 clinical trials in Britain, the United States, South Africa, and India, and it is expected to enroll nearly 50,000 participants [5]. The trial process, however, experienced a pause due to an undisclosed neurological illness in one of the study participants but, after a complete review process, the trials have recommenced [6]. Moreover, the previous investigations advocate the requirement of lower doses of vaccine delivered via a nasal spray. Therefore, the upcoming vaccine trials will involve the administration of aerosolized vaccines with the aid of a nebulizer which shall deliver the vaccine as airborne droplets [7].
BNT162b2 | BioNTech/FosunPharma/Pfizer: The phase 1 safety and immunogenicity trials of BNT162b1 and BNT162b2 validated the nomination of BNT162b2 for the Phase 2/3 clinical evaluation due to lower reactogenicity in older participants. BNT162b2 encodes for a full-length spike protein with two proline mutations to clasp the protein in the prefusion state to enhance its potency to elicit an immune response. The immunogenicity profile exhibited lower humoral responses in 65-85 year-olds than the 18-55 year-olds. However, the administration of the second dose intensified the nAb whose GMT surpassed the convalescent serum control. Furthermore, the vaccinated participants also displayed a concurrent activation of CD4+ and CD8+ T cell responses against the entire Spike protein. Moreover, phase 2/3 will evaluate the safety and efficacy of two 30 µg doses of BNT162b2 in a randomized, placebo-controlled clinical trial involving nearly 30,000 participants with varying backgrounds [8].
Gam-COVID-Vac and Gam-COVID-Vac-Lyo (Sputnik V) | Gamaleya Research Institute of Epidemiology and Microbiology: Gam-COVID-Vac (frozen) and Gam-COVID-Vac-Lyo (lyophilized) are the novel non-replicating adenovirus vector-based vaccines formulations against SARS-CoV-2. They are heterologous COVID-19 vaccines with two components, a recombinant adenovirus type 26 (rAd26-S) and recombinant adenovirus type 5 (rAd5-S) vectors containing the gene for spike glycoprotein. The phase 1 clinical trials for assessing the safety of the two components involved the administration of either rAd26-S or rAd5-S. Whereas, the phase 2 clinical trials involved the administration of prime-boost vaccination wherein, both the components were administered at an interval of 21 days. The critical monitoring process of the volunteers involved the evaluation of antigen-specific humoral immune response, antigen-specific cellular immune response, and variation in the neutralizing antibodies. The phase 1/2 studies have verified the safety and tolerance of the two formutations as mild and transient or no serious adverse events were observed. Furthermore, the antigen-specific IgG and neutralizing antibody responses have essentially exhibited seroconversion in 100% vaccine receiving participants. The volunteers also exhibited a proliferation of CD4+ and CD8+ cells which is indicative of the cell-mediated responses. Moreover, the vaccine candidate has been provisionally approved by the Decree of the Government of the Russian Federation [9].
The institute is currently recruiting for the double-blind placebo-controlled phase 3 clinical trials for nearly 40,000 subjects which will involve a critical assessment of virus-neutralizing activity, interferon-gamma activity, CD4+, and CD8+ cell count and ratio, and SARS-CoV-2 S-protein specific antibodies [10].
Whole-virus inactivated vaccine | Wuhan Institute of Biological Products & Sinopharm: The inactivated viral vaccine candidate with aluminum hydroxide (alum), a forerunner in the vaccine development program, has demonstrated immunogenicity and low rate of adverse reactions in the randomized, double-blind, placebo parallel controlled phase 1/2 clinical trials. Seroconversion was observed in 100% of participants with significant geometric mean titers of specific IgG antibodies after the second and third doses of the injection. The results of the clinical trials have also indicated a need for booster doses at longer intervals to maintain the higher antibody responses [11]. However, an extended trial and critical analysis of clinical data along with a prolonged follow-up of the intervention groups is required to understand the optimal interval between the primary shot and the subsequent booster shots. The vaccine candidate is currently in the phase 3 clinical trials in Bahrain for assessing the safety, clinical efficacy, and long-term safety of the vaccine candidate [12].
CoronaVac | SinoVac: CoronaVac, an inactivated vaccine, in a combined phase 1 and 2 clinical trial study did not lead to any severe side effects and triggered a robust immune response in 90% of the test subjects. The immune response exhibited in the elderly was slightly milder than the younger adults but the results were as per the study expectations. Furthermore, the vaccine tends to maintain its reactogenicity and stability for nearly 28 days at 37 degrees Celsius, 42 days at 25 degrees Celsius, and five months at 2-8 degrees Celsius. This will be beneficial in the vaccine distribution in the areas where the option for cold-chain storage is unavailable. The phase 3 clinical trials for attaining the regulatory approval for mass use are being conducted in Brazil and Indonesia. Moreover, 90% of the company's employees and their families are vaccinated with this experimental vaccine [13].
Ad5-nCoV | CanSino Biologics, Beijing Institute of Biotechnology: A single phase of immunization by 5 × 1010 viral vector particles was capable of inducing a significant immune response with a seroconversion rate to be as high as 97%. In 28 days post-vaccination, humoral responses attaining a peak against SARS-CoV-2 were observed along with rapid T-cell responses [14]. The vaccine candidate is currently undergoing the phase 3 clinical trials in multiple centers including Russia, Saudi Arabia, Mexico, etc., for the recruitment of nearly 40,000 volunteers. This potential candidate for emergency vaccination will be tested for its effectiveness in the phase III clinical trials which will involve the administration of 5 × 1010 viral particles in a single dose, in healthy adults. The process will involve quadruple masking of the participant, care-provider, investigator, and outcome assessor. Moreover, 50% of the total volunteers will be receiving the vaccine candidate, while the rest of the population shall be administered with a placebo. These measures will ensure a statistically accurate evaluation of the efficacy, safety, and immunogenicity of the Ad5-nCoV vaccine candidate [15]. Furthermore, the company has been bestowed with the patent on August 11, 2020 [16].
mRNA-1273 | ModernaTX, Inc., NIAID: Moderna Inc. is currently recruiting volunteers for the phase 3, quadruple blinded, randomized, stratified, and placebo-controlled clinical trials. The trials shall evaluate the safety and reactogenicity of the two shots (given after 28 days) of the candidate vaccine [17]. As of 25 September 2020, nearly 15,454 volunteers have been administered with a second shot for the trial. However, the company plans to recruit nearly 30,000 participants from various centers worldwide for conducting the study [18]. As per the study protocol, the primary objectives include the evaluation of efficacy, reactogenicity, the safety of the two-dose vaccine. While the secondary objectives are the comprehension of the efficacy of the vaccine to avert the serologically confirmed COVID-19 infection, regardless of prior SARS-CoV-2 infection in the entire population [19]. Moreover, the two-dose vaccination schedule of this vaccine is capable of generating a strong immune response providing immunity against the SARS-CoV-2 infection in the upper and lower airways in the challenge studies as conducted on non-human primates. Nonetheless, there was no evidence of vaccine associated enhanced respiratory disease (VAERD) [20].
NVX-CoV2373/ Matrix-M1™ | Novavax, Inc., Emergent BioSolution: NVX- CoV2373 is a recombinant and thermostable nanoparticle vaccine generated with mutations at the S1/S2 cleavage site along with certain substitutions in the full length SARS-CoV-2 spike glycoprotein. The vaccine candidate in the placebo-controlled, and observer blind phase 1 clinical trial was administered with/without the saponin-based adjuvant Matrix-M1™. The 35 days safety and immunogenicity analysis of the two-dose regimen exhibited an intense immune response which outpaced the geometric mean titers of the nAb in convalescent sera by four-fold. Apart from the exceptionally high magnitude of nAb, the vaccinated participants displayed induction of antigen-specific CD4+ cells. Moreover, the participants administered with the adjuvanted vaccine demonstrated a ten times better anti-spike IgG production response than the non-adjuvanted volunteers [21]. This recombinant, adjuvanted, nanoparticle vaccine is currently recruiting volunteers (HIV+ and HIV-) for the efficacy and safety studies in South Africa [15].
Ad26.COV2-S | Janssen Pharmaceutical Companies, Johnson & Johnson: Constructed using the Janssen AdVac® technology, it is one of the leading vaccine candidates against SARS-CoV-2. A recent interim study report of the first dose of blinded safety data depicts the rate of seroconversion to be as high as 100% in certain groups and exceptionally high GMTs varying with the vaccine dosage. Furthermore, the Th1 cytokine fabricating the S-specific CD4+ T cells were found to be soaring at 80% and 83% in the subsets of two cohorts. However, the absence of Th2 responses was symbolic of the Th1-skewed phenotype in the two groups of different dose levels. Nevertheless, the systemic adverse events and local adverse events observed for this adenovirus serotype 26 vectored S-protein vaccine candidates were mild and did not disrupt any regular activities i.e. no grade 4 adverse events were reported. The encouraging results of the phase 1/2a clinical trials foster the clinical development of the vaccine candidate [22].
The company is currently recruiting for the placebo-controlled, multicenter, phase 3 trial (ENSEMBLE) across three continents to study the safety and efficacy of a single dose vaccine. Moreover, the AdVac® technology ensures the stability of the vaccine for up to 2 years at -20 ℃ and at least three months at 2-8 ℃. Thus, the distribution of the vaccine if approved will not require the establishment of new infrastructure. Also, the company is scaling up the production of the vaccine to guarantee one billion doses every year, which is essential to eliminate the ongoing pandemic [23].
Conclusion
Out of the nine candidate vaccines under phase III trials currently, seven are projected to complete the trials by next year. The forerunner ChAdOx-1/Covishield/AZD1222 vaccine is scheduled to complete its trial by the end of 2020 and might be approved for use early next year. This will be a momentous development towards combatting the current pandemic be held by the whole world. It will also be an example of the development programs of vaccines for other diseases. Other than the vaccine candidates enlisted above, contenders from various biotechnology ventures and funding agencies including Sanofi Pasteur, Anhui Zhifei Longcom Biopharmaceutical, Bharat Biotech, Chinese Academy of Medical Sciences, etc. are also under clinical evaluations [3]. Apart from speeding up the research for the potent COVID-19 vaccine, the manufacturing capabilities must be developed to ensure the equitable distribution of the resulting vaccines. However, before the rolling out and approval of a vaccine capable of generating a long-lasting immunity against COVID-19 infection, we need to safeguard the vulnerable groups, educate and empower our communities to shield themselves, and avoid the amplifying events while ensuring the testing, isolating, and quarantining the contacts of the infected individuals.
The whole world is eagerly waiting for relief from this devastating pandemic, which will most probably come in the form of a vaccine and/or therapeutic regimen. But there also is a possibility that extensive spread of the virus leads to very effective herd immunity, which is already visible in countries like Italy, the UK, Spain, Brazil, the USA, etc. in which the infection peaked and is on the downside currently. The disease might vanish in a year or two like its earlier counterparts SARS and MERS. But as the lessons learned from research on SARS and MERS vaccines were handy in the current pandemic, lessons learned from COVID-19 vaccines will be pertinent in any future emergencies even if the current pandemic is by gone. This will help to overcome the limitations of a short duration of time for the safety and immunogenicity studies that accompany the clinical trials for the licensure of vaccines during an outbreak.
Acknowledgements
This work is not supported by any grant in particular. We rather would like to acknowledge Dr. Rakesh Kumar Gupta, Principal, Ram Lal Anand College, University of Delhi, for his continuous support and motivation and evaluating the manuscript. We also would like to thank Dr. Devendra Kumar for helping us with the plagiarism check of the document.
Conflict of Interest
Authors declare no conflict of interest in the publication of this manuscript.
References
- (2020) COVAX: CEPI's response to COVID-19 - CEPI.
- Kaur SP, Gupta V (2020) COVID-19 vaccine: A comprehensive status report. Virus Research 288: 198114.
- WHO (2020) Draft landscape of COVID-19 candidate vaccines.
- Folegatti PM, Ewer KJ, Aley PK, et al. (2020) Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. The Lancet 396: 467-478.
- (2020) Coronavirus (COVID-19) Vaccine status latest update: Oxford University, Pfizer, Moderna, Russia, Bharat Biotech Covaxin status check. The Indian Express.
- (2020) Coronavirus | Oxford vaccine trials to resume in UK. The Hindu.
- Researchers trial inhaled versions of Oxford and Imperial COVID-19 vaccine candidates. Reuters (2020).
- Walsh EE, Frenck R, Falsey AR, et al. (2020) RNA-based COVID-19 vaccine BNT162b2 selected for a pivotal efficacy study. MedRxiv 2020.08.17.20176651.
- Logunov DY, Dolzhikova IV, Zubkova OV, et al. (2020) Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. The Lancet 6736: 1-11.
- (2020) An open study of the safety, tolerability, and immunogenicity of "gam-covid-vac lyo" vaccine against COVID-19. ClinicalTrials.gov.
- Xia S, Duan K, Zhang Y, et al. (2020) Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes. JAMA 324: 951.
- (2020) Chinese Clinical Trial Register (ChiCTR) - A Phase III clinical trial for inactivated novel coronavirus pneumonia (COVID-19) vaccine. Vero cells.
- (2020) China's Sinovac coronavirus vaccine candidate appears safe, slightly weaker in elderly - world news. Hindustan Times.
- Zhu FC, Guan XH, Li YH, et al. (2020) Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet 396: 479-488.
- (2020) A Study Looking at the Effectiveness and Safety of a COVID-19 Vaccine in South African Adults. ClinicalTrials.gov.
- China grants the country's first COVID-19 vaccine patent to CanSino: State media. Reuters (2020).
- ModernaTX Inc (2020a) A Study to evaluate efficacy, safety, and immunogenicity of mRNA-1273 vaccine in adults aged 18 years and older to prevent COVID-19.
- ModernaTX Inc (2020c) COVE study: Participate to make a world of difference.
- ModernaTX Inc (2020b) Clinical study protocol Protocol Title: A Phase 3.
- Corbett KS, Flynn B, Foulds KE, et al. (2020) Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med 383: 1544-1555.
- Keech C, Glenn GM, Albert G, et al. (2020) First-in-Human Trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine authors, highest degree, and affiliation/institution. MedRxiv 2020.08.05.20168435.
- Sadoff J, Le Gars M, Shukarev G, et al. (2020) Safety and immunogenicity of the Ad26.COV2.S COVID-19 vaccine candidate: Interim results of a phase 1/2a, double-blind, randomized, placebo-controlled trial. MedRxiv 2020.09.23.20199604.
- PR Newswire (2020) (Johnson & Johnson initiates pivotal global phase 3 clinical trial of Janssen's COVID-19 Vaccine Candidate.
Corresponding Author
Vandana Gupta, Department of Microbiology, Ram Lal Anand College, University of Delhi, Benito Juarez Road, New Delhi 110021, India.
Copyright
© 2020 Kaur SP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.