Sleep Quality in Older Adults: A Review of Associated Mechanisms
Abstract
With the growing appreciation that sleeping habits interact with health and aging, it is now imperative to increase our understanding of exactly how sleep and age interact, influence one another, and contribute to a person's overall health and wellness. Recent studies on sleep and aging have produced some insight into the interplay among the mechanisms that govern sleep, such as circadian rhythms, neurodegenerative processes, neurological illnesses, and genetic factors that are associated with an aging population. The present review will provide an updated understanding of the relationship among aging, health, sleep deficits, circadian influences, and neurodegeneration. Gaining an understanding of these mechanisms may propel future research and treatment developments for age-related sleep deficiency and resulting health consequences.
Keywords
Sleep, Senescence, Aging, Circadian rhythms, Neurodegeneration, Neurodegenerative diseases, Elderly, Insomnia
Overview
Substantial growth of the elderly population is expected across the planet over the next few decades, with those over 60 years of age reaching about 2 billion, or 22% of the world's population by 2050 [1]. As one ages, numerous concerns commonly arise. One important aspect of healthy aging that can permeate other facets of life may be the duration and quality of one's sleep. The scientific consensus seems to affirm that getting adequate sleep is essential, although it is not currently practiced by the majority of adults [2]. This may be especially so in the case of older adults, a classification by the World Health Organization (WHO) which defines an older adult as one who is above the age of 60, and/or currently considered to be elderly or retired from an occupation by their nation of residence, while also appreciating that national and socioeconomic indicators relate to variations in life expectancy and health for the population [3]. As one advances in age, it has been found that they tend to fall asleep later, have reduced time spent within crucial rapid eye movement (REM) sleep, and have reduced overall duration and quality of sleep [4].
Relatedly, older adults typically need less sleep (7-8 hours per night) than younger adults (8-9 hours per night) and children (9-11 hours), according to the recommended sleep duration guidelines of the National Sleep Foundation [5]. Yet, many adults are sleeping fewer hours per night than recommended, and are often troubled by an inability to fall asleep or stay asleep for a full and restful night. The aforementioned sleep disturbances are core diagnostic criteria of insomnia, the most common form of sleep disorder. Symptoms of insomnia impact approximately 30% of adults, with approximately 10% reporting disturbed daytime functioning and/or stress as a result of impaired sleep which would warrant a formal diagnosis [6,7]. For example, a sleep study of 669 adults (mean age = 42) found that their sleep duration per night was self-reported as 6.8 hours, on average. The participant's self-reported average sleep duration was less than the recommended guidelines (by at least 1/5 of an hour for the minimum recommendation for older adults, and as much as 1.2 hours for the minimum recommendation for young adults). When these same individuals had their sleep durations recorded by a wrist actigraphy monitor (a non-intrusive wristwatch-like device which measures physiological sleep-related activity changes), they were found to average nearly a full-hour less in sleep duration throughout the evening than they originally reported, at just 6-hours duration [8]. New insights can begin to explain why aging adults tend to have poorer sleep quality, and sleep for less time overall, than is expected to be necessary for the benefits of fully recuperative sleep to be acquired.
As we age, the characteristics of sleep changes in several ways: The internal timing mechanisms of the body for sleep, known as circadian rhythms, shift towards earlier sleep and wake rituals; there is a tendency for sleep onset to take a greater amount of time to initiate; the amount of time spent sleeping declines; sleep is more often sporadic; sleepers are more likely to be awakened by their environments; deep sleep is reduced; a greater proportion of sleep is spent in the first two non-rapid eye movement (NREM) stages; all sleep stages are experienced with less duration and frequency; and there is more time spent fully conscious over the course of the evening. It is important to understand that, while these characteristics are true of the average older individual, they are not universally experienced. Also, some older adults acquire a portion of their sleep by napping during the daytime [9].
To investigate the relationship between nap qualities and mental health and performance and evening sleeping habits, researchers of the Sydney, Australia Brain and Mind Research Institute and Concord Medical School (2015) collaborated in a sleep and napping study monitoring 133 older adults (mean age = 65.5 years) considered at-risk for dementia by recording sleep via actigraphy and participant journaling. It was found that those who were recorded as napping for the greatest amount of time and/or most frequently throughout the day faced the most troublesome ill effects. They had the worst quality and duration of nighttime sleep, and had the poorest cognitive performance and the greatest amount of depressive symptoms, of compared to others participating in the study [10].
Health Outcomes and Sleep Deficits
Older adults sleeping for less than the recommended minimum of seven-hours per night may face troubling health effects. Poor sleep quality and reduced sleep duration is not only typical of diagnosable sleep disturbances (e.g., insomnia), but also of: Cognitive and memory impairment; bladder dysregulation; impaired hormonal output; mood and other psychosocial disorders; excessive weight gain and difficulty with weight loss; higher prevalence of prescription use; and greater overall pain experienced [9]. Many of these symptoms of sleep disturbance are often attributed as simply being signs of aging, because they are often found to be more commonly experienced in older populations. However, one needs to look beyond this overly general attribution and explore other reasons and consequences of this disturbance. For example, crucial to one's health is the functioning of metabolism, or the internal energy-regulation system of the body. Too little sleep has been found to impact one's metabolic efficiency, sometimes resulting in insulin resistance that becomes increasingly aggravated as sleep duration decreases [11]. A possible consequence of reduced metabolic functioning and impaired glucose management is a weakened immune system.
Reduced functioning efficiency of the immune system, in turn, may leave a person more susceptible to one or more of several classes of illnesses. For example, those who have fragmented sleep, or sleep for less than is recommended for their age bracket, are more likely to have dementia, heart disease, diabetes, mood disorders, and accidental injuries [12], as well as high blood pressure, obesity, and a higher mortality rate [8]. Moreover, in addition to the lengthy-list of potential consequences from lack of appropriate sleep, there are also many potential benefits from ensuring that one gets a continuous and restful night of slumber. Sleep serves many regulatory purposes in the body and brain, including improvements in metabolic, immunological, intero thermal, and stress regulation, as well as increased longevity [2,13].
Sleep and Circadian Rhythms
One's circadian rhythms regulate what can be described as the "internal biological clock". Circadian rhythms literally translate from Latin to rhythms "about the day", which is fitting, as the respondent biological clock gives the body an indication about what time of day it is [14]. Diurnal and nocturnal mechanisms related to waking and sleeping patterns are regulated by circadian rhythm sensitivity to external light-type and strength and internal glutamatergic activity [15]. This sensitivity allows the body and brain to appropriately shift hypothalamic activity and related hormone levels, and to vary one's stages of consciousness and sleep onset and duration. There are also transcription factors that control these biological rhythms and drive the activity underlying the mechanisms which regulate one's sleep and alertness levels.
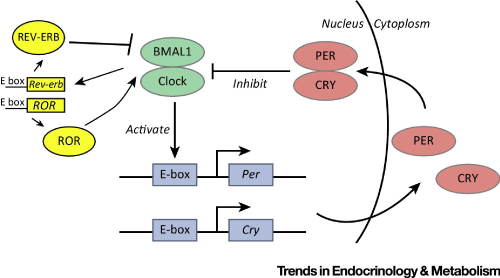
Circadian rhythms function on the basis of an interplay between environmental cues and neuro-molecular feedback mechanisms. These mechanisms are often initiated and propelled by sleep-regulating genes and transcription factors. There are several genes thought to be most prominent in the regulation of sleep. These genes are known as: Period (Per1, Per2, and Per3); Cryptochrome (Cry1 and Cry2); Bmal1; and Clock [11]. Bmal1 and Clock genes are known as master genes for sleep, and are especially crucial in regards to the suprachiasmatic nucleus (SCN), or the localized hypothalamic cells that regulate much of our circadian cycling [16]. Bmal1 and Clock act as sleep-associated transcription factors, fusing in daylight hours and later, initiating the transcribing of genes in the SCN. The genes transcribed by Bmal1 and Clock within the SCN repress their own expression during evening hours through the REV-ERB and ROR receptors (see Figure 1). This is thought to be in order to prime us for sleep [11]. The Period (Per) and Cryptochrome (Cry) genes are activated by the heterodimerization, or independent binding, of Bmal1 and Clock, which elicit circadian interplay promoters, called "E-boxes" of Per and Cry, thus forming an internal pattern of rhythmic gene expression related to alertness, sleepiness, and other biological functions, such as metabolic activity (again, see Figure 1). The activity of Clock and Bmal1 is halted during the dimming of light in the early evening hours by the protein products of Per and Cry interaction. Per and Cry products gradually deteriorate over the course of the night, allowing the Clock and Bmal1 factors to bind again at the onset of daylight [11].
One common feature of aging is a disruption of normal circadian patterns and related sleep-regulation [17]. Typical changes experienced by elderly individuals are often due to changes in sleep-cycle quantity and quality, and related circadian mechanisms. Some of the more frequently reported changes are the following: There is a shift towards earlier morningness alertness and a shift away from later eveningness alertness, such that one rises and sleeps earlier than in the past, with performance optimization shifting earlier in kind; there is more difficulty experienced with circadian phase changing (for example, from jet lag or altering one's typical schedule); there is a change in how the circadian clock regulates sleep, such that one's sleep cycles and sleep duration becomes fragmented and one is inclined to sleep 1-2 hours earlier than they had at a younger age; thermoregulation changes, such that there is a reduction in the downward shift in body temperature in the evenings, which would otherwise help one fall asleep; circadian rhythm regulating hormones (melatonin and cortisol) may experience a decline; circadian regulation of inflammation, fat metabolism, blood glucose levels, and metabolic detoxification reduces in efficiency; Clock genes alter in their expression, which leads to the Pergenes, that regulate circadian phase shifts, becoming flat, and Cry1, the gene that patterns circadian feedback, behaving more erratically [17].
Brain Areas Associated with Sleep
It should also be noted that common changes to the ways in which one sleeps when growing older may be related to the changes that occur within the central nervous system, which houses brain areas that play a crucial role in the regulation of sleep [9]. These areas appear to have an influence on the regulation of sleep and the monitoring of sensory signals related to patterning sleep. They are believed to function independently, while also affecting one another; as it has been found that a disruption in one will likely lead to changes in another [18]. These systems were found to falter more often in those who were older, as the neural cells and functional mechanisms associated with them were more likely to deteriorate as an individual's age progressed [9].
Some of the prominent sleep-regulating brain areas include: The thalamus, which regulates sensory input, such as light and darkness patterns; the cortex, which houses sleep-active cells that respond to deep, slow-wave sleep; the basal forebrain, which houses neurons that signal the cortex to activate REM phases of sleep [19]; the brain stem, which has arousal mechanisms that signal the cortex and facilitate transitioning during REM sleep; the pineal gland, which produces melatonin (a sleep-inducing hormone); and, prominently, the hypothalamus.
The hypothalamus serves several sleep functions, such as housing a circadian rhythm-regulating cluster of cells known as the suprachiasmatic nucleus (SCN), and the alertness regulating cluster of cells known as the ventrolateral preoptic nucleus (VLPO) that connect to arousal centers of the brain [20]. The preoptic hypothalamic region has been associated with sleep disturbance and fragmentation, as intermediate hypothalamic neurons (homologues to preoptic hypothalamic neurons) were found to be significantly reduced in in individuals who experienced the most disturbed and fragmented sleep patterns [21]. The cells of the SCN in the hypothalamus are responsible for regulating drowsiness and sleep-onset by signaling increased melatonin production, and by halting arousal messages in other brain areas. The SCN also serves as a beacon for signaling alertness before waking, as light signals change the ways in which the SCN regulates the internal mechanisms responsible for circadian rhythms [20]. As such, the SCN is thought to be the most crucial area of the brain in regulating circadian mechanisms and phase shifting. Removal of the SCN has been found to lead to permanently disturbed sleeping patterns and circadian cycling in rodent models [22]. It has also been found that lesioning or removal of the SCN leads to: Halted patterning in circadian mechanisms receiving input from the SCN; a lack of responding to day-night light shifts; and, in some cases, a total loss of circadian shifting toward sleep [18].
Sleep and Cognitive Performance
Undisturbed sleep appears to have substantive positive effects on cognitive performance. Increased sleep has been found to enhance long-term potentiation (a term describing faster and improved signaling between related neurons that is believed to enhance one's memory and learning over time; [9]. The sleep-regulating rhythms of the circadian cycle also have a strong association with properly balancing emotion-related neurotransmitters, such as dopamine and serotonin, which regulate calmness, enhance mood, and give rise to rewarding sensations [23]. In addition, improvements in sleep quality and duration positively correspond with enhanced performance on many mental tasks, such as: Working memory; the speed with which one processes incoming information; long-term recall; delaying one's impulses; and word fluency rate [24].
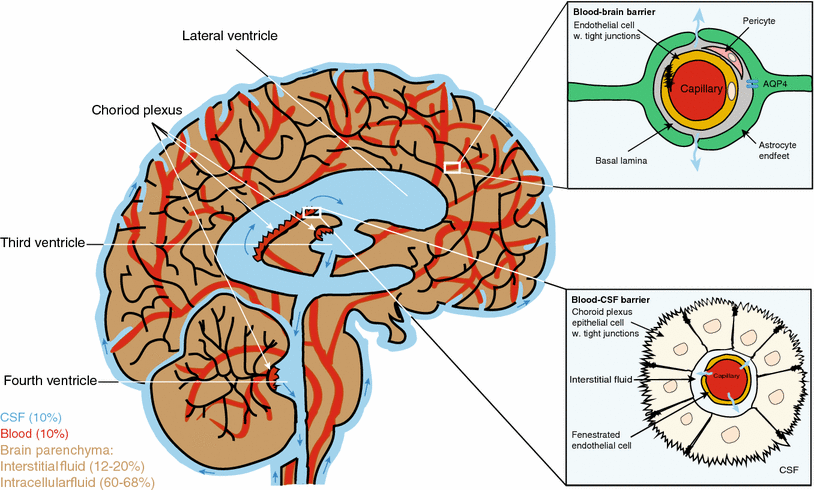
Sleep also allows for a clearing of potentially toxic debris via the brain's "glymaphatic system". Just as the lymphatic system transports toxins from many vital organs and ships them to the kidneys and liver to be metabolized, the glymphatic system utilizes a network of specialized tunnels and glial cells (hence, "Glymphatic") to transport proteins and toxic byproducts across the blood-cerebrospinal fluid barrier for an exchange with the interstitial fluid (ISF) for the clearing of potentially harmful molecules [25]. The glymphatic system removes waste products by transferring them out of one area (in this case the cerebrospinal fluid [CSF]), and into another; in this case, within the brain parenchyma, where the CSF exchanges molecules with the ISF beneath the epithelial cells of the brain's choroid plexus for transport to the cervical lymphatic system (see Figure 2) Without the glymphatic system, potentially toxic waste-byproducts and endogenous proteins, such as beta amyloid (β amyloid) and tau, which naturally collect in the nervous system, would otherwise continue to accumulate and may lead to future diseases, such as stroke and Alzheimer's disease, due to their potential triggering of neurondeterioration which concludes as neural death.
While we sleep, the brain effectively engages in a slight shrinking in overall mass. This has been found to strongly support the ability of the cerebrospinal fluid to filter, eliminate, and clear harmful proteins and waste by-products that accumulated over the course of the day [26]. These potentially damaging endogenous molecules are eliminated to a much lesser degree when an individual is awake, when the functions and energy of the body and brain are devoted to regulating an extensive amount of waking activity, such as: Decision-making and memory-formation; sensory input and perceptual output; behavioral tasks; organ optimization; and motor activity; just to name a few.
Sleep and Neurodegenerative Mechanisms
Neurodegenerative factors have been found to relate to age-linked sleep deficiency as well. Some of the most burdensome diagnoses common to senior citizens are those related to neurodegeneration, or the gradual functional and/or physical loss of crucial nervous system mechanisms. While sleep problems may be common features of old age, rapid and/or severe degeneration of the nervous system is not, and may lead to deficits in psychosocial, cognitive, and/or motor functioning and premature death in a sufferer. Yet, neurodegenerative diseases are far more common in older individuals, and are increasing in prevalence worldwide [27]. For example, dementia, a disorder characterized by chronic memory and cognitive impairments, impacts 5%-7% of those over the age of 60, and an estimated 35.6 million people worldwide, with a projection for that figure to be close to 115 million by 2050, as the total number of the world's elderly grows substantially [27]. This coincides with increases to lifetime longevity currently found in many countries.
These neurodegenerative diseases might be intimately connected with one's sleep and circadian rhythms. Often, the presence of a problem in one's sleep/wake patterns, circadian mechanisms, or nervous system functioning relates to deficits in one or both of the others. This is especially true for the characteristic nervous system patterns and symptoms of Alzheimer's dementia and Parkinson's disease, the two most common neurodegenerative illnesses in the world. Also, it has been reported that those with diagnosed Alzheimer's disease, an illness characterized by memory deficits, confusion, emotional dysregulation, and declines in cognitive and behavioral functioning, experience substantial neuronal loss within the suprachiasmatic nucleus (SCN). However, this cell loss may not be the source of sleep disturbances in those with neurodegenerative illnesses. Rather, the loss of cells may, in fact, be symptomatic of neuro-deteriorative features of dementia. The disease itself might be a source of impaired sleep. Indeed, post-mortem patients diagnosed with Alzheimer's disease typically exhibited a loss of neurons in their hypothalamus, the sleep hormone-regulating brain region. However, in other Alzheimer's models, such as β-amyloid expressing flies, a disruption to circadian cycles was present while neuron counts remained intact. Furthermore, it was noted that a dysfunctional sleep-wake pattern is related to dysfunctional circadian clock mechanisms [11]. The sources of these problematic sleep patterns are believed to be disturbed feedback of the circadian clock, although an interaction between the neuro-deterioration common in Alzheimer's disease and other forms of dementia, alongside the presence of faulty circadian mechanisms, may work in a combined and aggravating way, which leads to an impairment in sleep common to those with a neurodegenerative illness.
Parkinson's disease (PD), second only to Alzheimer's dementia in its prevalence as a neurodegenerative disease, impacts approximately 1% of individuals over the age of 60, and 4% of individuals over the age of 85 across the world [28]. PD is characterized by a gradual loss of dopaminergic cells within the midbrain's substantia nigra, and a buildup of disruptive intracellular Lewy Bodies, leading to impaired motor activity which worsens over time. PD is also typically associated with disturbed sleep, such as increased diurnal sleep, difficulties with falling and/or staying asleep and, most commonly, REM behavior disorder (RBD). RBD is characterized by an inability to initiate or maintain appropriate REM sleep, which accounts for up to a quarter of the amount of time spent sleeping [29]. As a consequence of losing aspects of the REM sleep stage, sufferers experience faulty motor activations. These activations are typically dormant during this neuroregenerative portion of the sleep cycle. It has been observed, in transgenic mouse models of PD, that there is reduced communication between neurons of the SCN, which may inhibit normal circadian output to brain areas responsible for sleep, relating to progressively disrupted sleep patterns [30]. It has also been found that the vast majority (81-90%) of those diagnosed with RBD go on to develop a neurodegenerative disease, typically Lewy Body dementia and/or PD [31].
It is also known that occluded sleep negatively influences future performance of mental tasks. This might be related to accumulation of an endogenous toxic protein, called β-amyloid (Aβ) which sleep helps to clear from the body. Aβ, a naturally occurring protein that is considered to be pathogenic can act as a biomarker of diseases like Alzheimer's dementia, due to an uncommonly high buildup of its plaques in those with the disease. Aβ has also been found to have a relationship with circadian cycling, falling during periods of sleep, and accumulating during periods of wakefulness [18]. An accumulation of β-amyloid typically coincides with greater quantities of tau protein neurofibrillary tangles, or distorted and collapsed microtubules within neurons. These neurofibrillary tangles can suffocate a neural cell, inhibiting its ability to communicate with other cells, and cause its death and the death of nearby neurons.
A high concentration of β-amyloid and tau proteins isa diagnostic feature of Alzheimer's disease; however, they have also been found in higher quantities in non-diagnosed, cognitively healthy adults who experience impaired sleep [32]. Researchers at the Washington University School of Medicine have also studied this phenomenon [33]. Adults, aged 35-65, who had no experience of disturbed sleep or neurodegenerative problems, were monitored for sleep activity before entering the school for a night's sleep in a small, dark, and quiet room. Here, their brainwave activity was recorded by an EEG to determine the sleep stage they were in as they slumbered. All participants wore headphones, but half were sent disruptive beeping noises once the EEG determined they had entered the third, slow-wave, sleep stage. This disturbance shifted their wave activity backward, "bumping" them out of body-restorative slow-wave sleep and preventing them from entering brain-restorative REM sleep. This half of participants reported far greater experiences of exhaustion the following day, compared to the other half, who were not disturbed by the beeping noises. A month passed, during which the sleep activity of all participants was monitored at home. Afterward, the other half of participants received a night of sleep disruption, while the first half (who were previously given a month of disrupted sleep), were provided with uninterrupted sleep. A spinal tap was then performed to measure β-amyloid and tau. It was found that, even without the presence of a pre-existing neurodegenerative illness, poorer sleep was related to greater overall levels of both β-amyloid and tau protein tangles. β Amyloid was increased by an average of 10% in those with a single night of sleep disturbance from the beeping noises during the previous evening, although tau levels were unchanged. However, those who slept poorly for more than a single night, according to the at-home monitor, also had increased levels of tau [33].
These are consistent with findings that β-amyloid levels change more rapidly than tau levels from disturbed sleep. They might suggest that the presence of sleep disturbance alone in a healthy adult might be a catalyst for the development of neurodegenerative symptoms, such as those seen in Alzheimer's dementia [33]. Other research, though, has suggested that tau proteins, β-amyloids, brain atrophy, memory problems, and depressive symptoms interact and influence sleep impairment in otherwise healthy populations [32]. As such, a relationship between neurodegeneration and disrupted sleep seems obvious. However, the cause and direction of the relationship is still not entirely clear. Faulty circadian-related genetic expression may play a role in this interaction.
Presenilin splits β-amyloid predecessor proteins. Presenilin genes (Presenilin1 and Presenilin2) are under circadian regulation and are considered potential precursors to Alzheimer's Disease when present in mutated forms [11]. Components of the Presenlin2 gene are activated by Clock and Bmal1 proteins. The disrupted ability to maintain a balance between appropriate levels of free radicals and antioxidant defenses, called oxidative stress, is often present across neurodegenerative diseases, and is believed to play a role in damage to DNA, faulty folding, and activity of intracellular proteins and cell death. Bmal1-deficient mice exhibit premature and rapid aging phenotypes: Including loss of hair and mass in bone, muscle, and subcutaneous fat; cataract development; and premature death. This was found to be potentially related to oxidative stress insofar as the introduction of an antioxidant (N-acetyl-L-cysteine) was found to partially reverse the progression of many of the afore mentioned symptoms [11].
Current Treatments and Future Directions
As difficulty sleeping in older adulthood is a well-documented phenomenon, and undesirable health ramifications often coincide with it, or are catalyzed by poor sleep, it is important to have an understanding of how to appropriately treat problems associated with disturbed sleep. An understanding of the potential influences on sleep dysfunction may allow vulnerable populations to prevent or overcome the consequences accrued from insufficient sleep, and may provide insights that allow them to attain adequate sleep. Currently, research suggests that enhancing sleep may be accomplished by introducing simple habits into one's routine. These can include introducing behavioral hypnotherapy, massage therapy, acupuncture, yoga, and/or relaxation techniques, such as breathing exercises, meditation, and other mindfulness-based practices [34].
The American Academy of Sleep Medicine (AASM) has proposed two basic treatments for older sufferers of circadian rhythm disorders and insomnia: One is called bright light therapy, which involves the use of intense light exposure, given in increments and in a pattern over the course of the day, in order to allow for a resetting of the internal circadian mechanisms; the other recommendation of the AASM for the treatment of age-related sleep problems is that of melatonin treatment [35]. Melatonin is a hormone produced in the brain by the pineal gland. When light shifts in the evening, the suprachiasmatic nucleus signals the pineal gland to produce greater amounts of melatonin in order to illicit drowsiness. When light returns in the morning, the pineal gland is further signaled to shut off production of melatonin, which rouses a person from sleep. It has been proposed that, along with circadian mechanism disturbances, age advancement leads to senescence, or age-related decline, of melatonin cycling and output [36]. Giving doses of melatonin to individuals in the evening at strategic times related to natural light shifts was found to be effective in treating adults diagnosed with Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), a disorder of disturbed circadian shifting representing patterning atypical to a 24-hour period, often leading to delayed sleep onset and other sleep disturbances [35].
The base of knowledge of the underlying mechanisms that contribute to the interplay between sleep quality and age progression has been enhanced by studies in molecular genetics, psychoneuroimmunology, and related biological subfields. Improper sleep has also been thoroughly studied and better explained by those surveying relevant populations and conducting behavioral and environmental studies on sleeping habits, aging, and related healthy lifestyles. Future studies may determine the role of underlying physiological mechanisms that interact with one another in a more specified and localized way, such as: Those of the circadian clock and its related genetic underpinnings; and how this mechanism specifically associates with environmental features, such as light/dark exposure, nootropic use, diet, exercise, meditation, and/or concentrated hypothalamic stimulation. A better understanding of particular changes in sleep-quality and sleep-duration in aging populations can provide healthcare professionals and patients alike the knowledge and strategies for developing a proper approach in managing better sleep and reducing many of the negative health effects related to sleep deficits and aging.
References
- World Health Organization (2014) 10 facts on ageing and the life course.
- Irwin MR (2015) Why sleep is important for health: A psychoneuroimmunology perspective. Annu Rev Psychol 66: 143-172.
- Beard JR, Officer A, de Carvalho IA, et al. (2016) The world report on ageing and health: A policy framework for healthy ageing. Lancet 387: 2145-2154.
- Yu J, Mahendran R, Abdullah FNM, et al. (2017) Self-reported sleep problems among the elderly: A latent class analysis. Psychiatry Res 258: 415-420.
- Hirshkowitz M, Whiton K, Albert SM, et al. (2015) National Sleep Foundation's sleep time duration recommendations: Methodology and results summary. Sleep Health 1: 40-43.
- Roth T (2007) Insomnia: Definition, prevalence, etiology, and consequences. J Clin Sleep Med 3: S7-S10.
- St-Onge MP, Grandner MA, Brown D, et al. (2016) Sleep duration and quality: Impact on lifestyle behaviors and cardiometabolic health: A scientific statement from the American Heart Association. Circulation 134: e367-e386.
- Lauderdale DS, Knutson KL, Yan LL, et al. (2008) Self-reported and measured sleep duration: How similar are they? Epidemiology 19: 838-845.
- Mander BA, Winer JR, Walker MP (2017) Sleep and human aging. Neuron 94: 19-36.
- Cross N, Terpening Z, Rogers NL, et al. (2015) Napping in older people 'at risk'of dementia: Relationships with depression, cognition, medical burden and sleep quality. J Sleep Res 24: 494-502.
- Mattis J, Sehgal A (2016) Circadian rhythms, sleep, and disorders of aging. Trends Endocrinol Metab 27: 192-203.
- Krueger PM, Friedman EM (2009) Sleep duration in the United States: A cross-sectional population-based study. Am J Epidemiol 169: 1052-1063.
- Cappuccio F, Miller MA, Lockley SW (2010) Sleep, health, and society: From aetiology to public health. Oxford University Press, USA.
- Vitaterna MH, Takahashi JS, Turek FW (2001) Overview of circadian rhythms. Alcohol Res Health 25: 85-93.
- Ruben MD, Hogenesch JB (2017) Circadian rhythms: Move over neurons-astrocytes mediate SCN clock function. Curr Biol 27: 350-352.
- Menet JS, Pescatore S, Rosbash M (2014) CLOCK: BMAL1 is a pioneer-like transcription factor. Genes Dev 28: 8-13.
- Hood S, Amir S (2017) The aging clock: Circadian rhythms and later life. J Clin Invest 127: 437-446.
- Musiek ES, Holtzman DM (2016) Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 354: 1004-1008.
- Brayet P, Petit D, Frauscher B, et al. (2016) Quantitative EEG of rapid-eye-movement sleep: A marker of amnestic mild cognitive impairment. Clin EEG Neurosci 47: 134-141.
- Saper CB, Scammell TE, Lu J (2005) Hypothalamic regulation of sleep and circadian rhythms. Nature 437: 1257-1263.
- Lim AS, Ellison BA, Wang JL, et al. (2014) Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer's disease. Brain 137: 2847-2861.
- Sumova A, Travnickova Z, Peters R, et al. (1995) The rat suprachiasmatic nucleus is a clock for all seasons. Proc Natl Acad Sci U S A 92: 7754-7758.
- Cools R, Nakamura K, Daw ND (2011) Serotonin and dopamine: Unifying affective, activational, and decision functions. Neuropsychopharmacology 36: 98-113.
- Wilckens KA, Woo SG, Kirk AR, et al. (2014) Role of sleep continuity and total sleep time in executive function across the adult lifespan. Psychol Aging 29: 658-665.
- Jessen NA, Munk AS, Lundgaard I, et al. (2015) The glymphatic system: A beginner's guide. Neurochem Research 40: 2583-2599.
- Fung CH, Vitiello MV, Alessi CA, et al. (2016) Report and research agenda of the American Geriatrics Society and National Institute on aging bedside‐to‐bench conference on sleep, circadian rhythms, and aging: New avenues for improving brain health, physical health, and functioning. J Am Geriatr Soc 64: e238-e247.
- Prince M, Bryce R, Albanese E, et al. (2013) The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement 9: 63-75.
- De Lau LM, Breteler MM (2006) Epidemiology of Parkinson's disease. Lancet Neurol 5: 525-535.
- Carskadon MA, Dement WC (2005) Normal human sleep: An overview. In: Kryger MH, Roth T, Dement WC, Principles and practice of sleep medicine. (4th edn), WB Saunders, USA, 13-23.
- Willison LD, Kudo T, Loh DH, et al. (2013) Circadian dysfunction may be a key component of the non-motor symptoms of Parkinson's disease: Insights from a transgenic mouse model. Exp Neurol 243: 57-66.
- Howell MJ, Schenck CH (2015) Rapid eye movement sleep behavior disorder and neurodegenerative disease. JAMA Neurol 72: 707-712.
- Fjell AM, Idland AV, Sala-Llonch R, et al. (2017) Neuroinflammation and Tau Interact with amyloid in predicting sleep problems in aging independently of atrophy. Cereb Cortex 1-11.
- Ju YS, Ooms SJ, Sutphen C, et al. (2017) Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain 140: 2104-2111.
- National Institutes of Health (2014) Sleep Disorders and Complementary Health Approaches. National Center for Complementary and Integrative Health.
- Auger RR, Burgess HJ, Emens JS, et al. (2015) Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). An update for 2015: An American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 11: 1199-1236.
- Reiter RJ, Rosales-Corral SA, Tan DX, et al. (2017) Circadian dysregulation and melatonin rhythm suppression in the context of aging. In: Jazwinski SM, Belancio VP, Hill SM, Circadian rhythms and their impact on aging. Springer, USA, 1-25.
Corresponding Author
Robert J Gatchel, Ph.D., ABPP, Department of Psychology, College of Science, The University of Texas at Arlington, 1225 West Mitchell, Box 19528, Arlington, TX 76019, USA, Tel: (323)361-2145.
Copyright
© 2018 Stephens J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.