Review: Effect of Rhizobium on the Yield of Leguminous Plants
Summary
Plants need the accurate combination of nutrients to live, grow and reproduce properly. If not, Plants suffer from malnutrition and show symptoms of being unhealthy and provide very low productivity. Too little as well as too much of any one nutrient can cause problems. Nitrogen is of the essential element for plant life. Its major use is provoking root growth and photosynthesis, as well as uptake of other nutrients. Naturally 78% of atmosphere gas on earth is nitrogen but in the earth's crust its composition is less than 1%. The small amount of reactive nitrogen in the soil determines biomass production in natural ecosystems.
Agriculture is one of the ways by which reactive nitrogen from the soil is utilized. Nitrogen absorption is performed during plant growth and then exported from the fields through harvesting mechanism. It is mandatory to be restored and recycle again by organic or mineral sources of nitrogen [1]. Major leading mechanism in nitrogen-fixing systems is Legumes, as they may derive up to 90% of their nitrogen from symbiotic N2 fixation (e.g. faba bean, lupin, soybean, ground nut). Fixation ability of legumes largely depends on cultivars and culture conditions. Though, on average fixation rate 200 to 300 kg N2/ha/year can be attained for most species [2].
Apart from nitrogen fixation Rhizobium serves as key role in increase resistance level with antagonistic activity of plants against pathogens and herbivores [3]. Additionally they have the ability to stimulate and enhance some growth parameters such as seed germination, emergence, seedling vigor, plant stand, and root and shoot growth, total biomass of the plants including seed weight [4].
Therefore directly through enhancing nutrient availability or indirectly by promoting plant resistant against their pathogen PGPR has positive effect on the yield of leguminous plants.
Introduction
The rhizobium of symbiotic micobiota in leguminous plant has tremendous importance in fixing unusable elemental form of N2 to useable one [5]. It is habitual fact that N2 is essential in growth and development of plants [6]. This symbiotic organisms now a day has been use as GMO and classical one (efficient stain selection and used as nitrogen fixation pursues) to boost agricultural productivity and environmentally friend production [7].
Nitrogen gas which is 80% of the earth's atmosphere is required by all living organisms for the synthesis of proteins, nucleic acids and other nitrogen-containing compounds. Even though it is richest gas of the atmosphere it is impossible to be used in this form by most living organisms until it has been fixed, that is reduced (combined with hydrogen), to ammonia. Green plants, the main producers of organic matter, use this supply of fixed nitrogen to make proteins that enter and pass through the food chain. Microorganisms (the decomposers) break down the proteins in excretions and dead organisms, releasing ammonium ions. These two processes form part of the nitrogen cycle. The nitrogen cycle is a sequence of progresses that converts nitrogen gas to organic substances and back to nitrogen in nature. It is a continuous cycle that is maintained by the decomposers and nitrogen bacteria [8].
Nitrogen is the nutrient which can play key role in plant survival, which is the most commonly deficient in soils, and is the first mentioned nutrient for reduction of agricultural yields throughout the world. It can be supplied to crops by biological nitrogen fixation (BNF), a process by which is becoming more important for not only reducing energy costs, but also in seeking more sustainable agricultural production. Nitrogen fixing micro-organisms could therefore be an important component of sustainable agricultural systems [9].
Rhizobium
Rhizobium is primary symbiotic fixer of nitrogen with the association of plant. There are 6 genera of bacteria (Rhizobium, Bradyrhizobium, Mesorhizobium, Sinorhizobium, Azorhizobium, and Allorhizobium) which have the ability to play a vital role of nitrogen fixation. Rhizobium species are specific to their host plant, so producers should be sure that strain of rhizobia is appropriate for the legume they are planting [10].
Rhizobia also used as commercial inoculants in legume species since them effectively ‘infect' and nodulate legumes. Even now, less than 15% of the roughly 20,000 classified species of legumes have been evaluated for nodulation. Many of the tropical forest legumes have not been evaluated but may have potential for development in future [11].
Members of the genus Rhizobium are aerobic, Gram-negative relatively large rods which are motile and have peritrichuous flagellation. Older cells contain prominent granules of polyhydroxy-butyrate which give them an enormous appearance. Within nodules they form irregularly shaped cells known as ‘bacteroids”; no fixation occurs in the absence of bacteroids. At the end of the growing season, the nodules occur in the absence of bacteroids. At this time, the nodules disintegrate; releasing bacterioids which, once in the soil, revert to rod-shaped cells and can survive there, sometimes for years. They invade roots of appropriate legumes and form new nodules when appropriate legumes are planted. Rhizosphere bacteria can enhance the plant growth and crop yield by different ways. The acronym PGPR has been widely used to group these microbes [8].
Rhizobium and Legumes Plant
One of the best ways by which reactive nitrogen from the soil utilized is through, Agricultural implementation. Nitrogen absorption from the fields is performed during plant growth and through harvesting mechanism [1]. Legumes are the major leading mechanism in nitrogen- fixing systems. They may derive up to 90% of their nitrogen from symbiotic N2 fixation (e.g. faba bean, lupin, soybean, ground nut). Fixation ability of legumes largely depends on cultivars and culture conditions. But on average fixation rate 200 to 300 kg N2/ha/year, can be attained for most species [2]. In addition to nitrogen fixation, Rhizobium serves a key role in promoting antagonistic activity of plants against pathogens and herbivores [3]. By doing so, they stimulate and enhance some growth parameters such as seed germination, emergence, seedling vigor, plant stand, root and shoot growth, total biomass of the plants including seed weight [4].
Balanced quantities and sufficient availability of nutrients is compulsory for optimum plant growth and productivity [12]. In developing nations all over the world, and especially among resource-poor farmers, soil infertility is the most important constraint limiting crop yield. In order to improved varieties and more productive cultural practices of developing nation's fertility restoration in these areas and use of Soil fertility of the mentioned area is mandatory. There are three well known mechanisms used for soil fertility restoration; adopting the concept of integrated soil fertility management encompassing a strategy for nutrient management-based on natural resource conservation, biological nitrogen fixation, and increased efficiency of the inputs [13].
Important components of integrated nutrients managements are called Biofertilizers. These potential biological fertilizers would contribute immense activity in productivity and sustainability of soil and also protect the environment as ecofriendly and also it is cost effective inputs for the farmers. Biofertilizers has multidirectional advantage for the users. For example cost effective, ecofriendly and renewable source of plant nutrients to supplement chemical fertilizers in sustainable agricultural system. Biofertilizers are products of different types of living cells (microorganisms) which when, they applied to seed, plant surface or soil, colonize the rhizosphere or the interior of the plant and promotes growth by converting nutritionally essential elements (nitrogen, phosphorus) from unavailable to available form through biological process such as nitrogen fixation and solubilization of rock phosphate [14]. There for these beneficial microorganisms in biofertilizers accelerate and improve plant growth and protect plants from pests and diseases [15].
Legume plants and their role
A legume plant taxonomically included under Fabaceae (Leguminosae) families which are the third biggest family of angiosperms [16]. We can classify legume plant into trees and crops, of the crops; alfalfa, clover, beans, peas, chickpeas, lentils, lupins, mesquite, carob, soybeanns, peanuts, and tamarind are some well-known in agricultural productivity and soil fertility living symbiotically with Nitrogen fixing bacteria's species [17]. Whereas from the side of trees the most and diversified ecologically important trees like acacia and Prosopis species are commonly found with different Rhizobuim. Those trees living symbiotically with N2 fixing bacteria's are commonly considered as soul soil fertility enhancer in the view of environmental protection reducing soil erosions [18].
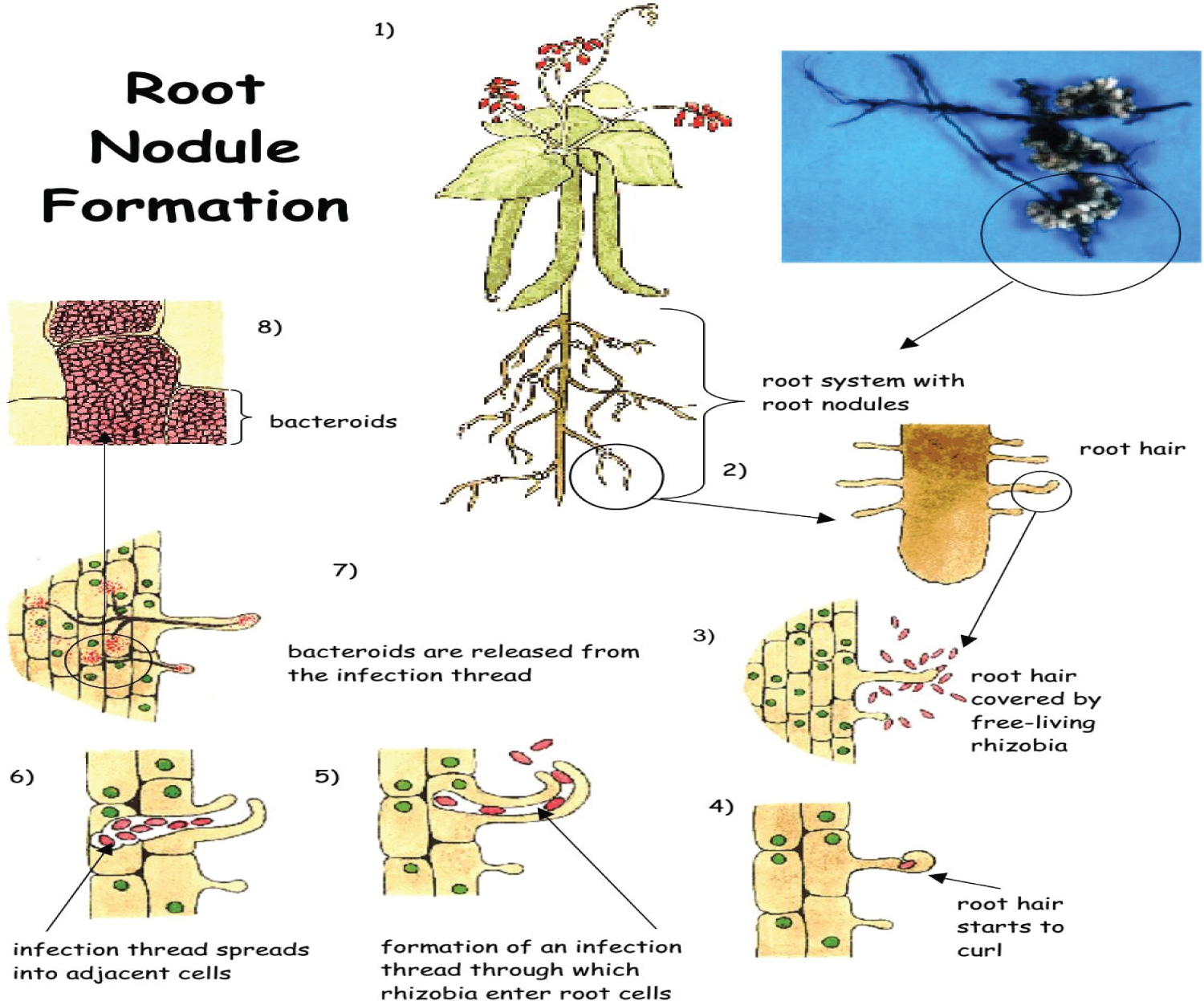
Nodule formation in legumes of rhizobia
According to Anelise Beneduzi [19], different aspects of the nodulation process by bacteria is controlled by specific genes. That means Rhizobium strain which infect certain species of legumes may not infect the others. For example the pea is the host plant to Rhizobium leguminosarum biovar viciae, whereas clover acts as host to R. leguminosarum biovar trifolii. Even if a strain is able to infect a legume, the nodules formed may not be able to fix nitrogen. There for Specificity genes determine which Rhizobium strain infects which legume.
Different stages of nodulation are directed by specific nod genes. At the first stage interaction between the host plant and free-living rhizobia is occurred which is the cause for release of a variety of chemicals by the root cells into the soil. Some of the releases encourage the growth of the bacterial population in the area around the roots (the rhizosphere). Then after reactions between compounds in cell wall bacterial and the root surface helps rhizobia to distinguish their correct host plant and it is responsible for the attaching them to the root hairs. During this interaction, Flavonoids which responsible for nod genes activator in the bacteria cell is secreted by the root cells in order to induce nodule formation. The whole nodulation process is controlled by highly complex chemical communications between the plant and the bacteria [19,20] (Figure 1).
Use of rhizobium as a biological fertilizer and their impact on yield
Bacteria categorized to the group of rhizobia are of considerable scientific and economic interest due to their ability to fix atmospheric nitrogen in leguminous plants [21]. Rhizobia encourage the formation of root nodules in leguminous plants through the production of speciûc signal molecules called Nod factors [22]. Inside the nodules, rhizobia produce ammonia and used as the source of nitrogen absorbed by host plants. This application reduces the need for application of chemical fertilizers in large [23]. Besides of nitrogen fixation efficiency, rhizobia also have a good potential of use as biological control agents against soil borne plant pathogens [24]. For instance Rhizobium strains are known to reduce disease severity caused by Fusarium solani, Pythium ultimum [25], Phytophthora clandestine [24], Fusariuum oxysporum, Rhizoctonia bataticola and Pythium sp., [26]. According to Ehteshamul-Haque and Ghaffar (1993) under field conditions; Sinorhizobium meliloti, Rhizobium leguminosarum and Bradyrhizobium japonicum used either as seed dressing or as soil drench reduced infection of Rhizoctonia solani, Macrophomina phaseolina and Fusarium spp., in both leguminous and non-leguminous plants.
The other essential uses of Rhizobium is Organic amendments nature they have in the soil, which have a positive effect on crop production and plant health and some of these effects have been related to the enhancement of soil suppressiveness against soil-borne pathogens [27].
There are also some justifications shows that application of botanical toxicants or plant products has reduced root knot disease and root-rotting fungi [28]. Organic amendment of soil has also enhanced the activity of biocontrol agents in the suppression of plant pathogens [29]. Among the various oil cakes, mustard oil cake is cheap and easy to decompose the soil, and serve as soil conditioner. Through reducing viruses and nematodes, mustard oil cakes additionally have the ability to increases plant survival rate and yields [30].
Soybean plants are subject to infection by several soil-borne pathogens that inducing root rot disease, which is considered one of the most important limiting factors against plant growth and yield [24]. Fusarium solani and Macrophomina phaseolina are among the most known pathogens affecting soybean [31]. Suppression of root diseases of soybean effectively combated with rhizobia [24].
Rhizobacteria importance on plant growth promoting
Plant growth-promoting rhizobacteria (PGPR) are naturally occurring soil bacteria that aggressively colonize plant roots and benefit plants by providing growth promotion [32]. The capacity of plant growth-enhancing bacteria is mainly through taking the action like; bio-controlling agents against phyto-pathogens and thus in some way they motivate plant growth by the help of different mechanisms including antibiotic production, depletion of iron from the rhizosphere, induced systemic resistance, production of fungal cell wall lysing enzymes, and competition for binding sites on the root [33].
Plant growth promoting bacteria can also directly enhance plant growth especially through fixing atmospheric nitrogen and supply it to plants; synthesize siderophores (a molecule which binds and transports iron in micro-organisms which can sequester iron from the soil and provide it to plant cells) and synthesize phyto-hormones such as auxins, cytokinins and gibberellins, which can increase different stages of plant growth; solubilize minerals such as phosphorus, making them available in large quantity for plant growth [34,35].
Biological nitrogen fixation performed yearly, up to 100 million tons of N for terrestrial ecosystems and from 30 to 300 million tons for marine ecosystems. Additionally, 20 million tons result from chemical fixation due to atmospheric phenomena. On the beginning of 19th century the first industrial production of Rhizobium inoculants began [36].
According to an FAO report, manufacture of N fertilizer for 2007 was 130 million tons of N, and this should further rise in the coming years. There for extensive use has a great impact on environment. A amount of added fertilizer is lost as a result of denitrification and leaching of soil by rainfall and irrigation [37]. The major source of water pollution caused by eutrophication is this leaching. As a consequence, extending application of biological nitrogen fixation by any means is an important issue [36]. Generally Rhizobium has positive impact on leguminous plants production and productivities via different mechanisms: of these nutrient accessibility and uptake.
Role of Rhizobium in nitrogen availability and uptake by legumes
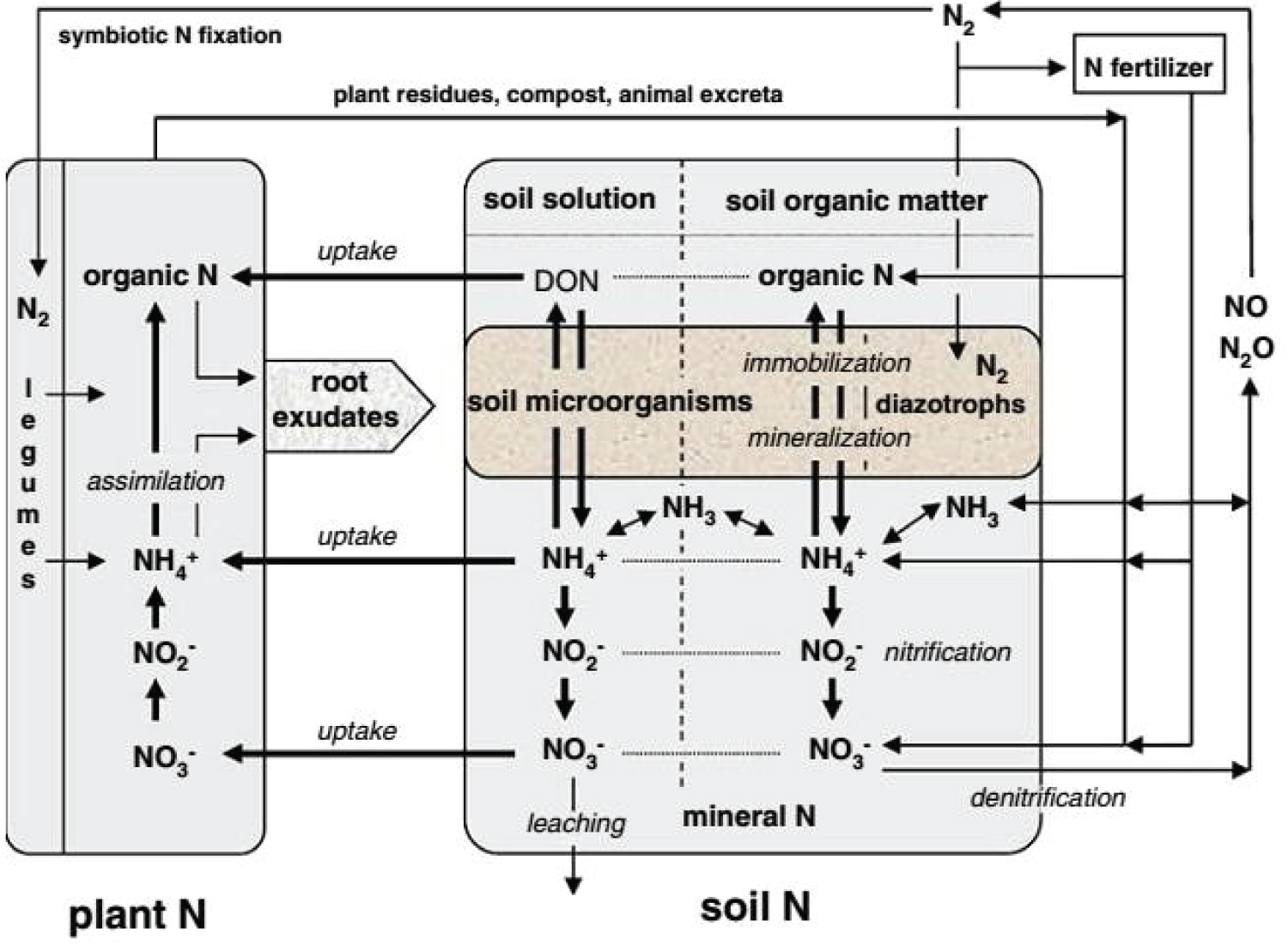
Unevenness in nitrogen (N) cycling, nutritional status, biological and physical properties of soil, incidence of pests and diseases, fluctuating climatic factors and abiotic stresses are the interlinked contributing factors for reduced agricultural productivity. In soil occurs nitrogen in forms of both organic and inorganic and with the in different seasonal changes and characterized by a heterogeneous distribution within the soil. Nitrogen inputs by the processes of fixation reactions (symbiotic microorganisms and potentially through free-living diazotrophs) and transformations of N between different pools have significant implications for plant growth and for the loss of N from soil systems. Mineralization of Organic forms of N to ammonium (NH4+) by Microbial-mediated and its succeeding nitrification to nitrate (NO3−) is a significant role for N availability and has pressure on root activity and rhizosphere dynamics. Mineral form of N is the form at which plant uptake or absorption of nitrogen is performed. According to evidences from researchers the soluble organic forms of N (e.g. low molecular weight compounds such as amino acids) may also play a significant role. The rhizosphore has particular significance in activity effect of up taking different N forms are directly influenced by soil pH in the immediate vicinity of the root and subsequent influence of this on nutrient acquisition, especially in relation to the availability of P and various micronutrients (e.g. Zn, Mn and Fe) [38].
Protons uptake that occur by the cause of NO3- influx changes the rhizosphore pH, this net release protons for NH4+ uptake, this can also bring about changes in the nature of substrates exuded from roots or the quantities of exudates released, and consequently may have major impact on the structure of microbial communities around the root .Mass flow and diffusion are the mechanism through which NO3− and NH4+ reach the root surface [38].
The concentrations nitrate in soil solution is typically present in the form of mM and, and it mobility is relative to orthophosphate, is more mobile and thus, this indicate it that it has the potential able to move in soil by up to several mm per day. On the other side ammonium has less motion or mobile since it eagerly adsorbs to the cation exchange sites in soil and but it has lower rates for both mass flow and diffusion. Since, diffusion and mass flow is the major pathway for inorganic N uptake and additionally it is difficult to differentiate diffusion from root interception, it is generally accepted that interception of N in soil solution following root extension accounts for a minute amount only of N taken up by plants [39,40] (Figure 2).
Benefits of legumes and actinorhizal for plants
The major secret which enables nitrogen fixers to adapt to the most extreme conditions and to colonize diverse ecological niches and having selective advantage is the concentration of fixed nitrogen which is one of the most limiting minerals for growth. Actually, the role of nitrogen-fixing symbiotic microorganisms which is mandatory in the life of plants, ensuring not only their nutrition, but also their defense against pathogens, pests, and adaptation to various environmental stresses [41].
Major leading mechanism in nitrogen-fixing systems is Legumes, as they may derive up to 90% of their nitrogen from N2 (e.g. faba bean, lupin, soybean, ground nut). Fixation ability of legumes largely depends on cultivars and culture conditions. There for on average fixation rate of 200 to 300 kg N/ha/crop can be attained for most species. For example among grain legumes, soybean represents more than 50% of the world oilseed production. Many actinorhizal plants, but also legumes, are capable of sustaining a mycorrhizal association as well, thus forming tripartite symbiosis and enhancing the success of these plants under poor soil conditions. Due to these properties, actinorhizal species can grow and improve soil fertility in disturbed sites and are used in re-colonization and reclaiming of eroded areas, sand dunes, moraines and areas of industrial waste and road cuts, and are planted following fires, volcanic eruptions and logging [42].
Some actinorhizal species has the ability to grow well under a range of environmental stresses such as high salinity, heavy metal and extreme pH. Because actinorhizal plant has comparable rates of nitrogen fixation to those found in legumes. For example Alders plants, are known to be beneficial at improving nutrient-poor soils [42]. The annual stands ranges input of N from N2 fixation in alder is performed from 20 to 300 kg N/ha, this is mainly depending on stand age, stand density and site conditions [41,42].
Growth promoting mechanisms of Rhizobium
Direct growth promoting mechanisms: PGPR has capacity to induce plant growth promotion by either direct or indirect modes of action. Direct mechanisms; by producing bacterial volatiles phyto-hormones which has stimulatory role and, reduction of the ethylene level in plant, upgrading plant nutrient status (liberation of phosphates and micronutrients from insoluble sources; non-symbiotic nitrogen fixation) and prompting disease-resistance mechanisms (induced systemic resistance) [43].
Indirect growth promoting mechanisms: Indirect type of Plant growth promoting is performed when Rhizobacteria act like biocontrol agents reducing diseases, and when they stimulate other beneficial symbioses, to protect the plant by degrading xenobiotics in inhibitory contaminated soils. Rhizosphore bacteria have also the ability to improve plant growth and crop productivity by various mechanisms [2].
Plant drought stress minimizing role of rhizobacteria
There are several breeding and genetic engineering approaches have been planned to increase the capability of plants to tolerate stress. The very recent one of the mechanisms implemented to plant to drought tolerance enhancement is application of plant growth promoting Rhizobacteria (PGPR) inoculation Arabidopsis thaliana which was published in Uppsala, Sweden [44]. After inoculation the result was shown that Arabidopsis thaliana inoculated with PGPR Paenibacillus polymyxa B2 could survive drought stress amazingly longer time when compared to the untreated control plant. Prof. Glick group studies in Canada [33] also reported the same results and reveals the mechanism of tolerance to drought stress. One of the methods by which plant tissue avoids sensitiveness towards dehydration is by maintaining high water potentials as long as possible.
The two major ways of drought avoiding mechanisms are: i) Water savers which conserve water and ii) Water spenders which absorb water fast enough to meet transpiration losses. Anatomical and morphological structure of plant helps them to increase water uptake and reduce water exaution.
For example water uptake could be enhanced through widespread root system with large active surface area enhanced due to inoculation of Rhizobium and then shoot/root ratio shifted in favor of the roots [45]. The type of drought tolerance is known in xerophytes. In each of the above mentioned mechanisms for plant drought tolerance and avoidance mechanisms Rhizobacteria has a vital role for success of each mechanisms [44].
Responsibility of Rhizobium in soil ecology and plant health and growth
Ecological roles of Rhizobacteria are mentioned within the definition of PGPR: PGPR play a key role in interaction of soil and plant root to form rhizosphore or bacteria colonizing roots in a competitive environment, for example in non-pasteurized or non-autoclaved field soils [46].
Additionally a single PGPR can also reveal multiple modes of action, including biological control and nutrient recycling. For example, inoculation of PGPR strain on ornamentals, forest trees, vegetables, and agricultural crops afford numerous effects on plant growth stages like; seedling germination enhancement, stand health, plant vigor, plant height, and shoot weight, nutrient content of shoot tissues, early bloom, chlorophyll content, and increased nodulation in legumes [47].
PGPR have the ability to influence the growth, yield, and nutrient uptake by an array of mechanisms and rising nitrogen fixation in legumes, provoke free-living nitrogen-fixing bacteria, amplify supply of other nutrients like; phosphorus, sulphur, iron and copper, produce plant hormones, improve other beneficial bacteria or fungi, control fungal and bacterial diseases and insect pests [48].
Biological nitrogen fixation role of Rhizobium
Nitrogen availability is probably the second most limiting factor in agricultural production, next to water availability. According to Mohamed Hemida Abd-Alla of 2014, from the total of 1015 tons of N2 gas avail in the atmosphere, only 3 × 109 tons of N2 per year is cycled or transformed. This transformations are achieved through different mechanisms (e.g., N2 fixation) biological which is the largest and account for about 60%, Lightening which accounts about 10% fixed nitrogen supply and fertilizer industry also perform significant amounts of chemically fixed nitrogen which is 25% of the Earth's newly fixed N2 [47].
The legume-Rhizobium symbiosis is the single most significant source of biologically fixed nitrogen in agricultural systems and without the need of on external sources of energy, by using sunlight which is free and renewable, and with few negative ecological effects [49].
Atmospheric nitrogen Fixation by the microorganism through a reductive process into ammonia is called as Biological nitrogen fixation (BNF). A variety of prokaryotic organism also have the ability to reduce the atmosphere N2 BNF which is accounts about 70% of the total N2 fixed in the biosphere [47]. The ability to reduce atmosphere N2 is restricted only to bacteria, which are belonging to the diverse groups. The root nodule associations were the first to be recognized for their ability to fix atmosphere N2. Rhizobium is the first group of organism realized for its potential of nitrogen fixation. The rhizosphere conditions favor the N2 fixation because it is carried out by heterotrophic bacteria that use organic compounds as source of electrons for the reduction of N2. Prominent microorganisms which are N2 fixers of the genera Rhizobium are, Bradyrrhizobium, Mesorhizobium, Allorhizobium, Sinorhizobium, and Mesorhizobium form symbiosis with legumes [2].
Decomposers digest proteins of dead organisms and their waste by the help of enzymes application, and releasing nitrogen in the form of ammonia. Widely used enzymes in the application for decomposition and digest proteins of are: - Proteinases break large proteins into smaller molecules, Peptidases break peptide bonds to release amino acids, Deaminases remove amino groups from amino acids and release ammonia [50].
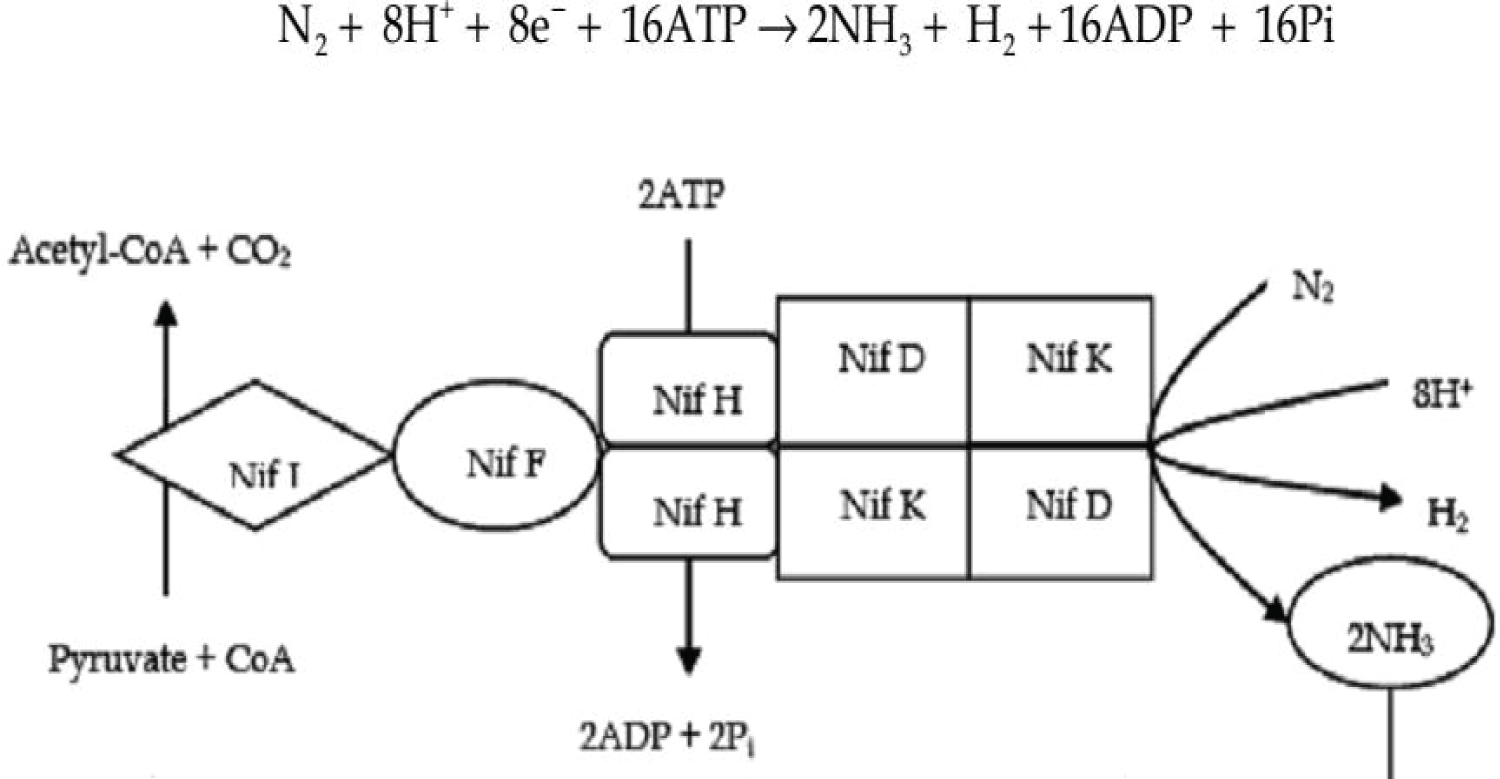
Biological nitrogen fixation processes: According to Kneip, et al., while biological nitrogen fixation (BNF) is performed, nitrogen is reduced in electron-transfer reactions. This does to synthesis of ammonia and release of hydrogen. After that ammonium which is produced used for the next synthesis of bio-molecules. For example synthesis of glutamine or glutamate for N-metabolism; NifJ: pyruvate flavodoxin/ferrodoxin oxidoreductase, NifF: flavodoxin/ferredoxin). This type of molecular nitrogen reduction and ammonium production is produced in all nitrogen-fixing organisms by the help of nitrogenase enzyme complex in an ATP- application and energy-consuming reaction [51,52] (Figure 3).
Advantage of replacing chemical fertilizer using Rhizobium
It is must to use necessary materials in agriculture to gain sufficient amount of agricultural production. The most widely applicable materials are fertilizer, pesticide, herb sides. Based on the production, process for production of fertilizer, it can be roughly categorized into three types: chemical, organic and bio-fertilizer. Each type of fertilizer can cause its own advantages and disadvantages. The advantages need to be integrated in order to achieve optimum performance and to realize balanced nutrient management for crop growth [12].
Influence of using chemical fertilizers on environment
1. Over use chemical fertilizer can result in negative effects such as leaching, pollution of water resources, damaging soil micro-flora and fauna and harmless insects, crop susceptibility to disease attack, acidification or alkalization of the soil or decline in soil fertility thus causing irreparable damage to the overall system.
2. Over application of N leads to softening of plant tissue resulting in plants that are more sensitive to diseases and pests.
3. They diminish the colonization of plant roots with mycorrihizae and inhibit symbiotic N fixation by Rhizobium due to high N fertilization.
4. They enhance the decomposition of soil organic material, which leads to degradation of soil structure.
5. Nutrients are easily lost from soils through fixation, leaching or gas emission and can lead to reduced fertilizer efficiency [12].
Build-up of disease suppressiveness
A further differentiation is made among specific disease suppressive soils; some soils retain their disease suppressiveness for prolonged periods and persist even when soils are left bare, whereas other soils develop suppressiveness only after monoculture of a crop for several years. Induction of suppressiveness by itself is remarkable, because for most plant species, successive monocultures will lead to a build-up of specialized plant pathogens. Nonetheless, development of disease suppressiveness in soils has been reported for various diseases, including potato scab disease caused by Streptomyces species, Fusarium wilt disease of several plant species, Rhizoctonia damping-off disease of sugar beet, and take-all disease of wheat [53].
Rhizobium and Its Role in Increasing Environmental Protection
Phytoremediation Role of Rhizobium
Phytoremediation is defined as the role of plants performance to extract, sequester, or detoxify pollutants. This remediation method has a significant role to mitigate or avoid environmental pollution and visually attractive, and also use full to maintained the structure of the soil [54]. Pollutants can be divided into two groups, the elemental pollutants and the organic pollutants [55].
Elemental pollutants Elemental pollutants are pollutants include contaminants like; toxic heavy metals and radionuclides. Compared to organic pollutants, only a little mechanism of remediation techniques are available for this types of pollutant, and role of plants to strip heavy metals from soil is an emerging tool [54,55]. The most important principles of phytoremediation are i) From soil the pollutants are extracted and trans located to the aboveground tissues, ii) The pollutant in the root system sequestered to prevent further spreading and leaching into soil or ground water, or iii) Changed into less toxic chemicals. For this kind of phytoremediation different plant species like; tobacco, sunflower, mustard, maize, and sand rocket, are used, depend on their ability to absorb or hyperaccumulate the pollutant [55].
Plants grown on soils which were naturally enriched with heavy metals natural has the capability of hyper-accumulate metals and this accumulation was thought to be a defense mechanism against herbivores [56]. But distribution of such plants is inadequate and the hyper-accumulating capability is contaminant specific. Bacterial genes introduction and its expression in plants resulting in enzymes involved in the conversion of xenobiotics is, when the legislative problems for using such plants are released, also a promising tool. Bizily and associates [57] mentioned that the use of Arabidopsis thaliana plants decomposed with bacterial genes in order to transform and detoxify organic mercury, and Iimura and associates [58] described the expression of a manganese-peroxidase gene in transgenic tobacco.
Organic pollutants the other group of pollutants which can be targeted for phytoremediation is organic pollutants, which include:- polychlorinated biphenyls, polycyclic aromatic compounds, nitroaromatics, or linear halogenated hydrocarbons. This group can be mineralized completely by the help of popular trees like:- willow, alfalfa, and different grass varieties. Organic compound which are pollutant in nature like:- tri-chloroethylene (TCE); the explosives 2,4,6-trinitrotoluene (TNT), hexahydro-1,3,5-trinitro- 1,3,5-triazine (RDX), glycerol trinitrate (GTN), and nitroglycerin can be degraded using plant enzymes shows the possibilities of phytoremediation. Actually the range of pollutants degradation can also be increased by construction of transgenic plants. For example tobacco plant construction harboring the mammalian cytochrome P450 2e1, provoke the ability to oxidize a wide range of pollutants such as TCE, ethyl bromide, carbon tetrachloride, chloroform, and vinyl chloride [59].
According to Shimizu and associates [60] report catechol-degrading gene cbnA, encodes an enzyme and participated in the break-down of the aromatic ring of chlorocatechol in plants successfully. For effective application of phytoremediation there are some limitations of the technique. These limitations are i) The time necessary for acceptable effects, ii) The limited depth of the root system, iii) The slow growth of plants, iv) The sensitivity toward some pollutants, v) The problem of being part of a food chain, and vi) The dependence on changes in the climate and winter dormancy [54].
Rhizobacterium can help plants to overcome these problems and can make plants deep rooting which lead to a more effective bioremediation process.
Five major subgroups of Phytoremediation
Phyoextraction - Removal of metals present in plant parts.
Phytodegradation - Degradation of contaminants by plant associated microbes and contribution to plant producing enzyme.
Rhizofilteration - Absorption of metals by plant roots from contaminated waters. Phytostabilization - immobilization and reduction in the mobility and bioavailability of catian an ion by plant roots and their associated microbes.
Phytovolatilization - Volatilization of contaminants from the soil into the atmosphere by plant parts [54].
Phytoremedation task of Rhizobium (Rhizoremediation): Combination of plants with some microorganisms enhances good organization of contaminants extraction; which is called rhizoremediation [61]. According to Chaudhry Q [62], both the plant and microbes are benefited from the association, the plants provide nutrients for microorganisms, this in turn, grow and multiply, rising the capacity of degradation by plants [63].
Rhizoremediation action of PGPR's on organic contaminants: Narasimhan K [35], Study is the first study in which PGPR was used to enhancing plant growth for the first time and PGPR is used as bio-control of plant diseases, but recently in more they can attract attention and apply on bioremediation. Bacteria those have capable of degrading certain kind of organic pollutant, such as polychlorinated biphenyls (PCBs) cannot survive in the near-starvation conditions found in soils, including the rhizosphore. Rhizoremediation or bioremediation with PGPR is the other application of Rhizobium in addition with plant growth prompting role and biocontrol action role of plant diseases [64].
Microorganisms can degrade inorganic compounds and even mineralize organic compounds in association with plants. Bacteria which can able to degrading certain kind of organic pollutant, such as polychlorinated biphenyls (PCBs) have been isolated from a range of sites and the pathways and encoding genes have also been well studied [65].
Rhizoremediation of metals facilitated by PGPR's: According to Michael, et al. of 2007 [66], used different plant species as chelating agents and this strategy that often works on a small scale in the laboratory but it is much less effective in the field; large number of plants have been tested for their ability to take up high levels of metals and then translocate those metals from roots to leaves and shoots, however, many so-called hyper-accumulating plants do not produce sufficient biomass to make this process efficient in the field. On other side using soil bacteria (often plant growth-promoting bacteria) as adjuncts in metal phytoremediation can significantly facilitate the growth of plants in the presence of high (and otherwise inhibitory) levels of metals [67].
Metal bioavailability can be enhanced through the addition of various chelating agents; this mostly conducted at the level of laboratory works on a small scale but is much less effective in the field. A large amount of plants have been checked for their performance to take up high levels of metals and then translocate those metals from roots to leaves and shoots. Those types of plants are called hyper-accumulating plants. In nature these plants do not produce sufficient biomass to perform this process in efficient in the field. So the use of soil bacteria (often plant growth-promoting bacteria) as adjuncts in metal phytoremediation can highly make favors growth of plants in the presence of high (and otherwise inhibitory) levels of metals [64].
According to Khan [68] bioremediation of trace metal contaminated soils is influenced and changed to conducive environment by rhizosphore microbes of plants which provide function of phytoremediation. The natural role of plant growth promoting Rhizobacteria (PGPR), Psolubilizing bacteria, mycorrhizal-helping bacteria (MHB) and arbuscular mycorrhizal fungi (AMF) maintaining soil fertility than conventional agriculture, horticulture, and forestry where higher use of agrochemicals minimize their significance. These microbe organisms provoke a concerted action when a particular population density is achieved, i.e. quorum sensing. AMF also recognize their host by signals released by host roots, allowing a functional symbiosis. AM fungi produce an insoluble glycoprotein, glomalin, which sequester trace elements and it should be considered for bio-stabilization leading to remediation of contaminated soils.
Plant-microbe association for Rhizoremediation
There are microbes (Endophytic bacteria) or bacteria colonizing and living internal tissues of plants (an intimate niche) without causing negative impact on their host. Except seed endophytes, primary site for most endophytes gain entry into plants is via the roots [69].
Additionally to their beneficial effects on plant growth, endophytes have considerable biotechnological potential to improve the applicability and efficiency of phytoremediation. Using both cultivation and cultivation-independent techniques, investigated the endophytes and Rhizobacteria with Thlaspi goes in genes, a hyper-accumulator of Nickel. Generally, most of the endophytes were cultivation- independent entophytes tolerated higher concentration of Ni than rhizosphore bacteria [69]. Although this system is promising in heavy metal remediation, the mechanisms by which endophytes promote metal accumulation are not well understood yet and the application of cultivation-independent microbe is very difficult.
Effect of Rhizobium on the yield of leguminous plants
Nitrogen is one of the essential substances in plant growth and a production of food and feed. Because it is the key elements in cellular synthesis like; enzymes, proteins, chlorophyll, DNA and RNA. Nitrogenase in rhizobial bacteroids are essential enzymes for fixing atmospheric N2 through symbiotic fixation action in nodulating legumes. This process of biological nitrogen fixation (BNF) and recycling nitrogen is currently utilized in agriculture, and it will be also provide promising yields for important in crop productivity for the future, especially in sustainable systems [70].
In Africa seasonally about 15-210 kg N2 per ha is fixed by legumes, on the other side about 43-581 kg of N2 fixed per hector per year by legumes tree. Non-legumes crops can also be benefited from fixed N2 by the action of rhizobia in legumes via direct transfer of biologically-fixed N to cereals growing in intercrops, or to subsequent crops rotated with symbiotic legumes [71]. In many low input grassland systems, the grasses depend on the N fixed by their legume counterparts for their N nutrition and protein synthesis, which is much needed for forage quality in livestock production. In addition to N2 fixation in legumes, rhizobia are also capable of contributing to growth promotion in non-legume species [70,71].
Role of Rhizosphore Bacteria for Legume Plants to Tolerate Abiotic and Biotic Stress
The application of bioinoculants plant growth-promoting Rhizobacteria such as Azospirillum, Agrobacteria, Pseudomonas, several Gram positive Bacilli is an environment-friendly, energy efficient and economically viable approach for reclaiming wastelands and increasing biomass production. According to Almadini 2005 beneficial effects of these bacteria in combination with Am fungi and their plant host through a different types of mechanisms like; (1) Effects on the receptivity of the root; (2) Effects on the root-fungus recognition; (3) Effects on the fungal growth; (4) Modification of the chemistry of the rhizospheric soil; and (5) Effects on the germination of the fungal propagules. So Am fungi and NFB often act synergistically on infection rate, mineral nutrition and plant growth [72].
Rhizosphore bacteria and abiotic stress
Photosynthetic plants can produce carbohydrates, after essential minerals are absorbed solubilized and by the help of root-associated microorganisms, commonly known as the rhizosphore microbes. These minerals have significant role in plant growth in optimum quantities of nitrate, phosphate, and other minerals which are often not available in free form or in limited quantities in the soil. Here is the significant role of root-associated beneficial microbes are clearly observed. The sessile nature of Plant has always been confronted with various abiotic and biotic stresses in their immediate environment. As a consequence, the continued existence of plants depends on their ability to rapidly adjust their physiology, development, and growth to reduce or mitigate the impacts of stress. All plants are known to perceive and respond to stress signals such as drought, heat, salinity, herbivory, and pathogens [73].
Rhizosphore bacteria and biotic stress
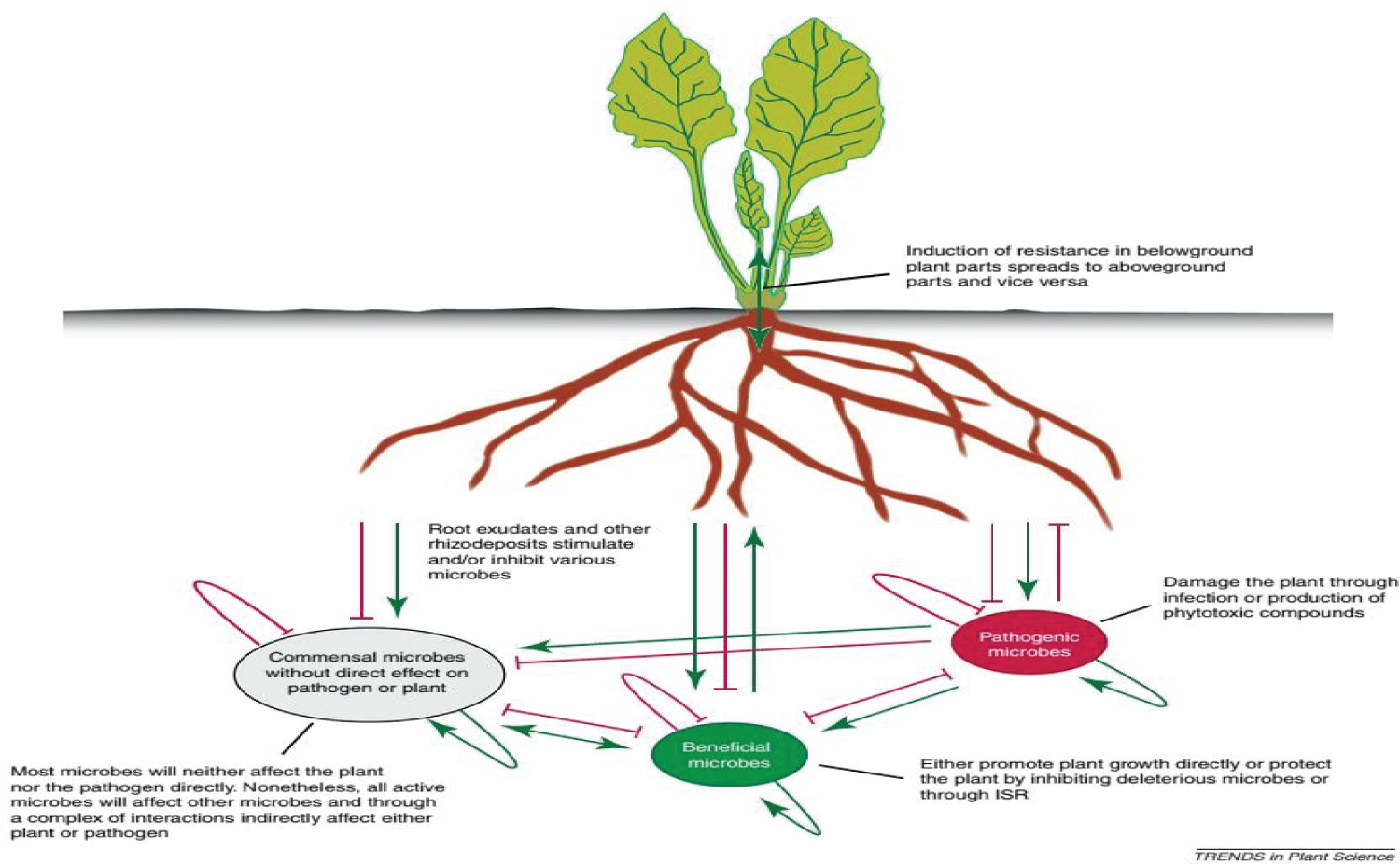
When the plant is attacked they release secondary metabolites and some other secretion. Since, in nature legume plants has the ability to form the interactions between a plant and its root microbiome, due to the change of host plant tissue and cell the rhizosphores bacteria chemicals like antibiotics, and hormones which has the role of defending pathogens. For example infection of citrus by Candidatus Liberibacter asiaticus, associated with Huang long bing, drastically altered the composition of citrus rhizosphore communities. Additionally, Verticillium dahliae infections affected the microbial composition of cotton rhizospheres. Changes in rhizosphore composition upon infection might be the result of the induced excretion of antimicrobial compounds by infected roots. In hairy root cultures of sweet basil (Ocimum basilicum), Pythium infection elicited the secretion of rosmarinic acid, a caffeic acid ester with antimicrobial activity. Infection of barley (Hordeum vulgare) roots by Fusarium graminum induced the exudation of phenolic compounds having antifungal activity. Infection of water melon plants by F. oxysporum enhanced the stimulation of Fusarium spore germination by root exudates. In the same study, association of the biocontrol bacterium Paenibacillus polymyxa SQR-21 decreased the germination- stimulatory effects of the root exudates [53] (Figure 4).
Antagonistic Activities of Sideropores, Bacteriocins, and Antibiotics on Legumes
Rhizobacteria in nature has the capability of producing siderophores and metabolites which has the nature of antibiotic and provokes disease defending mechanism for their host. The uptake of ferric ion via siderophore is largely used by pathogenic and non-pathogenic microorganisms from the soil, human body and marine environments [74].
The importance of siderophore is closely related to iron, which is an essential element for different biological processes. On the other hand, bacteria can produce a wide variety of compounds with antimicrobial activity used as defense systems. These include broad-spectrum antibiotics, lactic acid produced by lactobacilli, lytic agents such as lysozymes, numerous types of exotoxins and bacteriocins, which also have a bactericidal mode of action. Siderophores, bacteriocins and antibiotics are three of the most effective and well known mechanisms that an antagonist can employ to minimize or prevent phytopathogenic proliferation [48].
Conclusion
Bacteria of the genus Rhizobium and related genera can interact with host plants in a process called nodulation. The transformation of Rhizobium cells from vegetative bacteria into nitrogen-fixing bacteroids involves an alteration of cell fate, presumably with an underlying developmental pathway.
Rhizobium has fundamental role in environmental pollution protection (pollutant dicomposition) it may be organic compound or inorganic elements, mineraral solubility, plant diseases protection, capability of making avail essential and mandatory nutrients which detrmine plant growth and productivity, and they also can play a major role in absorbition or uptaking of minerals. Rhizobium can improve the availability and up take of N2, menerals and nutrient which are mandatory for pland survival and yeild.
Additionally these microbes are important in components of integrated nutrients managements are called Biofertilizers. These potential biological fertilizers would contribute immense activity in productivity and sustainability of soil and also protect the environment as eco-friendly and also it is cost effective inputs for the farmers. Bio-fertilizers have multidirectional advantage of cost effective, eco-friendly and renewable source of plant nutrients to supplement chemical fertilizers in sustainable agricultural system.
Biofertilizers are products of different types of living cells (microorganisms) which when, they applied to seed, plant surface or soil, colonize the rhizosphere or the interior of the plant and promotes growth by converting nutritionally essential elements (nitrogen, phosphorus) from unavailable to available form through biological process such as nitrogen fixation and solubilization of rock phosphate. There for these beneficial microorganisms in biofertilizers accelerate and improve plant growth and protect plants from pests and diseases.
The most widely used agricultural chemicals play a great role in increasing agricultural yields in sufficient amount. But on the other side these chemicals plays a significant role in environmental pollution, ecological and ecosystem destruction. So in order to stop or mitigate this destruction it is must to replace with eco- friendly materials and methods. There for utilization of Rhizobium in agricultural application is best solution.
Phytoremediation is the other advantage of rhizobium by which extract, sequester, or detoxify pollutants performed with the symbiosis relation plants. This remediation method has a significant role to mitigate or avoid environmental pollution and it has significant advantage in enhancing stress tolerance of plants.
Finally Rhizobacteria in nature also has the capability of producing siderophores and metabolites which has the nature of antibiotic and provokes disease defending mechanism for their host. The importance of siderophore is closely related to iron, which is an essential element for different biological processes. On the other hand, bacteria can produce a wide variety of compounds with antimicrobial activity used as defense systems.
Generally Rhizobacteria in nature has the ability to reduce agricultural chemical pollutants which can pollute soil and aquatic ecology a biotic and biotic stress; and has the ability to increase agricultural yields.
References
- Borlotti A, Vigani G and Zocchi G (2012) Iron deficiency affects nitrogen metabolism in cucumber (Cucumis sativus L.) plants. BMC Plant Biology 12: 189.
- Vega O, Walter N (2007) A review on beneficial effects of rhizosphere bacteria on soil nutrient availability and plant nutrient uptake. Rev Fac Nal Agr Medellín 60: 3621-3643.
- Orrell P, Bennett AE (2013) How can we exploit above-belowground interactions to assist in addressing the challenges of food security? Front Plant Sci 4: 432.
- Ravikumar R (2012) Growth effects of Rhizobium inoculation in some Legume plants. Int J Curr Sci 1: 1-6.
- Aczel M (2019) What Is the Nitrogen Cycle and Why Is It Key to Life?. Front Young Minds 7: 41.
- Leghari SJ, Wahocho GM, Laghari KH, et al. (2016) Role of nitrogen for plant growth and development: A review Advances in Environmental Biology 10: 209-218.
- Geetha SJ, Sanket JJ (2013) Engineering Rhizobial Bioinoculants: A Strategy to Improve Iron Nutrition. Scientific World Journal 2013: 15.
- De Moraes M, Mescher MC, Dean JM (2014) Plant Dependence on Rhizobia for Nitrogen Influences Induced Plant Defenses and Herbivore Performance. Int J Mol Sci 15: 1466-1480.
- Montañez A (2000) Overview and Case studies on Biological Nitrogen Fixation Perspectives and Limitations. FAO 243: 1.
- Sajid M, Rab A, Wahid FI, et al. (2011) Influence of Rhizobium Inoculation on Growth and Yield of Groundnut Cultivars. Sarhad J Agric 27: 573-576.
- FAO and UNEP (2020) The State of the World's Forests 2020: Forests biodiversity and people.
- Chen JH (2006) The combined use of chemical and organic fertilizers and/or biofertilizer for crop growth and soil fertility. Land Development Department, Bangkok, Thailand.
- Vielhauer Vlek PLG (2004) Nutrient management strategies in stressed environments In: Stressed ecosystems and sustainable agriculture, Oxford and IBH Publishing Co, New Delhi, India 67: 203-229.
- Radziah O, Panhwar QA (2014) Phosphate- solubilizing bacteria improves nutrient uptake in aerobic rice In: Phosphate Solubilizing Microorganisms. Springer 207-224.
- El-Yazeid AA, Abou-Aly HA, Mady MA, et al. (2007) Enhancing growth productivity and quality of squash plants using phosphate dissolving microorganisms (bio phosphor) combined with boron foliar spray. Res J Agric Biol Sci 3: 274-286.
- Gepts P, Beavis WD, Brummer EC, et al. (2005) Legumes as a model plant family Genomics for food and feed report of the cross-legume advances through genomics conference. Plant Physiology 137: 1228-1235.
- Azani N, Babineau M, Donovan Bailey C, et al. (2017) LPWG 2017 Phylogeny and classification of the Leguminosae. Taxon 66: 44-77.
- Shreeja D (2017) Symbiotic and Non-Symbiotic Nitrogen Fixing Bacteria. Soil Management 117-126.
- Beneduzi A, Ambrosini A, Luciane MP Passaglia (2012) Plant growth- promotingrhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet Mol Biol 35: 1044-1051.
- Long, Valerie Oke, Sharon R (2010) Bacteroid formation in the Rhizobium–legume symbiosis. Curr Opin Microbiol 2: 641-646.
- Vasileva V, Ilieva A (2012) Nodulation and nitrogen assimilation in legumes under elements of technology. Lap Lambert Academic Publishing.
- Boundless com (2021) The Legume-Root Nodule Symbiosis.
- Lin W, Lin M, Zhou H, et al. (2019) The effects of chemical and organic fertilizer usage on rhizosphere soil in tea orchards. PLoS One 14: e0217018.
- Noreen P, Parveen G, Shafique HA, et al. (2019) Role of Rhizobia in Suppressing the Root Diseases of Soybean under Soil Amendment. Sociedade Brasileira Da Ciência Das Plant as Daninhas 37: e019172336.
- Gulnez P, Urooj F, Asma H, et al. (2020) Role of rhizobia in suppressing the root rot and root knot disease of chili used alone or with pseudomonas aeruginosa. Pak J Bot 52: 1097-1104.
- Nautiyal, C shekhar (1997) Rhizosphere competence of pseudomonas sp NBR19926 and Rhizobium sp NBR19513 involved in the suppression of chick pea (Cicer ariettinum) pathogenic fungi. Lap Lambert Academic Publishing 2000: 120.
- Bailey KL, Lazarovits G (2003) Suppressing soil-borne diseases with residue management and organic amendments. Soil & Tillage Research 72: 169-180.
- Hafiza AS, Viqar S, Jehan A, et al. (2015) Role of Antagonistic Microorganisms and Organic Amendment in Stimulating the Defense System of Okra against Root Rotting Fungi. Pol J Microbiol 6: 157-162.
- Garbeva P, Postma J, van Veen JA, et al. (2006) Effect of above-ground plant species on soil microbial community structure and its impact on suppression of Rhizoctonia solani AG3. Environmental Microbiology 2: 233-246.
- Rafi H, Dawar S, Marium T (2016) Combined effect of soil amendment with oil cakes and seed priming in the control of root rot fungi of leguminous and non-leguminous crops. Pak J Bot 48: 1305-1311.
- Marquez N, Giachero ML, Declerck S, et al. (2021) Macrophomina phaseolina: General Characteristics of Pathogenicity and Methods of Control. Front Plant Sci 12: 634397.
- Saharan BS, Nehra V (2011) Plant Growth Promoting Rhizobacteria: A Critical Review. Life Sciences and Medicine 2011.
- Glick BR, Tirosh T, Mayak S (2004) Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci 166: 525-530.
- Amora-Lazcano E, Guerrero-Zúñiga LA, Rodriguez-Tovar A, et al. (2010) Rhizospheric plant-microbe interactions that enhance the remediation of contaminated soils. Plant and Cell Physiology.
- Narasimhan K, Basheer C, Bajic VB, et al. (2003) Enhancement of plant-microbe interactions using a rhizosphere metabolomics driven approach and its application in the removal of polychlorinated biphenyls. Plant Physiol 132: 146-153.
- Mosier k (2002) Environmental challenges associated with needed increases in global nitrogen fixation. Nutr Cycl Agroecosyst 63: 101-116.
- FAO (2008) Current world fertilizer trends and outlook 2011/ 2012 Food and agricultural organization of the United Nations Rome.
- Marschner (2010) Mineral nutrition of higher plants. Academic Press London.
- George TS, Gregory PJ, Hocking PJ, et al. (2008) Variation in root-associated phosphatase activities in wheat contributes to the utilization of organic P substrates in-vitro but does not explain differences in the P-nutrition of plants when grown in soils. Environ Experim Bot 64: 239-249.
- Richardson AE, Barea JM, McNeill AM, et al. (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321: 305-339.
- Wheeler M (2010) Current potential uses of actinorhizal plants in Europe Academic press Inc San Diego: 365-389.
- Wall B (2010) Nitrogen-fixing actinorhizal symbioses. Springer Dordrecht 147-166.
- Kloepper JW, Antoun H (2001) Plant Growth Promoting Rhizobacteria (PGPR) In: Brenner S and Miller JH Eds Encyclopedia of Genetics Academic Press New York, 1477-1480.
- Timmusk S, Wagner EG (2000) The plant-growth-promoting rhizobacterium Paenibacillus polymyxa induces changes in Arabidopsis thaliana gene expression: a possible connection between biotic and abiotic stress responses. Mol Plant Microbe Interact 12: 951.
- Sadras V, Calderini D (2009) Crop Physiology, Edn 1, Elsevier.
- Kloepper JW, Ryu C-M, Farag MA, et al. (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA 100: 4927-4932.
- Hoben P, Somasegaran, H J (2003) Methods in legume-rhizobium technology.
- Wertz JE, Riley MA (2002) Bacteriocins: Evolution ecology and application. Annu Rev Microbiol 56: 117-137.
- Abd-Alla MH, Issa AA, Ohyama T (2014) Impact of Harsh Environmental Conditions on Nodule Formation and Dinitrogen Fixation of Legumes. Environmental Science.
- Iwata K, Yu SS, Noor N, et al. (2012) Ammonia Accumulation of Novel Nitrogen Fixing Bacteria. Biotechnology - Molecular Studies and Novel Applications for Improved Quality of Human Life.
- Kneip C, Lockhart P, Voss C, et al. (2007) Nitrogen fixation in eukaryotes-New models for symbiosis. BMC Evol Biol 7: 1471-2148.
- Elmerich C, Franche C, Lindström K (2009) Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant and Soil 321: 35-59.
- Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17: 478-486.
- Khan AG, Kuek C, Chaudhry TM, et al. (2000) Role of plants mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 41: 197-207.
- Meagher RB (2000) Phytoremediation of toxic elemental and organic pollutants. Curr Opin Plant Biol 3: 153-162.
- Gabriel P, Abdul R, War M, et al. (2012) Mechanisms of Plant Defense against Insect Herbivores. Plant Signal Behav 7: 1306-1320.
- Bizily SP, Rugh CL, Meagher RB (2000) Phytodetoxification of hazardous organomercurials by genetically engineered plants. Nat Biotechnol 18: 213-217.
- Iimura Y, Ikeda S, Sonoki T, et al. (2002) Expression of a gene for Mn-peroxidase from Coriolus versicolor in a transgenic tobacco generates potential tools for phytoremediation. Appl Microbiol Biotechnol 59: 246-251.
- Doty SL, Shang TQ, Gordon MP, et al. (2000) Enhanced metabolism of halogenated hydrocarbons in transgenic plants containing mammalian cytochrome P450 2e1. Proc Natl Acad Sci USA 97: 6287-6291.
- Shimizu S, Kobayashi H, Masai E, et al. (2001) Characterization of the 450-kb linear plasmid in a polychlorinated biphenyl egrader Rhodococcus sp RHA1. Appl Environ Microbiol 67: 2021-2028.
- Newman LA, Reynolds CM (2004) Phytodegradation of organic compounds. Curr Opin Biotechnol 15: 225-230.
- Chaudhry Q, Blom-Zandstra M, Gupta SK, et al. (2005) Utilizing the synergy between plants and rhizosphere microorganisms to enhance breakdown of organic pollutants in the environment. Environ Sci Pollut Res 12: 34-48.
- Siciliano SD, Germida JJ (1998) Mechanisms of phytoremediation: biochemical and ecological interactions between plants and bacteria. Environ Rev 6: 65-79.
- Zhuang X, Chen J, Shim H, et al. (2007) New advances in plant growth-promoting rhizobacteria for bioremediation. Environ Int 33: 406-413.
- Singh A, Ward O (2004) Applied Bioremediation and Phytoremediation. Soil Biology 243: 115-134.
- Michael WHE, Ebel M, Schaeffer A (2007) Chelate assisted phytoextraction of heavy metals from soil Effect mechanism toxicity and fate of chelating agents. Chemosphere 68: 989-1003.
- Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28: 367-374.
- Khan Abdul G (2005) Role of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. J Trace Elem Med Biol 18: 355-364.
- Idris R, Trifonova R, Puschenreiter M, et al. (2004) Bacterial communities associated with flowering plants of the Ni hyperaccumulator Thlaspi goesingense. Appl Environ Microbiol 70: 2667-2677.
- Dakora FD, Viviene NM (2004) Potential use of rhizobial bacteria as promoters of plant growth for increased yield in landraces of African cereal crops. Afr J Biotechnol 3: 1-7.
- Dakora F, Keya S (2003) Contribution of legume nitrogen fixation to sustainable agriculture in Sub-Saharan Africa Soil Biol Biochem 29: 809-817.
- Almadini AM, Rabie GG (2005) Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr J Biotechnol 4: 210-222.
- Hirt H (2009) Plant Stress Biology: From Genomics to Systems Biology, Wiley West Sussex.
- Maksimov IV, Abizgil'dina RR, Pusenkova LI (2011) Plant growth promoting rhizobacteria as alternative to chemical crop protectors from pathogens (Review). Appl Biochem Microbiol 47: 373-385.
Corresponding Author
Abdissa Deberssa Gutama, Department of Biology, Dilla University College of Natural and computational Science, PO Box 419, Dilla, Ethiopia.
Copyright
© 2022 Gutama AD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.