The Usefulness of Serum Procalcitonin in Determining the Severity of Spreading Odontogenic Infections in Patients Seen at Ile-Ife, Nigeria
Abstract
Background: The high morbidity, mortality and cost of treatment of spreading odontogenic infections makes it critical to find diagnostic tools that can facilitate prompt and accurate management decision-making.
Materials and methods: This is a prospective cohort study of 63 patients being managed for spreading odontogenic infection at the Obafemi Awolowo University Teaching Hospitals Complex, Ile Ife, Nigeria. Serum procalcitonin levels were recorded at three time points: At presentation, day-four and day-eight after treatment has commenced. The severity of spreading odontogenic infections was determined using the criteria described by Flynn, et al. A Mann-Whitney U test was used to compare the serum procalcitonin at different time points. A Receiver Operating Characteristic (ROC) curve was plotted to determine the diagnostic accuracy. The sensitivity, specificity, negative predictive value, positive predictive value, positive likelihood ratio and negative likelihood ratio of serum-procalcitonin as a diagnostic tool for spreading odontogenic infections were calculated.
Results: Serum procalcitonin levels at presentation ranged from 51.8 pg/ml to 484.3 pg/ml with a mean value of 169.9 ± 108.0 pg/ml and median (interquartile range) value of 129.3 (81.5) pg/ml. There was an increase in serum procalcitonin level with increased severity of odontogenic infections, the relationship was statistically significant (H = 40.665, df = 2, p = < 0.001). The diagnostic accuracy of serum procalcitonin was 0.60 (95% CI = 0.40-0.77 ; p = 0.33) at a cut-off point of 168.3 pg/ml. Serum procalcitonin had moderate sensitivity (53.3%) and specificity (75.0%), high negative predictive value (83.7%), low positive predictive value (40.0%), a 1.6 positive likelihood ratio and a 0.5 negative likelihood ratio.
Conclusion: There was a significant increase in serum procalcitonin level with increased severity of odontogenic infections. Thus, serum procalcitonin could differentiate between low to moderate severe and high severe spreading odontogenic infections. It can be used as an adjunct to traditional clinical methods of determining severity of spreading odontogenic infections.
Keywords
Serum, Procalcitonin, Spreading, Odontogenic, Infections
Introduction
Spreading odontogenic infections constitute one of the major cases managed by maxillofacial surgeons globally. Odontogenic infections are associated with high mortality and morbidity rate due to the late presentation and associated co-morbidities in some of these patients [1]. Prompt decision making about the severity of the infection needs to be made to reduce the risk of severe morbidity and mortality. Management of cases spreading odontogenic infections entails understanding the aetiology, grading the severity, supportive/medical management and surgical management [2]. Traditionally, management of a spreading odontogenic infection rely on patients' clinical parametres at presentation which has relatively low sensitivity (55%) but moderate specificity (73%) [3] and laboratory investigations such as white blood cell count. White blood cells are not specific and reliable for bacterial infection alone and has a lower sensitivity 81.2% and specificity 64.3% compared to procalcitonin with 90.3% sensitivity and 98.1% specificity in infection in other parts of the body [4,5]. Microscopy, culture and sensitivity test as well as blood culture are also useful but it takes some days for the results to be available. Recently, inflammatory biomarkers have been employed in the management of infections in other parts of the body [6].
The use of procalcitonin, a pro-hormone of the thyroid hormone calcitonin as a sensitive measure of the severity of odontogenic infection had been proposed [7]. The serum procalcitonin level in healthy individuals is below detectable level (< 0.1 ng/mL) but may increase to 1000 ng/mL in sepsis [8]. It has a half-life of 20-30 hours; it is stable in plasma and no plasma enzymes are able to breakdown procalcitonin once it enters the circulation. Serum procalcitonin rises within 2-6 hours of bacterial infection and rapidly peaks within 6-24 hours [9]. The rapidity of result availability compared to other investigations for bacterial infections made it a useful tool in infection of bacterial origin. Previous studies had focused on the role of serum procalcitonin in sepsis generally, with the exclusion of nosocomial infection in newborns hospitalised in intensive care unit. However, little is known on the role of serum-procalcitonin in management of severe spreading odontogenic infections [9-11]. This study aimed to evaluate the usefulness of serum-procalcitonin in the management of patients with spreading odontogenic infections. This study contributes to the literature on the use of serum-procalcitonin in the management of spreading odontogenic infections [12].
Materials and Methods
This was a prospective cohort study conducted in Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Nigeria involving 63 patients with odontogenic infections. The study was approved by the Research Ethics Committee of the Institution, with protocol number ERC/2018/11/11. The patients were enlisted via the outpatient clinic of the Department of Oral and Maxillofacial Surgery and the Accident and Emergency Unit of the hospital from August 2019 to June 2020. The inclusion criteria adopted for the study were: Patients with spreading odontogenic infection arising from carious lesion, periodontal lesion, fractured tooth, retained tooth; patients in whom the infection had spread beyond dentoalveolar segment; patients that consented to the study. Pregnant women, patients who completed any form of antibiotics (prescribed or self-medicated) within one week prior to presentation, patients who underwent major surgical procedures within one month of presentation and patients with recent clinical manifestation of malaria with a positive malaria rapid diagnostic test 72 hours prior to presentation were excluded. The rapid diagnostic test (RDT) was done to establish the presence of plasmodium parasite in the blood using the SD BIOLINE Malaria Ag P.f/Pan (05FK60) RDT kit [13].
The fascial spaces involved were clinically determined and the degree of severity of the fascial spaces was scored according to Flynn, et al. [2].
Score 1: Infection involved vestibular, subperiosteal, infraorbital, buccal, and canine spaces.
Score 2: Infection involved any of these spaces: submandibular, sublingual, pterygomandibular, submasseteric, superficial and deep temporal spaces.
Score 3: Involvement of the lateral pharyngeal, retropharyngeal, pretracheal, mediastinal danger spaces and intracranium.
The Flynn score for each patient was the aggregate sum of the scores of all the anatomical spaces involved in the patient. The mean severity score (MSS) and standard deviation (SD) of all the patients were determined followed by stratification of patients into three severity groups namely; low, moderate and high. Scores lower than MSS minus SD were classified as low severity; scores between MSS minus SD and MSS plus SD as moderate severity and scores above MSS plus SD as high severity group. The patients were either managed as in-patients or out-patients. Criteria for in-patient management were: involvement of more than one facial space, potential for airway compromise, high grade fever of at least 38.3 °С, presence of medical co-morbidity, need for general anesthesia, need for intravenous medication or rehydration. All other patients were managed on outpatient basis.
Five millilitre of blood was collected from the antecubital fossa of each patient at three-time points (D1, D2 and D3) and transported to the laboratory for serum procalcitonin assay. The 'D1' was at presentation, 'D2' and 'D3' were Days 4 and 8 respectively after the commencement of treatment. The serum was analysed with Bioassay Laboratory Technology human procalcitonin ELISA kit. Serum level of procalcitonin was determined with bioassay laboratory technology human procalcitonin ELISA kit [14].
Statistical methods
The data collected was analyzed using IBM-SPSS version 25. Shapiro-Wilk test was used to test for normality of data distribution. Continuous variables were summarized using median and interquartile range. Data were expressed in tables and graphs. The relationship between the level of serum procalcitonin and the severity of the spreading odontogenic infections as described by Flynn, et al. [1] was analyzed using Kruskal Wallis test.
The diagnostic accuracy of serum procalcitonin in spreading odontogenic infections was determined with a Receiver Operating Characteristics Curves (ROC). The Area under Curve (AUC) was determined at a cut-off point. The sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio and negative likelihood ratio of serum procalcitonin at the cut-off point were determined. The confidence interval was set at 95%, with significance at p < 0.05.
Results
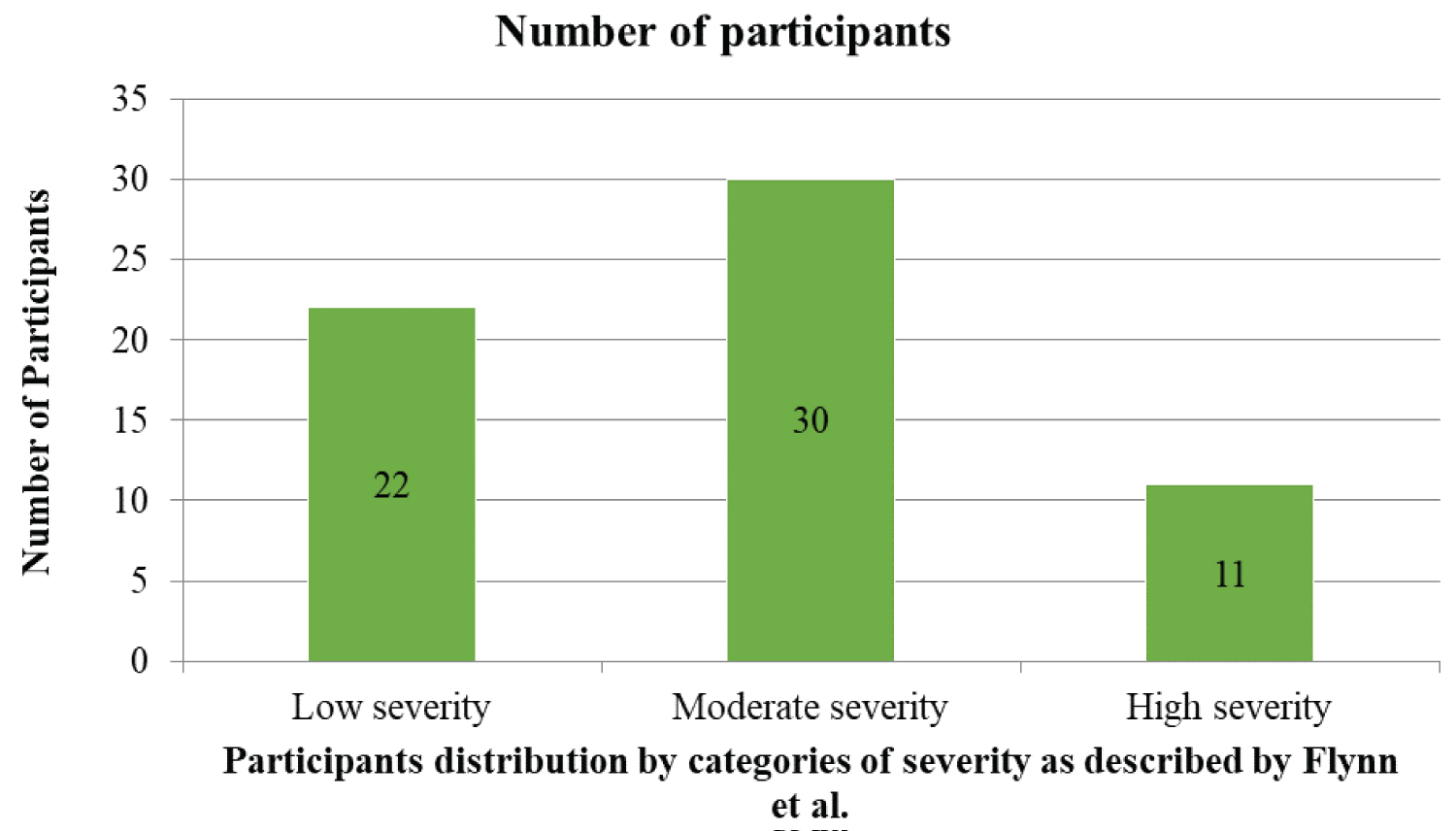
A total of 63 patients participated in the study and there was no lost to followup. The median (IQR) value of serum procalcitonin was 129.3 (81.5) pg/ml. The mean severity score was 6.0 ± 3.0. Based on this scoring, 22 (34.9%) of the patients had low severity, while it was moderately severe in 30 (47.6%). Eleven (17.5%) had a higher severity score. Details are as shown in Figure 1. Table 1 showed that serum procalcitonin level increased with increased severity of odontogenic infections and the relationship was statistically significant (H = 40.665, df = 2, p = < 0.001). The median serum-procalcitonin were 108.8 (37.5) pg/ml for patients with low severity, 183.0 (79.7) pg/ml for moderate severity and 365.8 (166.0) pg/ml for highly severe spreading odontogenic infections.
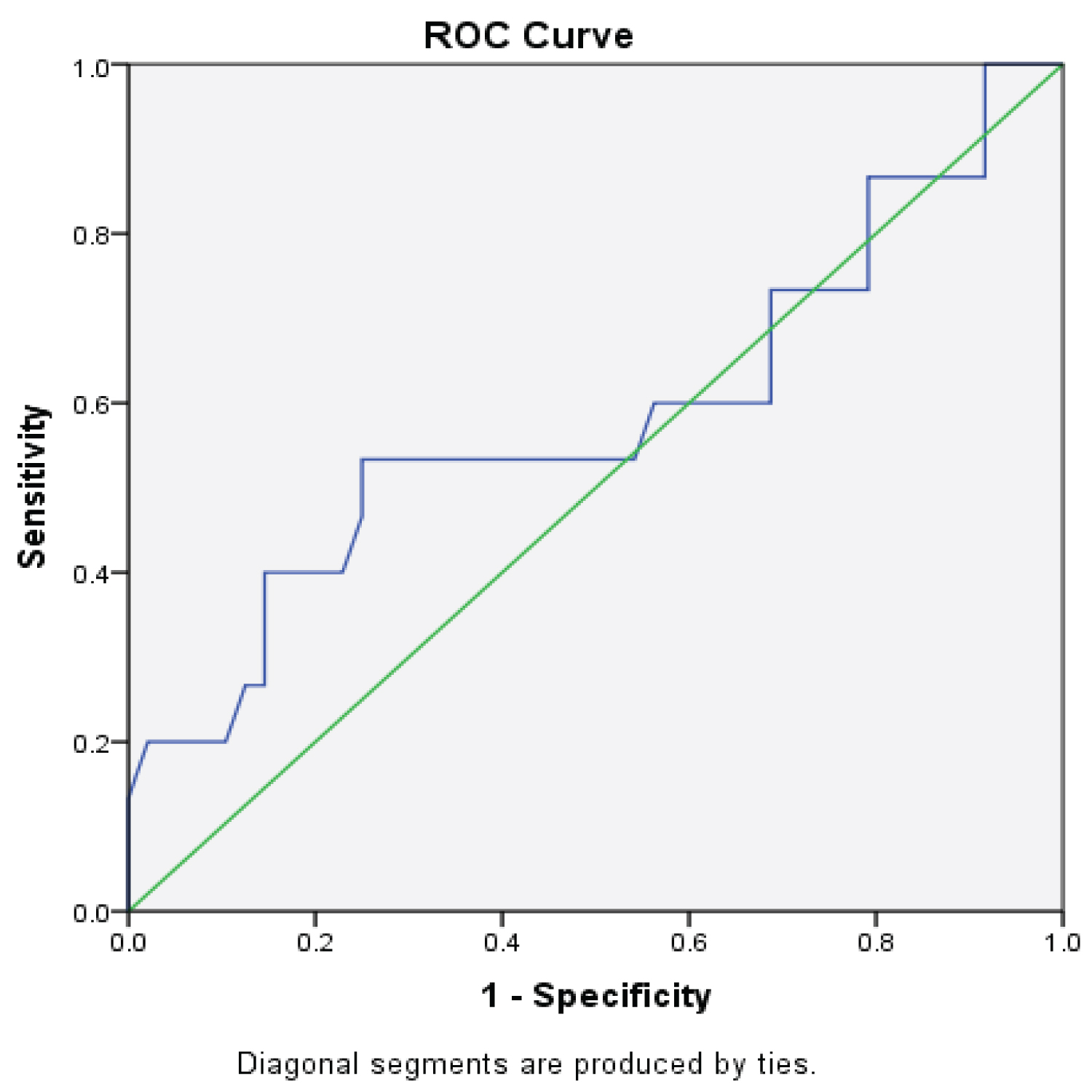
The effect of possible confounding variables on the level of serum procalcitonin at presentation was checked using a multiple linear regression analysis. There was no significant effect of any of the variables on the serum procalcitonin level at presentation. The accuracy of serum procalcitonin (Area under the Curve) was 0.60 (95% CI = 0.40-0.77, p = 0.33). The optimal cut-off point was 168.3 pg/ml. Therefore, the value of 168.3 pg/ml and above indicated high severe spreading odontogenic infections. The cut-off value had a sensitivity of 53.3% and specificity of 75.0% as shown in the Receiver Operating Characteristic (ROC) curve in Figure 2. The negative predictive value was 83.7%, and the positive predictive value was 40.0%. The positive and negative likelihood ratios of serum-procalcitonin were 1.6 and 0.5 respectively at the cut-off value as shown in Table 2.
Discussion
In this current study, the median serum-procalcitonin level at presentation (D1) was within the normal range in more than nine out of ten patients; only two patients with elevated values (greater than the study's reference value of 82-460 pg/ml). The median serum-procalcitonin level showed decline by day 4 (D2) and further declined at day 8 (D3) after institution of treatment in all the three severity groups. This suggests that the degree of systemic inflammation in the patients was low and rapidly dissipated following institution of treatment. This is not unexpected as most of the patients had mild to moderate spreading odontogenic infections. The cut-off value therefore divided the three groups into two arms of high severe versus low and moderate severe spreading odontogenic infections. While a baseline elevated level is highly suggestive of high severe spreading odontogenic infection, serum procalcitonin level may not be very efficient in differentiating between low and moderate severe spreading odontogenic infections. Once treatment is initiated, a decline in the serum-procalcitonin level should be anticipated and failure of rapid decline is a pointer to non-resolution of the spreading odontogenic infection.
Bertolus, et al. [12] reported a median serum-procalcitonin level which is much lower than the median level of 129.30 pg/ml obtained in this study. The reason for the difference in levels of serum procalcitonin reported in this study and that of Bertolus, et al. [12] could be associated with the variation in patients' recruited into the two studies. This study recruited more severe cases of spreading odontogenic infections in term of the types of infection such as cervicofascialnecrotising fasciitis while study by Bertolus, et al. [12] was limited to cases of facial cellulitis.
In this study, a marginal increase in serum-procalcitonin level above the laboratory reference value was noted in two patients. Bertolus, et al. [12] also reported that six out of their seventy-eight patients had serum-procalcitonin values slightly above the clinical threshold. The reason for this marginal increase of serum-procalcitonin in spreading odontogenic infections could be due to the lesser degree of systemic manifestations observed in spreading odontogenic infections compared with infections arising from visceral organs of the body in which an exponential increase in the level of serum-procalcitonin was reported [6,12,15].
Serum procalcitonin levels increased significantly with severity of infection in the patients (p < 0.001). There was no significant effect of age and gender on the level of serum procalcitonin in the patients. Similar to this, Li, et al. [6] reported no association between age or gender and the level of procalcitonin in critically ill patients with ventilator-associated pneumonia.
The serum-procalcitonin levels in the different degrees of severity of spreading odontogenic infections were lower compared to other visceral organs. The level of rise of serum-procalcitonin in local infections with signs of systemic manifestation is low compared to the reported values in systemic infections arising from core body organs like liver, pancreas, lungs [10]. This implies that though Flynn, et al. [1] recognised three severity groups (low, moderate and high), the level of rise in these levels of severities may not be the same as in similar levels of severities in other systemic infections. Odontogenic infections belong to this category of local infections (it usually arises from the dental and periodontal tissues) with signs of systemic manifestations [12]. A similar finding of low level of serum procalcitonin was observed in study that assessed procalcitonin level among patients with limb cellulitis [16].
The accuracy of serum-procalcitonin in predicting severity of spreading odontogenic infections was determined by a Receiver Operating Characteristic (ROC). The Area under the Curve (AUC) was determined alongside the sensitivity, specificity, negative predictive value and positive predictive value at cut off point of 168.3 pg/ml. The AUC for detection of severe spreading odontogenic infections in this group is 0.60 (p = 0.33, CI = 0.40-0.77). Bertolus, et al. [12] also reported an area under the receiver operating curve of 0.61 (p = 0.10, CI = 0.47-0.71) in patients that required surgical drainage as depicted by the severity of their infections. All the patients in this group had surgical drainage or decompression. Kim, et al. [17] on the other hand reported a higher area of 0.93 under the curve in their patients. Most of their patients presented with sepsis syndrome which could have impacted on the higher diagnostic accuracy.
The sensitivity of 53.3% and specificity of 75.0% at a cut-off point of 168.3 pg/ml was noted in this group of patients. Bertolus, et al. [12] reported a lower sensitivity of 30.0% and higher specificity of 100% at a cut-off value of 0.1 µg/L (100 pg/ml). The varied degrees of severity of the spreading odontogenic infections in the two studies could account for the differences in sensitivity, specificity and cut-off values. Bertolus, et al. [12] study was limited to patients with facial cellulitis while the current study included patients with necrotizing cervicofacial fasciitis. These diagnostic variables are very important in evaluating the diagnostic accuracy of a test and are more useful clinically than just the sensitivity and specificity. The positive and negative likelihood ratios obtained suggest that the use of serum-procalcitonin in conjunction with the traditional clinical method of assessment of severity as described by Flynn, et al. [2] have a slightly better diagnostic value in the management of patients with spreading odontogenic infections. This finding is corroborated by Bertolus, et al. [12] who concluded that serum-procalcitonin have an independent diagnostic value in spreading odontogenic infections. There was a significant increase in serum procalcitonin level with increased severity of odontogenic infections. Thus, serum procalcitonin could differentiate between low to moderate severe and high severe spreading odontogenic infection. It can be used as an adjunct to traditional clinical methods of determining severity of spreading odontogenic infections.
Funding
Not applicable.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the 1994 Declaration of Helsinki and its later amendments or comparable ethical standards.
References
- Botha A, Jacobs F, Postma C (2015) Retrospective analysis of etiology and comorbid diseases associated with ludwig's angina. Ann Maxillofac Surg 5: 168-173.
- Flynn TR, Shanti RM, Levi MH, et al. (2006) Severe odontogenic infections, part 1: Prospective report. J Oral Maxillofac Surg 64: 1093-1103.
- Miller WD, Furst IM, Sàndor GK, et al. (1999) A prospective, blinded comparison of clinical examination and computed tomography in deep neck infections. Laryngoscope 109: 1873-1879.
- Magrini L, Gagliano G, Travaglino F, et al. (2014) Comparison between white blood cell count, procalcitonin and c reactive protein as diagnostic and prognostic biomarkers of infection or sepsis in patients presenting to emergency department. Clin Chem Lab Med 52: 1465-1472.
- Liu S, Hou Y, Cui H (2016) Clinical values of the early detection of serum procalcitonin, c-reactive protein and white blood cells for neonates with infectious diseases. Pak J Med Sci 32: 1326.
- Li B, Zhao X, Li S (2015) Serum procalcitonin level and mortality risk in critically ill patients with ventilator-associated pneumonia. Cell Physiol Biochem 37: 1967-1972.
- Maruna P, Nedelnikova K, Gurlich R (2000) Physiology and genetics of procalcitonin. Physiol Res 49: S57-S62.
- Assicot M, Bohuon C, Gendrel D, et al. (1993) High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 341: 515-518.
- Vijayan AL, Vanimaya, Ravindran S, et al. (2017) Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care 5: 51-57.
- Yan ST, Sun LC, Jia HB, et al. (2017) Procalcitonin levels in bloodstream infections caused by different sources and species of bacteria. Am J Emerg Med 35: 579-583.
- Riedel S (2012) Procalcitonin and the role of biomarkers in the diagnosis and management of sepsis. Diagn Microbiol Infect Dis 73: 221-227.
- Bertolus C, Schouman T, Aubry A, et al. (2016) Is procalcitonin a useful biomarker for the risk stratification of facial cellulitis?. J Craniomaxillofac Surg 44: 995-997.
- f/Pan SBMA. p. 6.
- Bioassay Technology Laboratory-Human Procalcitonin ELISA Kit E0977Hu.
- Baboudjian M, Tellier BG, Bisceglie MD, et al. (2020) The prognostic value of serum procalcitonin in acute obstructive pyelonephritis. World J Urol 1-7.
- Brindle R, Ijaz A, Davies P, et al. (2019) Procalcitonin and cellulitis: Correlation of procalcitonin blood levels with measurements of severity and outcome in patients with limb cellulitis. Biomarkers 24: 127-130.
- Kim DY, Lee YS, Ahn S, et al. (2011) The usefulness of procalcitonin and c-reactive protein as early diagnostic markers of bacteremia in cancer patients with febrile neutropenia. Cancer Res Treat 43: 176-180.
Corresponding Author
Taofeek Akinniyi, Department of Oral and Maxillofacial Surgery, Obafemi Awolowo University Teaching Hospitals' Complex, Ile - Ife, Osun State, Nigeria.
Copyright
© 2023 Akinniyi T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.