Removing Biofilms in the Isthmus by Endodontic Surgery
Abstract
Biofilms are multicellular communities which are composed of prokaryotic and/or eukaryotic cells embedded in matrix. More than 500 bacterial species have been identified in dental biofilms, which pose a harmful risk to dental health. Isthmus is a ribbon-shaped communication between two root canals which contains pulp tissue. It is almost impossible to be instrumented and cleaned completely in root canal treatment. It is not unusual to find a very narrow isthmus containing multi-layered microbial condensation and necrotic pulp tissues in the segment of the roots with periapical radiolucent lesions. Therefore, biofilm in isthmus cannot be neglected when we track the possible failure causes of root canal treatment. Since apical periodontitis has been identified as a biofilm-induced disease, efforts should be made not only to investigate the incidence pattern of isthmus in the roots of maxillary and mandibular teeth, but also to find more efficient ways to remove biofilms in isthmus. This review outlined the classification and incidence of isthmus, biofilm characteristics in isthmus as well as the treatment of removing biofilms in it by endodontic surgery.
Keywords
Biofilm, Isthmus, Endodontic surgery
Introduction
Biofilms are matrix-enclosed microbial aggregate that adhere to each other and/or to biological or non-biological surfaces [1]. It has been reported that the number of bacteria species which are identified so far in dental biofilms is over 500. These bacteria have been surviving and evolving in the environment of the oral cavity, including tooth surfaces, root canals, and even isthmuses with lower oxygen level and oligotrophic conditions [2]. Countless specific pathogens, including Gram-negative bacteria, extracellular matrix, antimicrobial agents and host defense mechanisms represent a tremendous threat to the dental health of human beings. As for endodontists, apical periodontitis is a biofilm-induced disease and bacterial biofilms can be easily detected in the apical root canals, especially in isthmuses, ramifications and lateral canals which are not easy to be accessed with instruments [3,4]. A mixed microbial population of cocci, rods, and filamentous organisms can be recognized, and the flora is encased in extracellular matrix and mixed with necrotic pulp tissue, organic materials and dentinal chips [5]. This review summarized laboratory outcomes of biofilms in isthmus in terms of morphology, composition and characteristics. In addition, the classification and incidence of isthmus are also outlined. More importantly, surgical treatment to remove biofilms in isthmus are fully introduced, which provides a high success rate and satisfactory prognosis for apical periodontitis.
Definition of Isthmus
An isthmus is geographically defined as a narrow strip of land connecting two larger land areas. In medical nomenclature, isthmus indicates a narrow anatomic passage connecting two broader and larger cavities or tissue structures [6]. The isthmus had been called anastomosis [7]. Weller [8] described the canal isthmus as a narrow, ribbon-shaped communication between two root canals that contained pulp tissue. The American Association of Endodontists (AAE) gave the newest definition for isthmus in the 2003 Edition of the Glossary of Endodontic Terms: Isthmus (anastomosis) is a thin communication between two or more canals in the same root [9].
Classification of Isthmus
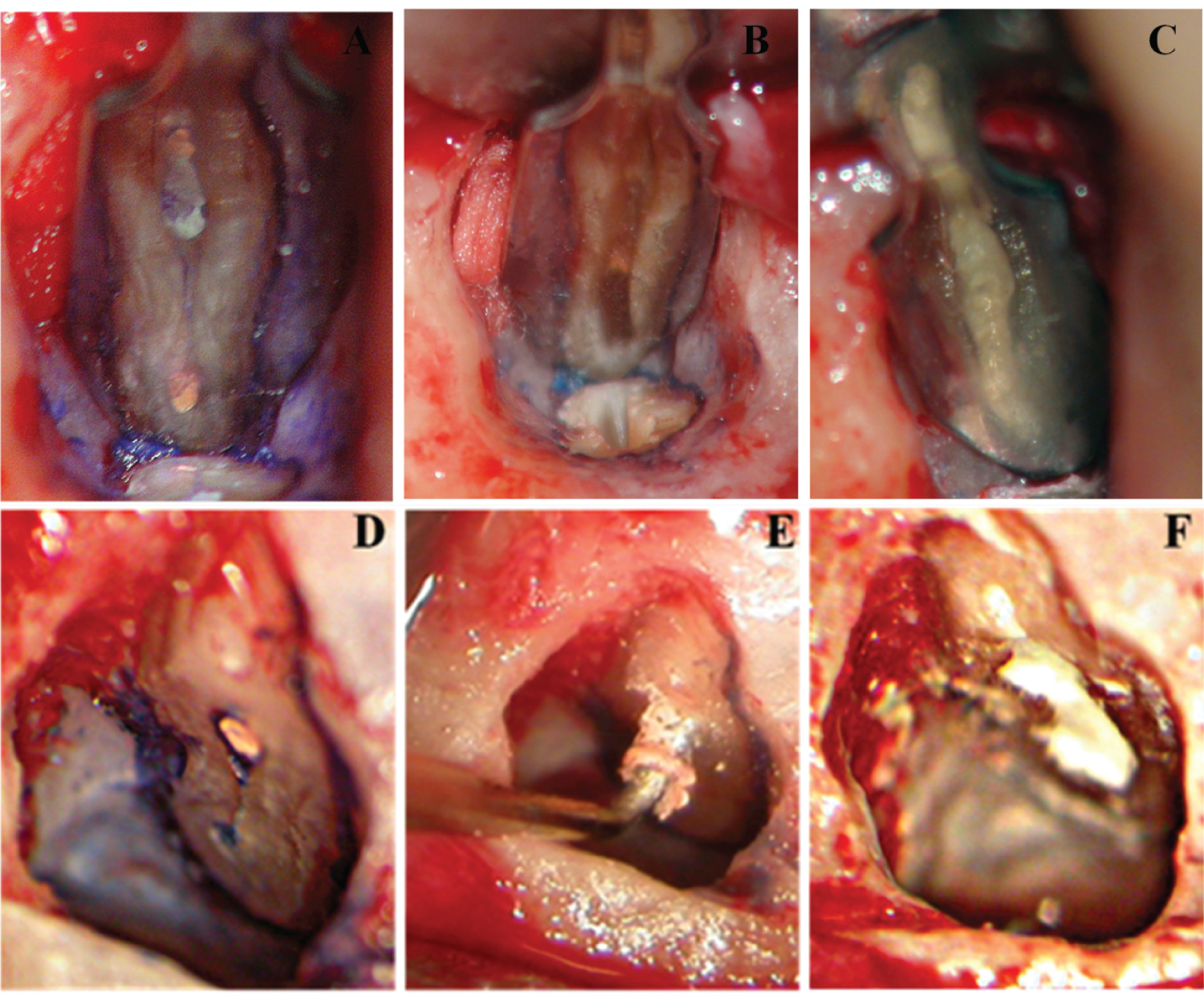
Isthmuses were classified into five different types by Hsu and Kim [10]. Type I was defined as no notable communication between the two canals. Type II was defined as a hair-thin connection between the two main canals. Type III differed from the Type II only with the presence of three canals instead of two. Incomplete C-shapes with three canals were also included in this category. Type IV had an isthmus with extended canals into the connection. Type V was recognized as a true connection or wide corridor between the two main canals. Weller [8] distinguished two types of isthmuses: The complete (Figure 1A) and partial isthmus (Figure 1D). A complete isthmus was one with a continuous, narrow connection between the two main root canals. A partial isthmus was defined as an incomplete communication with one or more patent openings between the two canals.
Incidence of Isthmus
The incidence of canal isthmus is related to the type of tooth and the level to apex within a given root [7]. Isthmuses have been found to be present in all types of roots in which two canals are normally found, including the mesiobuccal roots of maxillary molars, the mesial and distal roots of mandibular molars, the maxillary and mandibular first premolars and mandibular incisors [11].
To minimize bacteria leakage from the apical ramifications and lateral canals and avoid reinfection, the infected root should be resected during apical surgery. The isthmus which is present in the root should be properly treated. Weller reported that the incidence of isthmus in mesiobuccal root of maxillary first molar was the highest in the apical 3 to 5 mm level [8]. According to the frequency of apical ramifications and lateral canals, a 3-mm apical resection of the root-end will reduce 98% of the apical ramifications and 93% of the lateral canals [12]. Therefore, at least 3 mm root end was suggested to be amputated to eliminate the majority of apical ramifications and lateral canals. In this review, the incidence of partial and complete isthmuses at 3 mm level from the original apex was provided for endodontists as a reference of clinic practice.
Data on the incidence of canal isthmuses was extracted from various studies and summarized in Table 1 [13-15] and Table 2 [16-20]. If the incidence of isthmus in the same root was reported by more than one study, an overall rate was calculated based on data from all the included studies.
Biofilm in Isthmus
Biofilms are community of microorganisms which are embedded in a matrix composed of water, extracellular macromolecules and polysaccharides [1,21]. The overall physical, chemical and biological features of the environment around the biofilm determine its structure and characteristics in terms of gene expression, adhesion and metabolism, etc. Therefore, features of endodontic biofilms significantly differ from other biofilms in nature [22].
Mounting evidence has verified that apical periodontitis is a biofilm-induced disease and bacterial biofilms can be easily checked out in the apical root canals, especially in main root canals, isthmuses, ramifications and lateral canals of both untreated and treated teeth with apical lesions [3,4]. A mesial root of a failing endodontically retreated mandibular first molar showed a complex and multispecies biofilm around the isthmus areas between the mesiobuccal and mesiolingual canals when examined with transmission electron microscope (TEM). In addition, it is usual to find bacterial biofilms harbouring in the dentinal tubules and in interstitial areas of the dentin or collagen fibers [23]. Biofilms in isthmuses have four features. Firstly, biofilms are seen covering the walls of isthmuses in both untreated and treated canals and clogging all the way along the isthmus, regardless of the infiltration of neutrophils, suggesting that significant biofilm populations may be missed when only neutrophiles containing areas were investigated [22,23]. Secondly, small bacterial flocs and planktonic cells are observed in those biofilm-absent areas. Usually, they are observed in the lumen of the main canals, isthmuses and ramifications. They intermix with necrotic pulp tissue or possibly suspended in a fluid phase [22,23]. Bacterial flocs may originate from growing cell aggregates or detach from biofilms [24]. It was reported that both isthmus biofilms and planktonic bacteria play the role in the pathogenesis of acute apical periodontitis [1,4]. Thirdly, the flora consists of a mixed microbial population of cocci, rods, and predominantly filamentous organisms, which shows morphologic and structural diversities [5]. The overall physical, chemical and biological features of the isthmus environment, availability of nutrients and time of infection also affect the features of biofilm in this narrow area [22,23]. Fourthly, membrane vesicles (MVs) were observed to attach to the cell wall surface where they break open. Many Gram-negative bacteria produce external membrane vesicles during normal growth. During their formation, MVs entrap several periplasmic components including alkaline phosphatase, phospholipase C, proelastase, protease, peptidoglycan hydrolase, lipopolysaccharides of the cell membrane, adhesins, toxins, and other immunomodulatory compounds. No reports have yet verified that the MVs from isthmus biofilms contain isthmus-related periplasmic components and induced periodontitis. When the biofilm bacteria left in isthmuses cause periodontitis via the leakage to periapical tissues, MVs are regarded as one of the potential reasons. [23,25,26]. The virulence factors in MV components and high inflammatory capability cannot be overlooked during the initial phases of infection. They are able to modulate host defense and irritate inflammatory response.
Impact on Root Canal Treatment
Numerous failed cases requiring for apical surgery or intentional replantation shows that one of the main causes of failure is the high prevalent biofilm bacteria in isthmus which was mismanaged or failed to be completely instrumented and irrigated [11-13,27]. In clinical practice, isthmus preparation is important in both non-surgical and surgical endodontic procedures. Some narrow biofilm-covered isthmus walls may remain untouched by instruments and irrigants, therefore act as a bacterial reservoir. It is especially true when the root canal is irregular, flattened, or oval in cross-section [28-30]. Isthmus poses a challenge to root canal preparation and disinfection because poor accessibility increases possibilities of incomplete cleaning, therefore reduce the rate of healing [31].
Nair [5] suggested 50% specimens in both hand file treated and NiTi treated roots revealed microbes. This finding was in agreement with the results of a study by Dr. Trope [32]. As for the isthmus, 10 out of 11 specimens showed the presence of microorganisms within. It indicated that the microbes in these inaccessible areas existed primarily as biofilms which cannot be removed by instruments and irrigation in one-visit treatment.
Treating Isthmus by Endodontic Surgery
To achieve a favourable prognosis and high rate of healing, management of the isthmus should never be neglected. Modern endodontic surgery has evolved in microsurgery era. As microscope, micro instruments, ultrasonic tips and biologically compatible root-end filling materials were introduced in the last decade, endodontists could get better understanding of the apical anatomy, do microsurgery within small osteotomies and therefore get significantly higher success rate. There has been an ongoing controversy about when we should choose root canal retreatment or apical microsurgery once an apical lesion was appeared or had not healed at all after at least 6 months of initial root canal therapy. Generally, when the cases with true periapical cyst or/and complex root canal anatomy, or the root canal with post restorations in it and it is difficult to get access to apex coronally, or the tooth has been restored with crown which is in good condition, or any cases which is expected to be less time consuming, less cost, more predictable and better prognosis with modern endodontic surgery will definitely benefit from it.
Microscopy has been using in daily practice with surgical and non-surgical cases by endodontists who benefited from high magnification and illumination. Especially during the apical surgery procedures endodontists took advantages of microscopes not only to identify root apices and anatomical structures such as isthmuses, fractures and lateral canals, but also to make smaller osteotomies and shallower resection angles to conserve cortical bone and root length as much as possible. Methylene blue is helpful to identify isthmuses and other anatomical details after a 3-mm resection of root apices with the help of microscopes [12].
Ultrasonic tips have fundamentally changed the ways of treating isthmuses in apical surgery. The most recent developments are the Zirconium coated and diamond coated tips e.g. KiS tips [12,27]. Diamonds that were adhered to the surface of the tips increased the efficiently of isthmus preparation and gutta-percha removement. The ultrasonic preparation could preserve the integrity of the root apex rather than cut it into a bevel which increased the risk of leakage. The Kis tips have a pre-curved tip which is carefully designed to match various angles of both maxillary and mandibular roots when doing retro-preparations. The management of a partial or complete isthmus will be more efficient with the co-axial preparation tips within the root canal (Figure 1E). Degerness reported that 80% of the accessory canals and canal isthmuses were present within 3.6 mm from the apex. A minimum root resection level of 3.12 in average is essential to expose the isthmus area for endodontists awareness and cleaning during endodontic retrograde root resection, cleaning and retro filling [15]. Kim suggested that at least 3 mm of the root-end should be removed to reduce 98% of the apical ramifications and 93% of the lateral canals based on their anatomical study of the root apex (Figure 1B) [12,27].
A 3 mm class I cavity prepared by ultrasonic instruments should be retrofitted with a material that guarantees a leakage-controlled seal. A number of studies showed that IRM and SuperEBA had favourable results and were proved to be superior to amalgam in terms of saleability and biocompatibility [33-36]. More recently, mineral trioxide aggregate (MTA) has been suggested as the most biocompatible material and ideal root-end filling material with many outstanding and incomparable properties. MTA showed the outstanding capacity of consistently inducing bone, dentin and cementum formation. It can also increase the regeneration of periapical tissues including periodontal ligament and cementum in vivo, which allow healthy periodontal tissues to contact the filling material directly without the presence of inflammation (Figure 1C and Figure 1F) [12,37-39].
Summary
A full understanding of the characteristics of biofilm in isthmus and the complexity of isthmuses structures represented a challenging task for both lab researches and clinical practice. On the one hand, Biofilm community appears to be incredibly adaptive and persistent in hostile environments and anti-bacteria irrigating solutions, On the other hand, the anatomical structures of isthmuses are irregular and hard to be located and instrumented coronary. Currently, with the application of microscopes in endodontic surgery, we are able to observe the resected surface of the root more clearly, identify the shape, connection and type of isthmus, prepare it with ultrasonic tips and seal it with appropriate materials. The improvements in this clinic technology could increase the rate of healing and guarantee a promising prognosis.
References
- Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol 2: 95-108.
- Kroes I, Lepp PW, Relman DA (1999) Bacterial diversity within the human subgingival crevice. Proc Natl Acad Sci USA 96: 14547-14552.
- Chavez de Paz LE (2007) Redefining the persistent infection in root canals: Possible role of biofilm communities. J Endod 33: 652-662.
- Siqueira JF, Rocas IN (2009) Community as the unit of pathogenicity: An emerging concept as to the microbial pathogenesis of apical periodontitis. Oral Surg Oral Med O 107: 870-878.
- Nair PNR, Henry S, Cano V, et al. (2005) Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after "one-visit" endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 99: 231-252.
- Merriam Webster (1993) Merriam Webster’s Collegiate Dictionary. (10th edn), Springfield, Springfield, Mass, USA.
- Vertucci FJ (1984) Root canal anatomy of the human permanent teeth. Oral Surg Oral Med Oral Pathol 58: 589-599.
- Weller RN, Niemczyk SP, Kim S (1995) Incidence and position of the canal isthmus. Part 1. Mesiobuccal root of the maxillary first molar. J Endod 21: 380-383.
- Choudary TM, Kiran C (2010) Isthmuses of the mesial root of mandibular first molar-An spiral computed tomographic study. Endod 22: 48-52.
- Hsu YY, Kim S (1997) The resected root surface. The issue of canal isthmuses. Dent Clin North Am 41: 529-540.
- Mannocci F, Peru M, Sherriff M, et al. (2005) The isthmuses of the mesial root of mandibular molars: A micro-computed tomographic study. Int Endod J 38: 558-563.
- Kim S, Kratchman S (2006) Modern endodontic surgery concepts and practice: A review. J Endod 32: 601-623.
- Villegas JC, Yoshioka T, Kobayashi C, et al. (2004) Frequency of transverse anastomoses with and without apical communication in Japanese population teeth. Aust Endod J 30: 50-52.
- Arx TV (2005) Frequency and type of canal isthmuses in first molars detected by endoscopic inspection during periradicular surgery. Int Endod J 38: 160-168.
- Degerness RA, Bowles WR (2010) Dimension, anatomy and morphology of the mesiobuccal root canal system in maxillary molars. J Endod 36: 985-989.
- Uma Ch (1970) Canal and isthmus morphology in mandibular incisors-An in vitro study. Endod 16: 7-11.
- Peiris R, Takahashi M, Sasaki K, et al. (2007) Root and canal morphology of permanent mandibular molars in a Sri Lankan population. Odontology 95: 16-23.
- Peiris HRD, Pitakotuwage TN, Takahashi M, et al. (2008) Root canal morphology of mandibular permanent molars at different ages. Int Endod J 41: 828-835.
- Al-Qudah AA, Awawdeh LA (2009) Root and canal morphology of mandibular first and second molar teeth in a Jordanian population. Int Endod J 42: 775-784.
- Tonelli SQ, Sousa-Neto MD, Leoni GB, et al. (2021) Micro-CT evaluation of maxillary first molars: Interorifice distances and internal anatomy of the mesiobuccal root. Braz Oral Res 35: e060.
- Allison DG (2003) The biofilm matrix. Biofouling 19: 139-150.
- Ricucci D, Siqueira JF (2010) Biofilms and apical periodontitis: Study of prevalence and association with clinical and histopathologic findings. J Endod 36: 1277-1288.
- Carr GB, Schwartz RS, Schaudinn C, et al. (2009) Ultrastructural examination of failed molar retreatment with secondary apical periodontitis: An examination of endodontic biofilms in an endodontic retreatment failure. J Endod 35: 1303-1309.
- Hall-Stoodley L, Stoodley P (2009) Evolving concepts in biofilm infections. Cell Microbiol 11: 1034-1043.
- Li Z, Clarke AJ, Beveridge TJ (1998) Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria. J Bacteriol 180: 5478-5483.
- Kuehn MJ, Kesty NC (2005) Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev 19: 2645-2655.
- Kim S (1997) Principles of endodontic microsurgery. Dent Clin North Am 41: 481-497.
- Siqueira JF, Araujo MC, Garcia PF, et al. (1997) Histological evaluation of the effectiveness of five instrumentation techniques for cleaning the apical third of root canals. J Endod 23: 499-502.
- Wu MK, Van der Sluis LWM, Wesselink PR (2003) The capability of two hand instrumentation techniques to remove the inner layer of dentine in oval canals. Int Endod J 36: 218-224.
- Paqué F, Balmer M, Attin T, et al. (2010) Preparation of oval-shaped root canals in mandibular molars using nickel-titanium rotary instruments: A micro-computed tomography study. J Endod 36: 703-707.
- Teixeira FB, Sano CL, Gomes BPFA, et al. (2003) A preliminary in vitro study of the incidence and position of the root canal isthmus in maxillary and mandibular first molars. Int Endod J 36: 276-280.
- Dalton BC, Orstavik D, Phillips C, et al. (1998) Bacterial reduction with nickel-titanium rotary instrumentation. J Endod 24: 763-767.
- Szeremeta-Browar TL, VanCura JE, Zaki AE (1985) A comparison of the sealing properties of different retrograde techniques: An autoradiographic study. Oral Surg Oral Med Oral Pathol 59: 82-87.
- Bondra DL, Hartwell GR, MacPherson MG, et al. (1989) Leakage in vitro with IRM, high copper amalgam, and EBA cement as retrofilling materials. J Endod 15: 157-160.
- Pitt Ford TR, Andreasen JO, Dorn SO, et al. (1994) Effect of IRM root end fillings on healing after replantation. J Endod 20: 381-385.
- Ford TR, Andreasen JO, Dorn SO, et al. (1995) Effect of Super-EBA as a root end filling on healing after replantation. J Endod 21: 13-15.
- Torabinejad M, Ford TR, McKendry DJ, et al. (1997) Histologic assessment of mineral trioxide aggregate as a root-end filling in monkeys. J Endod 23: 225-228.
- Thomson TS, Berry JE, Somerman MJ, et al. (2003) Cementoblasts maintain expression of osteocalcin in the presence of mineral trioxide aggregate. J Endod 29: 407-412.
- Baek SH, Plenk H, Kim S (2005) Periapical tissue responses and cementum regeneration with amalgam, SuperEBA, and MTA as root-end filling materials. J Endod 31: 444-449.
Corresponding Author
Li Peng, State Key Laboratory of Oral Diseases and National Clinical Research Center for Oral Diseases and Department of Cariology and Endodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, China, Tel: 18010563611, Fax: 86-28-85501481
Copyright
© 2022 Wang X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.