The Effects of Particulate Dentine Compared to Bio-Oss® on Bone Regeneration
Abstract
Objective: To compare particulate dentine to Bio-Oss® in terms of bone regeneration, inflammatory response and foreign-body reaction.
Methods: A total of 32 male Sprague Dawley rats were subjected to a surgical creation of a calvarial defect of a 5 mm diameter. The rats were divided into 4 groups of 8 rats. The first group received no graft material in the defect and was named the control group. The 2nd group received human particulate dentine. The 3rd group received a mixture of human particulate dentine and Bio-Oss®. The 4th group received Bio-Oss® only. The groups were compared histologically in terms of reduction of the mean of greatest diameter of bone defect, new bone formation, number of lymphocytes to assess the degree of inflammation and number of giant cells present to assess foreign-body reaction.
Results: Dentine group and mixture group showed significant reduction of the largest diameter between 4 and 8 weeks. Only the mixture group showed a significant reduction of the greatest diameter than the control group at 8 weeks. All three test groups showed significantly higher new bone formation than the control at 8 weeks (P< 0.05). Significantly less inflammation in Bio-Oss® and control group was observed at 8 weeks than the other 2 groups. No significant difference in foreign body reaction was observed among the test groups.
Conclusion: No significant difference in new bone formation, reduction of defect size or foreign-body reaction was found between Bio-Oss®, particulate dentine and a mixture. However, Bio-Oss® showed significantly less inflammation at 8 weeks.
Clinical Relevance: Particulate dentine could be used as an alternative for Bio-Oss® in bone augmentation procedures.
Keywords
Bio-Oss, Dentine, Bone Regeneration
Introduction
The bone defects resulting from various pathological (dentofacial trauma, infections, neoplasia) or developmental abnormalities represents a major challenge for periodontists and maxillofacial surgeons. Different techniques were developed in order to restore bone defects including the use of bone grafts, membranes or both [1]. A graft is needed to induce new bone formation when restoring a critical or severe bony defect.
Several bone graft materials were suggested to treat bone defects. The ideal bone graft must be biocompatible, bioresorbable, osteoconductive, osteoinductive, easy to use, cost effective and structurally similar to bone [2] Autograft is known to be the gold standard for the reconstruction of a bony defect [3] as it possess an osteogenic potential, produces good bony regeneration, however, it is not without disadvantages like a limited harvest volume, donor-site morbidity [4], extended surgery time, and resorption of the graft material. Several studies have shown that also onlay bone block grafts are prone to resorption and a large part of the bone graft can be lost during the healing period [5]. In an attempt to overcome these limitations, other substitutes of less resorption prone material such as deproteinized bovine bone and biphasic calcium phosphate have been attempted for clinical use. Growth factors that promote healing and regeneration are mostly used along with the grafting materials and those used in dentistry include platelet concentrates and recombinant growth factors. These agents are broadly used when bone-healing mechanisms are affected by the patient's medical conditions, or when the graft only provides mechanical support and does not provide stimulation of cell growth and differentiation. Montanari and colleagues [6] demonstrated that platelet-rich fibrin membrane was able to reduce the healing period and improve bone regeneration.
Bio-Oss® is a well-documented bovine-derived xenograft and experiments on its use can be traced to more than 25 years ago. It has been used in the treatment of periodontal osseous defects, guided bone regeneration and sinus augmentation procedures, and associated with high success rates and effectiveness, low resorption and integration with no local rejection in grafting procedures [7,8]. Despite providing an osteoconductive scaffold for bone formation, Bio-Oss® does not have any osteoinductive properties, which may require adding growth factors to enhance bone formation in certain defects which incur extra cost to the treatment.
Researchers and clinicians have become interested in the use of human dentin from extracted teeth in the context of serving as graft material. Dentine has a similar composition to bone [9] both being mineralized tissues and almost similar in chemical components (collagen, non-collagenous proteins, and hydroxyapatite crystals). Moreover, dentine matrix contains bone morphogenetic proteins (BMPs) which induces and enhances osteogenesis [10]. Similar to bone, dentine also contains proteins including osteopontin, bone sialoproteins, osterix and osteocalcin. Therefore, dentine can be considered an effective alternative bone grafting material [11] Non collagenous proteins of dentine are recognized to be involved in bone calcification [12].
Dentine grafts are either block or particulate grafts [13] and the latter has been used as a bone substitute in the treatment of large mandibular defects [14], maxillary sinus augmentation [15], socket preservation [16], and guided.
Tissue regeneration [17]. It has been shown that dentine graft materials have osteogenic potential while resorbing with minimal inflammation [18]. It was shown that particulate dentine does not elicit a strong immune reaction [19] and could be either demineralized or used without demineralization for bone augmentation purposes. It was shown that partially demineralized dentine performs better than un-demineralized dentine and completely demineralized dentine [20]. Nevertheless, demineralization of dentine could take one week using hydrocholoric acid (HCL) [21] or 14 days using Ethylenediaminetetraacetic acid (EDTA) [22]. While using an autogenous dentine graft eliminates foreign-body reaction due to genetic homogeneity [13], still it has the disadvantages of a limited harvest volume, and you need to sacrifice a tooth to gain the graft. Accordingly, many studies have investigated the use of particulate dentine grafts of xenogeneic origin and showed different degrees of success in bone defects regeneration [10,23,24].
Tissue regeneration [17]. It has been shown that dentine graft materials have osteogenic potential while resorbing with minimal inflammation [18]. It was shown that particulate dentine does not elicit a strong immune reaction [19] and could be either demineralized or used without demineralization for bone augmentation purposes. It was shown that partially demineralized dentine performs better than un-demineralized dentine and completely demineralized dentine [20]. Nevertheless, demineralization of dentine could take one week using hydrocholoric acid (HCL) [21] or 14 days using Ethylenediaminetetraacetic acid (EDTA) [22]. While using an autogenous dentine graft eliminates foreign-body reaction due to genetic homogeneity [13], still it has the disadvantages of a limited harvest volume, and you need to sacrifice a tooth to gain the graft. Accordingly, many studies have investigated the use of particulate dentine grafts of xenogeneic origin and showed different degrees of success in bone defects regeneration [10,23,24].
Materials and Methods
Experimental model and protocol
Thirty-two healthy male Sprague-Dawley (SD) rats were obtained from the animal house of the Jordan University of Science and Technology (JUST)- Irbid, Jordan and used for this study. The animals were housed in the same room but were kept in separate cages. The room had a 12-hour light/dark cycle and had a temperature of 22-24°C. Food and water were available for the rats ad libitum. All procedures were approved by the Animal Care and Use Committee before the start of the actual study (Project number 275-2017).
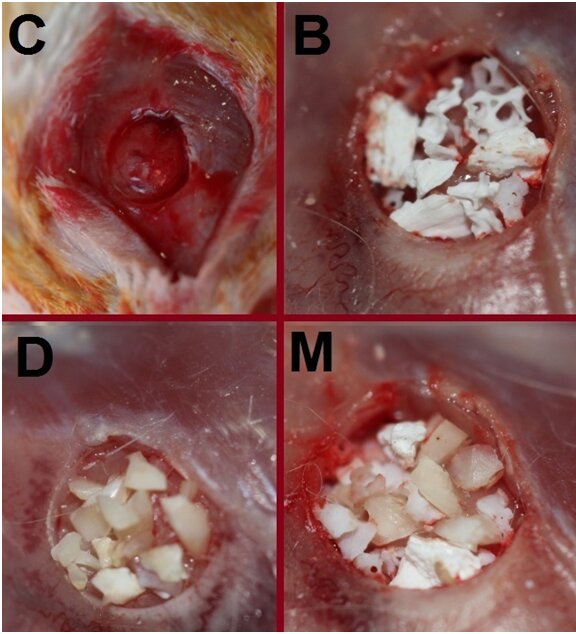
The animals were randomly divided into four groups of eight rats in each. Randomization sequence was created using Excel 2010 (Microsoft, Redmond, WA, USA) with a 1:4 allocation using random block size 4. Each animal underwent a surgery of creating a defect of a diameter of 5 mm in its calvarium. Different materials or no materials were placed in the defects as following: the first group received no bone graft material and was named the control (C) group. The second group received particulate dentine and was named the dentine (D) group. The third group received a mixture of dentine and Bio-Oss® and was named the mixture (M) group. The fourth group received only Bio-Oss® and was called the Bio-Oss® (B) group. The mixture group received an equal amount of Bio-Oss® and dentine by volume. Each group was randomly divided into two subgroups of four rats. The rats of the first subgroup were sacrificed at 4 weeks while the rats of the second subgroup were sacrificed at 8 weeks.
Particulate Dentine Preparation
Human extracted wisdom teeth were obtained from the oral and maxillofacial departments at JUST and the University of Jordan. Only sound extracted wisdom teeth were used in this study. The teeth were decoronated and root planed. Pulpal tissue was removed using stainless steel K files #10 and #15. The teeth were then autoclaved and pulverized by the means of mortar and pestle. The resulting dentine particles were re-sterilized in an autoclave and were ready to be used in the surgical procedure. Dentine particles were not demineralized because we wanted to mimic a clinical scenario where time is an important factor. Using a periodontal probe, only particles with a diameter of 1-2 mm were used in the study as this was the diameter of the Bio-Oss® particles we intended to use.
Surgical Procedure
Surgical procedures were performed in all rats in a period of two weeks. All surgical procedures were done under sterile conditions. The rats were anesthetized according to their weight with a mixture of ketamine (60 mg/kg) (10 % Ketasol; Richter Pharma AG, Wels, Austria) and Xylazine hydrochloride (10 mg/kg) (Rompuns, Bayer, Leverkusen, Germany). Using the aforementioned dose, some animals died either immediately or soon after anesthetic injection. Therefore, an adjustment was made by reducing the doses to 53 mg/kg for ketamine and 6.67 mg/kg for xylazine.
After the establishment of general anesthesia, the rat's head was shaved using an electric shaver. The skin was then disinfected with a povidone iodine solution (HiGeen Ltd Company, Hungary). Just before the incision in the skin was made, a 0.3-0.4 ml of local anesthesia was administered subcutaneously. Octocaine® (Lidocaine 2% and epinephrine 1:100,000, Novocol Pharmaceutical of Canada, Inc.) was administered along the sagittal area of the skin so that epinephrine helps in bleeding control. A skin incision around 25 mm long was done using a 15C blade from the mid-nasal bone area to the posterior nuchal line over the linea media. A cutaneous flap was raised, and the periosteum was incised and lifted bilaterally using a small periosteal elevator exposing the cranium.
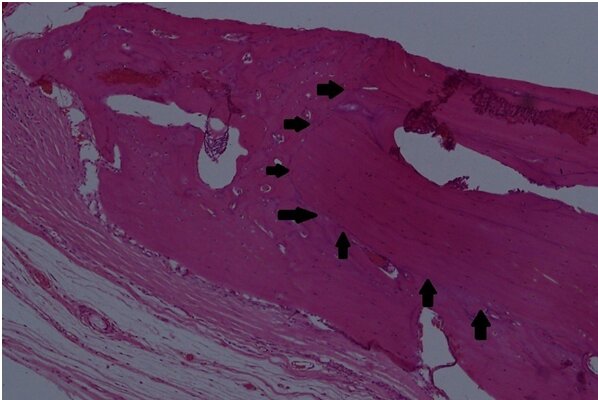
After the cranium was exposed, a 5-mm diameter defect was created using a trephine drill with a 5 mm internal diameter (Helmut Zepf, Seitingen-Oberflacht, Germany) in a slow-speed handpiece (NSK, Tohcigi, Japan) attached to a micromotor. A continuous saline irrigation was administered to prevent bone overheating. In order not to damage the brain, a small periosteal elevator was used to gently elevate the round bone segment that was cut by the trephine drill. The bone substitutes were then placed in the rats allocated in groups D, M or B. Bio-Oss® particles with a diameter of 1-2 mm were used in this study (Figure 1). 5-0 polyglycolic acid (PGA) sutures (PGA, ACUFIRM, Germany) were used to suture the periosteum. Skin closure was done using a 4-0 PGA sutures (PGA, ACUFIRM, Germany). The sutured area was again disinfected with povidone iodine solution. The rats were housed at room temperature and observed until they woke up and resumed their normal activities.
Sacrifice and Tissue Harvest
Based on their allocated subgroups, the rats were sacrificed by at either 4 or 8 weeks. The sacrifice was done using an overdose of diethyl ether. Subsequently, neck dislocation at the level of cervical vertebrae was done to make sure that the spinal cord was separated from the brain. The skin was dissected and the area of the previously created surgical defect was harvested along with the surrounding bone and soft tissues using bone scissors. The specimens were fixed in 10% formalin and were ready to be assessed.
Histological examination
The specimens were decalcified with 10 % hydrochloric acid for 1 week. The samples were then processed and paraffin-embedded for routine histological examination. The largest diameter was agreed upon by two examiners. The specimens were cut along the largest diameter and four sections of a thickness of 5 microns were prepared for each rat. The sections were stained with hematoxylin and eosin and were ready for examination under the light microscope.
Reduction of the largest diameter
A 27 mm reticle crosshairs eye piece micrometer (Nikon, Tokyo, Japan) was used to measure the widest diameter of the defect under light microscopy. The measurements were recorded and then compared to each other. The largest diameter was measured for all sections.
Amount of new bone formation
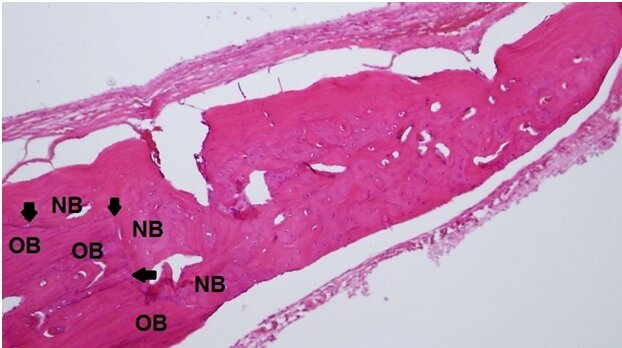
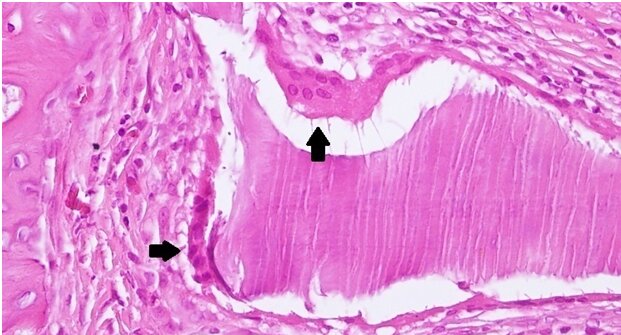
Under a x100 magnification, a random digital microscopic image was captured at one of the edges of the defect for each rat yielding four images per subgroup. Upon evaluation, new bone formation was determined to be the bone that shows a change in pattern from lamellar to woven bone and is located near the defect (Figure 2). Using the ImageJ software (NIH, Bethesda, MD), the area of new bone formation was calculated in squared pixels for each sample.
Degree of inflammation and foreign body reaction
Under a x 400 magnification, digital microscopic images of four randomly selected areas within the defect were captured for each rat using an Olympus DP20-5 camera mounted on an Olympus BX50 light microscope (Olympus Corporation, Tokyo, Japan). The presence of lymphocytes and plasma cells was considered representative of inflammation, with the higher number being interpreted as a higher degree of inflammation. An average of 30-60 inflammatory cells per slide was considered a low inflammation, an average of 60-90 inflammatory cells per slide was considered a moderate inflammation and an average over 90 cells per slide was considered representative of high inflammation. The demarcation between the groups was agreed upon after consulting a histopathologist. The same images were used to count giant cells for all specimens. The presence of giant cells was considered to be representative of foreign-body reaction with the higher number being interpreted as a higher degree of foreign-body reaction.
Statistical analysis
IBM SPSS Statistics 23.0 (SPSS Inc. Chicago, IL) was used for all statistical analyses. A two-way ANOVA analysis was used. Bonferroni test was used for multiple comparisons. Means and standard deviations were calculated. A p-value of less than 0.05 was considered statistically significant.
Results
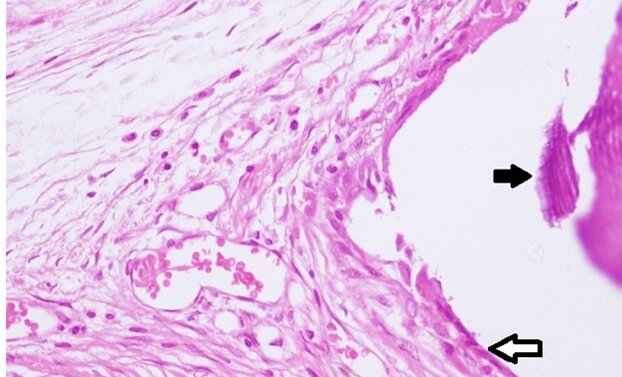
Generally, the defect size in all groups appeared smaller at 8 weeks compared to 4 weeks and the edges of the bony defects showed remodeling (i.e. woven bone with reversal lines and sometimes osteoblastic rimming). Sections from the (C) group at 4 weeks showed fibrous bridging of the body of the defect by moderately cellular fibro-collagenous connective tissue with mild to moderate infiltration by chronic inflammatory cells (lymphocytes and plasma cells) (Figure 3). At 8 weeks, (C) group showed less inflammatory cells and the defect appeared smaller.
For the (D) group, the defects showed fibrous bridging of the body of the defect at 4 weeks. The fibrous bridge contained chips of dentine surrounded by severe chronic granulomatous infiltration showing histiocytes, plasma cells and some multinucleated giant cells (Figure 4). At 8 weeks, the dentine chips appeared smaller as they were being resorbed. The inflammatory reaction was moderate to severe.
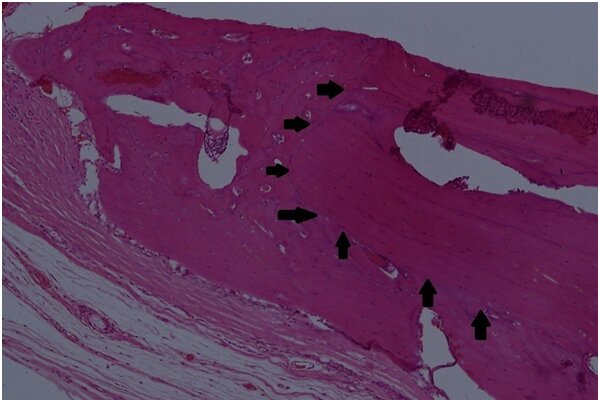
The (M) group showed evidence of fibrous bridging at 4 weeks. The fibrous bridge contained fragments of nonviable bone graft material in addition to dentine fragments. Both types of fragments were surrounded by chronic granulomatous inflammation of varying degrees ranging from moderate to severe. Inflammatory cells were higher in number around dentine than around Bio-Oss. Multinucleated giant cells were sometimes observed adjacent to bone and dentine fragments (Figure 5). At 8 weeks, the (M) group also showed more inflammatory cells around dentine than around Bio-Oss particles as for the (B) group, some Bio-Oss® particles appeared smaller than at 4 weeks indicating resorption.
Reduction in the largest diameter
Among different groups, no statistically significant difference in the largest diameter of the defect was found between any of the groups at 4 weeks. However, a statistically significant difference was found only between the mixture group (M) and the control group (C) at 8 weeks favoring the mixture group (Table 1). All groups showed a smaller largest diameter of the defect 8 weeks after the surgery than 4 weeks after it. However, only the dentine (D) and the mixture (M) groups showed a significant reduction in the largest diameter between 4 and 8 weeks (Table 1).
New bone formation
Among the rats that were sacrificed at 4 weeks, a significant difference favoring (B) group and the (M) group over the (C) group was found. The (D) group exhibited more new bone formation than the control (C) group. However, this difference was not statistically significant. Among the rats that were sacrificed at 8 weeks, a significant difference favoring all three test groups over the control (C) group was found. Bio-Oss showed the highest new bone formation at 8 weeks, however the difference between the group (B), (D) and (M) was not statistically significant. Among the same group of rats, all groups, including the control group, showed statistically significant increase in new bone formation at 8 weeks (Table 2). It is noteworthy to mention that new bone formation was found on the superior and inferior parts of the defects and not only towards the center (Figure 6).
Degree of inflammation
Among the rats that were sacrificed at 4 weeks, (C) and (B) groups showed significantly lower lymphocytic infiltrate than the (D) group. The (M) group showed higher number of inflammatory cells than the (C) and the (D) groups. However, the difference was not statistically significant. There was no significant difference in the number of lymphocytes between the (B) group and the (C) group either. Among the rats that were sacrificed at 8 weeks, control group (C) exhibited a significantly lower number of inflammatory cells than all other three groups including the Bio-Oss (B) group. The (B) group showed a significantly lower number of lymphocytes than the (M) and the (D) groups. The difference between the (D) group and the (M) group was not significant (Table 3). Among the same group of rats, only the control (C) group showed a significantly lower number of inflammatory cells at 8 weeks (Table 3).
Foreign-body reaction
Among the rats that were sacrificed at 4 weeks, control (C) group showed a significantly lower number of giant cells than all other three groups. The difference among the other three groups was not statistically significant. Among the rats that were sacrificed at 8 weeks, similar findings were found to those sacrificed at 4 weeks. Control (C) group showed a significantly lower number of giant cells than the other three groups. The difference among the other three groups was not statistically significant. Within the same groups, no statistically significant difference was found between 4 and 8 weeks in any of the groups.
Discussion
Bone regeneration surgeries are commonly performed by periodontists to regenerate defects caused by periodontal disease and enhance implant sites. One of the major drawbacks in bone regeneration surgeries is the additional cost as the patient needs to pay the price of a bone graft and a membrane. Finding a material that is cheaper but still shows clinically positive results would be of paramount importance.
Animal studies are usually conducted to investigate the effectiveness of bone regeneration materials. One of the commonly used methods to test bone regenerations in animals is the critical size defect (CSD) which is defined as a bone defect that does not heal totally through the lifetime of the animal unless an intervention is used [25] CSD was later redefined as a defect that does not heal within the duration of a study [26]. Inconsistent data were reported regarding the CSD in healthy rats with the proposed diameter of the defect varying from 4 mm to 8 mm [1,27-30]. However, most studies showed that a defect with a diameter of 5 mm is the CSD in healthy rats [28-30]. The rat calvarium allows for a reproducible defect that can be generated quickly and does not require fixation for stabilization of the skeleton as is generally required for femoral defects.
Regarding the materials used in the study, the largest reduction in the largest diameter of the defect was found in the mixture group at eight weeks followed by the dentine group. In terms of new bone formation, all test groups had significant new bone formation at 8 weeks compared to the control group. However, there was no statistically significant difference between dentine, Bio-Oss® and mixture groups in terms of reduction of the largest diameter or in terms of new bone formation.
Our results showed that Bio-Oss® had the highest amount of new bone formation, this was in agreement with previous studies which reported increased new bone formation around Bio-Oss® alone compared to Bio-Oss combinations with Plaster of Paris [31], particulate dentine [31] or Ceramic bone [32].
There was a significant difference in the reaction due to the presence of a foreign body over time, for all groups.
With small decreases. Which could indicate acceptance of the implants with the increase of the time in contact. Non-demineralized dentine was used in this study. Mordenfeld and colleagues (2011) [33] found new bone formation around demineralized xenograft dentine after one month while no bone formation was seen when using non-demineralized dentine grafts. On the other hand, Al-Asfour and colleagues (2013) [34] in 6 months follow up study showed that new bone formation does occur around non-demineralized dentine, but it requires longer time. Our study probably could show different results with longer follow up since non-demineralized dentin using long periods of time.
Mild inflammation was seen around Bio-Oss® in comparison with dentine and mixture groups. In fact, the lymphocytic infiltrate in the Bio-Oss® group was comparable to that of the control group at 4 weeks. This can be explained by the low antigenicity of the deproteinized bovine bone compared to the collagen-rich dentine. Our results come in agreement with a previous study [31] that found less inflammation in the rats that received Bio-Oss® as compared to those that received a mixture of particulate dentine and plaster of Paris. Moreover, Piatelli and colleagues (1999) [8] reported no inflammatory reaction to Bio-Oss® by humans at 6 months, 9 months and 18 months. We found a mild inflammation at 4 weeks in rats that was comparable to the control group. However, the inflammatory cells in the Bio-Oss® group were significantly higher than the control group at 8 weeks. Rokn et al. [32] reported that Bio-Oss® group elicited the second lowest inflammatory reaction, only second to the control group.
The higher level of inflammation seen around the dentine and mixture groups may have interfered with new bone formation at 4 weeks and 8 weeks as explained by Kamal and colleagues (2017) [35]. The mean lymphocytic infiltrate that was diminishing with time in all groups may indicate that a longer waiting time at 12 weeks or more may have resulted in lower inflammation and higher new bone formation [33-35].
Foreign body reaction was seen in all groups except the control group with no significant difference among them. This was in agreement with previous study [32] as they did not show any significant difference in terms of foreign body reaction among test groups. However, this is in disagreement with the results of previous study [31] that showed a lower giant cell (foreign-body reaction) to Bio-Oss® as compared to particulate dentine. Piatelli, and colleagues (1999) [8] found that giant cells were still adjacent to Bio-Oss® particles four years after implantation in humans. Our results showed no statistically significant reduction in the number of giant cells in any of the study groups.
Limitations
In this study we used human dentine with human proteins, and it was grafted in rats. Bone production comparison is going to be overshadowed by an immune response generated by the human proteins in a rat. Further studies using rat's dentine is recommended to negate or minimize the foreign protein reaction. Furthermore, it would have been more appropriate to analyze the efficiency of obtaining the particulate dentin with a size of 1-2 mm using only a spraying method such as mortar.
Future studies are needed to compare groups of demineralized and non-demineralized dentine particulates in terms of new bone formation and level of inflammation with larger number of rats and over a longer period.
Conclusions
Within the limitations of this study, we concluded that the inferiority of particulate dentine and a mixture of it with Bio-Oss® to Bio-Oss® alone in new bone formation is not statistically significant, particulate dentine showed significantly more inflammation than Bio-Oss® alone at 8 weeks and that there are no statistically significant differences among all three test groups in terms of foreign body reaction.
References
- Mulliken JB, Glowacki J (1980) Induced osteogenesis for repair and construction in the craniofacial region. Plast Reconstr Surg 65: 553-560.
- Greenwald AS, Boden SD, Goldberg VM, et al. (2001) Bone-graft substitutes: Facts, fictions, and applications. J Bone Joint Surg Am 83: 98-103.
- González-García R, Naval-Gías L, Rodríguez-Campo FJ, et al. (2008) Vascularized free fibular flap for the reconstruction of mandibular defects: Clinical experience in 42 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106: 191-202.
- Laurie SW, Kaban LB, Mulliken JB, et al (1984) Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg 73: 933-938.
- Pistilli R, Felice P, Piatelli M, et al. (2014) Blocks of autogenous bone versus xenografts for the rehabilitation of atrophic jaws with dental implants: preliminary data from a pilot randomised controlled trial. Eur J Oral Implantol 7: 153-171.
- Marco Montanari, Michele Callea, Izzet Yavuz, et al. (2013) A new biological approach to guided bone and tissue regeneration. BMJ Case Rep 9: bcr2012008240.
- Richardson C R, Mellonig J T, Brunsvold MA, et al. (1999) Clinical evaluation of Bio-Oss®: A bovine-derived xenograft for the treatment of periodontal osseous defects in humans. J Clin Periodontol 26: 421-428.
- Piatelli M, Favero GA, Scarano A, et al. (1999) Bone reactions to an organic bovine bone (Bio-Oss) used in sinus augmentation procedures: A histologic long-term report of 20 cases in humans. Int J Oral Max Impl 14: 835-840.
- Berkovitz B K, G Holland, Bernard Moxham (2017) In: Oral Anatomy, Histology and Embryology (5th edn). Elsevier Health Sciences Chapter 9.
- Gomes MF, Abreu PD, Morosolli AC, et al. (2006) Densitometric analysis of the autogenous demineralized dentin matrix on the dental socket wound healing process in humans. Braz Oral Res 20: 324-330.
- Yeomans JD, Urist MR (1967) Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch Oral Biol 12: 999-1008.
- Ritchie HH, Ritchie DG, Wang LH (1998) Six decades of dentinogenesis research: Historical and prospective views on phosphophoryn and dentin sialoprotein. Eur J Oral Sci 106(Suppl 1): 211-220.
- Park SM, Um IW, Kim YK, et al. (2012) Clinical application of auto-tooth bone graft material. J Korean Assoc Oral Maxillofac Surg 38: 2-8.
- Kima SG, Yeoa HH, Kimb YK (1999) Grafting of large defects of the jaws with a particulate dentin-plaster of Paris combination. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 88: 22-5.
- Jeong KI, Kim SG, Kim YK, et al. (2011) Clinical study of graft materials using autogenous teeth in maxillary sinus augmentation. Implant Dent 20: 471-475.
- Kim YK, Kim SG, Kim KW, et al. (2011) Extraction socket preservation and reconstruction using autogenous tooth bone graft: Case report. Plast Reconstr Surg 33: 264-269.
- Kim YK (1996) The experimental study of the implantation of toothash and plaster of Paris and guided tissue regeneration using Lyodura. J Korean Assoc Oral 22: 297-306.
- Andersson L, Ramzi A, Joseph B (2009) Studies on dentin grafts to bone defects in rabbit tibia and mandible; development of an experimental model. Dent Traumatol 25: 78-83.
- Kim YK, Kim SG, Lee JH (2001) Cytotoxicity and hypersensitivity test of tooth ash JKAMPRS 23: 391-395.
- Koga T, Minamizato T, Kawai Y, et al. (2016) Bone regeneration using dentin matrix depends on the degree of demineralization and particle size. PloS one 11: e0147235.
- Yagihashi K, Miyazawa K, Togari K, et al. (2009) Demineralized dentin matrix acts as a scaffold for repair of articular cartilage defects. Calcified Tissue Int 84: 210-220.
- Chun SY, Acharya B, Lee HJ, et al. (2011) Composite scaffold with demineralized dentin particle and poly (lactic co-glycolic acid) for cranial bone regeneration. J Tissue Eng Regen M 8: 306-313.
- Al-Asfour A, Farzad P, Al-Musawi A, et al. (2017) Demineralized xenogeneic dentin and autogenous bone as Onlay grafts to rabbit tibia. Implant Dent 26: 232-237.
- Ku HR, Jang HS, Kim SG, et al. (2007) Guided tissue regeneration of the mixture of human tooth-ash and Plaster of Paris in dogs. Key Eng Mater 330-332: 1327-1330.
- Schmitz J P, Hollinger J O (1986) The critical size defect as an experimental model for cranio mandibulo facial nonunions. Clin Orthop Relat Res 205: 299-308.
- Gosain AK, Santoro TD, Song LS, et al. (2003) Osteogenesis in calvarial defects: Contribution of the dura, the pericranium, and the surrounding bone in adult versus infant animals. Plast Reconstr Surg 112 :515-527.
- Takagi K, Urist M R (1982) The reaction of the dura to bone morphogenetic protein (BMP) in repair of skull defects. Ann Surg 196: 100-109.
- Artz Z, Kozlovsky A, Nemcovsky CE, et al. (2008) Histomorphometric evaluation of natural mineral combined with a synthetic cell-binding peptide (P-15) in critical-size defects in the rat calvaria. Int J Oral Max Impl 23: 1063-1070.
- Donos N, Graziani F, Mardas N, et al. (2011) The use of human hypertrophic chondrocytes-derived extracellular matrix for the treatment of critical-size calvarial defects Clin Oral Implants Res 22: 1346-1353.
- Vajgel A, Mardas N, Farias BC Petrie A, et al. (2014) A systematic review on the critical size defect model. Clin Oral Implants Res 25: 879-893.
- Su-Gwan K, Hak-Kyun K, Sung-Chul L (2001) Combined implantation of particulate dentine, plaster of Paris, and a bone xenograft (Bio-Oss®) for bone regeneration in rats. J Craniomaxillofac Surg 29: 282-288.
- Rokn AR, Khodadoostan MA, Ghahroudi AARR, et al. (2011) Bone formation with two types of grafting materials: A histologic and histomorphometric study Open Dent J 5: 96-104.
- Mordenfeld A, Hallman M, Lindskog S (2011) Tissue reactions to subperiosteal onlays of demineralized xenogenous dentin blocks in rats. Dent Traumatol 27: 446-451.
- Al-Asfour A, Farzad P, Al-Musawi A, et al. (2017) Demineralized xenogeneic dentin and autogenous bone as onlay grafts to rabbit tibia. Implant Dent 26: 232-237.
- Kamal M, Andersson L, Tolba R, et al. (2017) Bone regeneration using composite non-demineralized xenogenic dentin with beta-tricalcium phosphate in experimental alveolar cleft repair in a rabbit model. J Transl Med 15: 263.
Corresponding Author
Dr. Rola Al Habashneh, Department of Preventive Dentistry, Faculty of Dentistry, Jordan University of Science & Technology, Irbid 22110, Jordan.
Copyright
© 2021 Habashneh RA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.