One-Stage Primary Palatoplasty in Non-Syndromic Isolated Cleft Palate - Surgical Technique and Morbidity Evaluation
Abstract
Initial repair of a cleft palate, a procedure often performed in cleft centers all over the world, belongs to the basic skills of a cleft surgeon. The aim of this study was to present the surgical technique of our one-stage method of palatoplasty performed during the second half of the first year of life in patients suffering from isolated cleft palate and to evaluate it's short and long term morbidity. The study included 120 non-syndromic patients 41 (34.2%) males and 79 (65.8%) females operated on according to the same method within a time span of 24 months. The assessment was based on medical documentation recorded up to the 10th year of life. The following mean values were calculated in regard to the moment of the procedure: age was 8.6 months, duration of surgery was 66 minutes (range was 30-135), and length of hospitalization following operation was 2.6 days (range 1-8). The incidence of severe velopharyngeal insufficiency, oronasal fistulas and persistent otitis media with effusion requiring surgical intervention was 2.5%, 3.3% and 18.3%, respectively. Two patients had surgical wound dehiscence, one of them revealed upper airway infection. It is worth pointing out that the presented surgical technique, apart from being relatively save, is simple, not time consuming and does not require any special equipment, whatsoever.
Keywords
Isolated cleft palate, One-stage palatoplasty, Surgical technique, Morbidity
Abbreviations
VPI: Velopharyngeal Insufficiency; OME: Otitis Media with Effusion
Introduction
The treatment of patients with a cleft palate requires the involvement of several specialists such as pediatric surgeons, otolaryngologists, orthodontists, speech-language therapists and maxillofacial surgeons. It seems that the most critical element of treatment is the initial surgical repair of a cleft palate called primary palatoplasty because it influences feeding, hearing, breathing, speech and maxillary development. The procedure was first described in 1764 by a French dentist, LeMonnier [1]. During its long history, the surgical technique of palatoplasty has been developed and different protocols have been applied. At this time, it is appropriate to mention the contribution of great and well-known surgeons, such as Bernhard von Langenbeck, Theodor Billroth, Victor Veau, Janusz Bardach, Fenton Braithwaite, Otto Kriens.
Some ideas regarding surgical treatment of clefts evolved overtime, while others, which were once promising, did not appear to be as successful as previously expected and were finally abandoned. In fact, the efficacy of cleft surgery evolves as well and consequently, older reports about the subject are not fully relevant and up-to-date anymore. Our surgical protocol of treatment of patients suffering from isolated cleft palate is composed of the primary palatoplasty according to one-stage method performed during the second half of the first year of life. The simplicity of this method and the satisfactory results that we can observe in our everyday clinical practice encouraged us to present it. The aim of the study was to present our one-stage surgical technique of palatoplasty of isolated cleft palate and to evaluate its short and long term morbidity. The treatment outcome based on craniofacial development, dental arch relationship and speech evaluation will be the subject of the separate article to be followed.
Materials and Methods
Patients
This retrospective study evaluated data of all non-syndromic patients who suffered from isolated cleft palate and consecutively underwent primary palatoplasty about 10 years ago at our center. According to the surgical protocol applied at our department, it is recommended that isolated cleft palates be operated by the one-stage method in the second half of the first year of life. Consequently, patients who were operated at a different age did not meet the inclusion criteria, just like those whose palatoplasty was secondary or whose medical record was incomplete.
Surgical technique
Preoperative antibiotics were given to all patients intravenously and continued for 1 day postoperatively. Ceftriaxone (50 g per 1 kg of body weight) was routinely administered.
Although the evaluated palatoplasty was performed at that time by 5 surgeons belonging to our cleft team, all patients were operated on according to the same surgical technique standard and any differences resulted only from the individual extent of the palatal cleft defect.
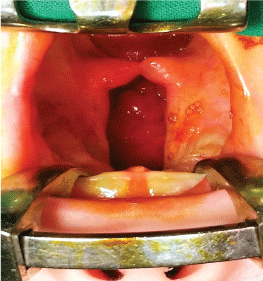
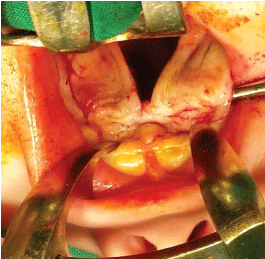
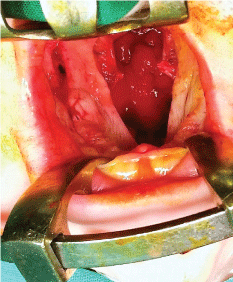
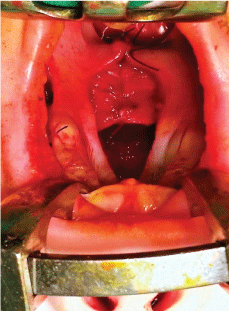
After meticulous infiltration of the tissues to be operated on with local anesthetic (Figure 1), the incisions are made on the border between the oral and nasal mucosa and along the cleft fissure in the area of the soft and hard palate. It is worth mentioning that deeper dissection of the palatal muscles should be avoided as much as possible in order to minimize damage to the delicate muscle tissue which is needed later on during speech rehabilitation and therapy. Additionally, two short lateral incisions are made on the palatal surface at the base and behind the alveolar process of the maxilla. These lateral incisions are here mainly for obtaining a better approach. The palatal periosteum is dissected (Figure 2), the palatal neurovascular bundles are gradually elongated and the bipedicle mucoperiosteal palatal flaps are elevated and mobilized (Figure 3). The pterygoid hamuli of the sphenoid bone, around which tensor veli palatini muscles run, can be easily approached through the posterior portion of the lateral incisions and gently broken in order to release tension in the transversal plane. That maneuver helps to gain the anatomical continuity of tensor veli palatini muscle during its suturing at the following stage of operation.
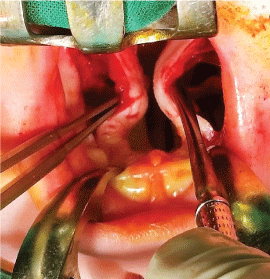
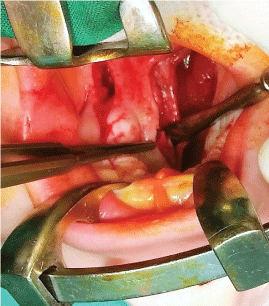
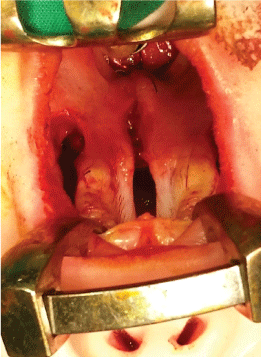
As a consequence of cleft malformation, the fibers of the palatal muscles are separated and pathologically attached to the posterior and medial bone edges of the horizontal palatal laminas, instead of being joined together in the midline. That is why transection of the soft palate aponeurosis (Figure 4) is performed on both sides, which results in the release and automatic posterior repositioning of the soft palate muscles by 8-12 mm on average (Figure 5). These muscles are sutured together in the midline during the next stage of the procedure, granting them more transversal direction. At the same time, the soft palate mucosa is easily stretched out in the posterior direction, which enables an approximation of the posterior edge of the reconstructed soft palate towards the pharynx in accordance with the natural function of the palatal muscles.
The edges of the nasal mucosa in the region of the hard palate are sutured together to reconstruct the floor of the nasal cavity bilaterally in grades 3 and 4 of palatal cleft according to Jensen, et al. classification [2]. Apparently, this is not performed in cases of incomplete cleft palates with preserved anatomical continuity of the nasal cavity.
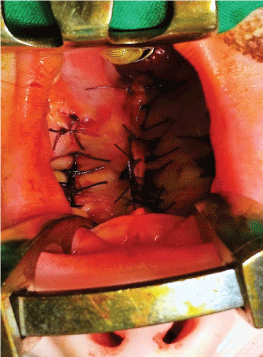
Next, the soft palate is sutured layer by layer - nasal mucosa, palatal muscles and oral mucosa (Figure 6 and Figure 7) along the medial edges of the palatal flaps in the region of the hard palate. Towards the end, the lateral incisions are sutured as closely as possible in order to minimize scar formation (Figure 8).
Methodology
The source of information about consecutively performed procedures which took place in the Operating Theatre of our center was the Theatre Register. The obtained data was verified, completed and duly extended according to the individual medical record of each patient. The following variables were recorded: gender, diagnosis, cleft type and its extent, patient's age at the operation, operation duration, and additional procedures performed together with palatoplasty, length of hospitalization following palatoplasty, any postoperative complications reported at follow-up appointments and any secondary surgical repairs if performed over the following 10 years. A special attention was payed to the information about the potential need for Velopharyngeal Insufficiency (VPI) repairs, ear ventilation tube placements, any orthodontic conditions requiring subsequent surgical intervention.
Additionally, the medical record was reviewed in regard of orthodontic, speech language, phoniatric, and otology and audiology appointments. The speech examination apart from clinical examination always included perceptual speech evaluation based on standardized speech test appropriate for the Polish language inventory which was specially designed for the purposes of the assessment of cleft palate patients [3]. Thus, the collected documentation obligatorily included information about articulation, resonance, intelligibility, voice disorders, audible nasal emission, and compensatory facial grimaces. Additionally, as part of speech documentation in our center the video recordings are made routinely at the age of 5, 10 and 15 years. In cases with the signs of VPI, the examination was deepened by phoniatric evaluation on the basis of nasofiberoscopy.
All calculations were performed using Microsoft Excel 97-2003 software.
Results
There were 216 primary palatoplasties of isolated cleft palate performed in a time span of 24 months (from January 3rd 2005 to December 28th 2006). The selection process excluded 34 syndromic cases, 53 cases operated at different ages other than those recommended and 9 cases with incomplete or missing medical documentation. Finally, 120 cases 41 males (34.2%) and 79 females (65.8%) operated on during the second half of the first year of life were included into the study group for further evaluation.
Detailed information which was obtained after evaluating the material is presented in Table 1. The mean duration of surgery of the primary palatoplasty in isolated cleft palate was estimated to be 66 minutes and ranged significantly from 30 to 135 minutes. However, it should be pointed out that some additional procedures were performed apart from palatoplasty during that time as well, for example upper lip frenuloplasty in 22 cases, tongue frenuloplasty in 9 cases, excision of hard palate polyp in 1 case and skin mole excision in 1 case. There was not any special equipment like surgical microscope or loupes used during any of the operations in the study group whatsoever.
The incidence of the registered complications within the collected material is presented in Table 2. The material included three patients with persistent VPI following primary palatoplasty which persisted for 2 years of observation, despite subsequent intensive speech therapy that was focused on improvement of velopharyngeal sphincter function. As a consequence, in these cases, secondary pharyngoplasty was applied as the method of choice, obligatory before the 6th year of life - upper pedicled flap pharyngoplasty in two cases and sphincter pharyngoplasty in one case. Decision about the procedure has always been made by a surgeon, speech pathologist and phoniatrician on the basis of nasofiberoscopy. Three patients in the study group were affected by oronasal fistula formation and two others by surgical wound dehiscence. All these patients underwent secondary palatal repairs by the 3rd year of age.
The prophylactic ear ventilation tube placement was not applied in the study group, instead this procedure was performed when the persistent symptoms of Otitis Media with Effusion (OME) appeared. That was reported in 22 children in the study group.
There have been no needs for blood transfusion as a result of excessive bleeding during intra or postoperative period nor have there been any postoperative wound infection requiring prolonged antibiotic therapy in this series of patients. The study did not reveal any of general complications such as respiratory failure or pneumonia.
Discussion
The presented procedure of palatoplasty of isolated cleft palate should be part of the basic skills in the armamentarium of a cleft surgeon; it is simple, time efficient and does not require any special equipment, whatsoever. The presented surgical technique became a method of choice in cases of isolated cleft palate in our center. Currently, we operate over one hundred cases of isolated cleft palate per year.
The efficacy of palatoplasty and its morbidity may rely on several factors. It has been well documented in the literature that even the smallest details of a surgical technique are able to influence the palatoplasty results [4-6]. On the other hand, the same surgical method applied in different types of cleft defects might result in various outcomes [7,8]. In order to ensure the most reliable evaluation results, all efforts were made to obtain as homogenous a study group as possible. The surgical technique of palatoplasty to treat isolated cleft palate differs slightly from that in unilateral or bilateral cleft lip and palate due to the different anatomical extend of these defects. That is why the present study is based on consecutive primary palatoplasties in isolated cleft palates exclusively, and performed at the same age according to the same surgical technique, regardless the preoperative cleft anatomy. The principle of collecting data regarding cases operated on by a single surgeon, often mentioned in the literature is pointless in regard to the multimember cleft team, and should be applied only to small centers where there is only one specialist designated for cleft surgery; otherwise, a risk of a bias coming from the lack of random selection of patients operated on equally by each surgeon in the team may appear. Naturally, the most difficult cases, including syndromic clefts, are referred to the most experienced member of a team, while the least difficult to the beginners. Therefore, all patients operated on in our operating theater were included in the study. The same surgical technique carried out by all members of the operating team who adhered to the same standard contributes to better homogeneity of the presented material.
The need for speech-related surgical procedures following primary palatoplasty in isolated cleft palate in the literature varies significantly. It seems that with time some progress might be observed, based on the results that have been reported. Inman and coworkers noted that 29.2% of patients with isolated cleft palate require subsequent pharyngoplasty [8]. More recently published rates of pharyngoplasty requirements have been 19.4% [9], 20.2% [6] and 12.1% [10]. It was also indicated that the quality of speech is significantly dependent on the age at primary palatal repair [11,12]. The evaluation, performed about 10 years after the primary palatoplasty, seems to be quite reliable as it was proven that speech does not become stable before 10 years of age and that patients with cleft palate should be carefully followed until they are beyond this age [13].
The incidence of fistulas following palatoplasty inisolated cleft palates described in the literature similarly varies greatly and is indicated as follows: 4.7% [8], 5.4% [14], 6.6% [15], 9.6% [10], 15.2% [16] or even 21% [17] of cases. This complication was proven to more likely occur in cases of combined cleft lip and palate than cleft palate alone [10,14,15]. A meta-analysis, which included 11 published studies occurring between 2000-2012 and comprising 2505 cases, indicated the incidence of oronasal fistulas following primary palatal surgery as being around 4.9% [18]. Therefore, the presented method of primary palatoplasty can be deemed as successful since it required subsequent correction by secondary pharyngoplasty in barely 2.5% of cases and was complicated by oronasal fistula formation in 3.3% of cases.
OME is caused by Eustachian tube dysfunction [19] of which the cause is multifactorial in patients with cleft palate [20]. Therefore OME is not stricto senso the complication of palatoplasty. However, palatoplasty is documented to decrease the frequency of middle ear disease and to improve hearing and Eustachian tube function compared with untreated cleft palate [21-23] and from that point of view the incidence of OME can be perceived as the measure of palatoplasty efficacy. Although, OME is a very common finding in children with cleft palate [24], it was proven that the tendency to develop OME is reduced by the early term of palatal surgery (between 6 months and 1 year of age) [25]. Surprisingly low incidence of OME referred for ear ventilation tube placement in the presented material (even with certain reservations about the objectivity of indications for such referral) seems to confirm that opinion.
Finally, one needs to mention here that although maxillary development is far from being completed at the 10th year of life, nevertheless no patients in the study group were judged to have developed maxillary deformity attributable to the primary palatoplasty.
In conclusion, the morbidity evaluation in the study group points out that the presented treatment method is relatively save. Moreover, it does not require any special equipment and allows the palatoplasty to be completed, on average, in only 66 minutes; which together with a short length of hospitalization following the operation (around 2-3 days) contributes to its high cost efficiency.
Funding
The study was performed as part of a major statutory project of Department of Pediatric Surgery, Institute of Mother and Child in Warsaw, Poland.
Competing Interests
The authors declare no conflicts of interest.
Ethical Approval
The study was approved by the Bioethics Committee of the Institute of Mother and Child in Warsaw (number 31/2016).
Patient Consent
The informed consent of legal guardians of all patients was obtained. All research on enrolled participants has been conducted according to the principles expressed in the Declaration of Helsinki.
Acknowledgement
All authors have viewed and agreed to the submission of this article.
References
- Iyer VS (1967) Isolated cleft palate. Cleft Palate J 4: 124-128.
- Jensen BL, Kreiborg S, Dahl E, et al. (1988) Cleft lip and palate in Denmark, 19760-1981: Epidemiology, variability, and early somatic development. Cleft Palate J 25: 258-269.
- Zdunkiewicz-Jedynak D, Hortis-Dzierzbicka M (2000) Linguistic basis of assessment and documentation of speech disorders in children with cleft lip and/or palate. Warsaw University Publishing, Warsaw.
- Chen Q, Zheng Q, Shi B, et al. (2011) Study of relationship between clinical factors and velopharyngeal closure in cleft palate patients. J Res Med Sci 16: 945-950.
- Becker M, Hansson E (2013) Low rate of fistula formation after Sommerlad palatoplasty with or without lateral incisions: an analysis of risk factors for formation of fistulas after palatoplasty. J Plast Reconstr Aesthet Surg 66: 697-703.
- Lithovius RH, Ylikontiola LP, Sándor GK (2014) Frequency of pharyngoplasty after primary repair of cleft palate in northern Finland. Oral Surg Oral Med Oral Pathol Oral Radiol 117: 430-434.
- Bicknell S, McFadden LR, Curran JB (2002) Frequency of pharyngoplasty after primary repair of cleft palate. J Can Dent Assoc 68: 688-692.
- Inman DS, Thomas P, Hodgkinson PD, et al. (2005) Oro-nasal fistula development and velopharyngeal insufficiency following primary cleft palate surgery- an audit of 148 children born between 1985 and 1997. Br J Plast Surg 58: 1051-1054.
- Andersson EM, Sandvik L, Tørdal IB, et al. (2010) Pharyngoplasty after primary repair of clefts of the secondary palate. Scand J Plast Reconstr Surg Hand Surg 44: 26¨-30.
- Ha S, Koh KS, Moon H, et al. (2015) Clinical outcomes of primary palatal surgery in children with nonsyndromic cleft palate with and without lip. BioMed Research International 2015: 185459.
- Haapanen ML, Rantala SL (1992) Correlation between the age at repair and speech outcome in patients with isolated cleft palate. Scand J Plast Reconstr Surg Hand Surg 26: 71-78.
- Marrinan EM, LaBrie RA, Mulliken JB (1998) Velopharyngeal function in nonsyndromic cleft palate: Relevance of surgical technique, age at repair, cleft type. Cleft Palate Craniofac J 35: 95-100.
- Park S, Saso Y, Ito O, et al. (2000) The outcome of long-term follow-up after palatoplasty. Plast Reconstr Surg 105: 12-17.
- Hardwicke JT, Landini G, Richard BM (2014) Fistula incidence after primary cleft palate repair: A systematic review of the literature. Plast Reconstr Surg 134: 618e-627e.
- Lithovius RH, Ylikontiola LP, Sándor GK (2014) Incidence of palatal fistula formation after primary palatoplasty in northern Finland. Oral Surg Oral Med Oral Pathol Oral Radiol 118: 632-636.
- Becker M, Svensson H, Sarnäs KV, et al. (2000) Von Langenbeck or Wardill procedures for primary palatal repair in patients with isolated cleft palate - speech results. Scand J Plast Reconstr Surg Hand Surg 34: 27-32.
- Lu Y, Shi B, Zheng Q, et al. (2010) Incidence of palatal fistula after palatoplasty with levator veli palatini retropositioning according to Sommerlad. Br J Oral Maxillofac Surg 48: 637-640.
- Bykowski MR, Naran S, Winger DG, et al. (2015) The rate of oronasal fistula following primary cleft palate surgery: A meta-analysis. Cleft Palate Craniofac J 52: e81-e87.
- Braganza RA, Kearns DB, Burton DM, et al. (1991) Closure of the softpalate for persistent otorrhea after placement of pressure equalization tubes in cleft palate infants. Cleft Palate Craniofac J 28: 305-307.
- Smith LK, Gubbels SP, MacArthur CJ, et al. (2008) The effect of the palatoplasty method on the frequency of ear tube placement. Arch Otolaryngol Head Neck Surg 134: 1085-1089.
- Smith TL, DiRuggiero DC, Jones KR (1994) Recovery of eustachian tube function and hearing outcome in patients with cleft palate. Otolaryngol Head Neck Surg 111: 423-429.
- Arnold WH, Nohadani N, Koch KH (2005) Morphology of the auditory tube and palatal muscles in a case of bilateral cleft palate. Cleft Palate Craniofac J 42: 197-201.
- Bluestone CD, Beery QC, Cantekin EI, et al. (1975) Eustachian tube ventilator function in relation to cleft palate. Annals of Otology, Rhinology & Laryngology 84: 333-338.
- Lithovius RH, Lehtonen V, Autio TJ, et al. (2015) The association of cleft severity and cleft palate repair technique on hearing outcomes in children in northern Finland. J Craniomaxillofac Surg 43: 1863-1867.
- Kuşcu O, Gşnaydın RÖ, İcen M, et al. (2015) The effect of early routine grommet insertion on management of otitis media with effusion in children with cleft palate. J Craniomaxillofac Surg 43: 2112-2115.
Corresponding Author
Andrzej Brudnicki, Department of Maxillo-facial Surgery, Institute of Mother and Child, Kasprzaka Street 17a, 01-211 Warsaw, Poland, Tel: 0048223277110.
Copyright
© 2017 Brudnicki A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.