Prediction of Retinal Ischemia with Optical Coherence Tomography Angiography in Branch Retinal Vein Occlusion
Abstract
Herein, we aimed to assess the ability of optical coherence tomography angiography (OCT-A) to show ischemic and non-ischemic differentiation in two patients with ischemic and non-ischemic branch retinal vein occlusion (BRVO). Two patients aged 67 and 39 presented to our hospital with an acute vision loss. Fundus Fluorescein Angiography (FFA) revealed non-ischemic inferior temporal BRVO in the first case ((67-year-old) and ischemic inferior temporal BRVO in the second case (39-year-old). Ischemic case showed similar retinal non-perfusion areas on OCT-A compared to FFA images. The OCT-A is a promising method in evaluating non-ischemic, ischemic differentiation and complication management of BRVO.
Keywords
Optical coherence tomography angiography, Branch retinal vein occlusion, Retinal ischemia
Introduction
Retinal vein occlusions are the second most common cause of visual impairment in the retinal vascular disease [1]. The prevalence in the community varies between 0.3% and 1.6% [1-4]. Branch retinal vein occlusions (BRVO) are observed in arteriovenous crossing areas, and the macular edema and retinal ischemia are the main complications [2,5]. Although Fundus Fluorescein Angiography (FFA) is still the gold standard method for differential diagnosis of the ischemic and non-ischemic BRVOs, it is an invasive examination and side effects such as fluorescein-related hypersensitivity reactions and renal dysfunction may occur [6].
Optical Coherence Tomography Angiography (OCT-A) is a new method that refers to the intravascular erythrocyte movements to view the retinal vascular network non-invasively. Unlike FFA, since any contrast material is not used, there are no side effects and pathologies such as ischemia and neovascularization can be indicated [7-10].
In this case report, we aimed to compare the findings of FFA and OCT-A in two patients with ischemic and non-ischemic BRVO, and to show that ischemic and non-ischemic differentiation can be determined by both methods and that OCT-A can be used instead of FFA in this group of patients.
Case Report
Case 1
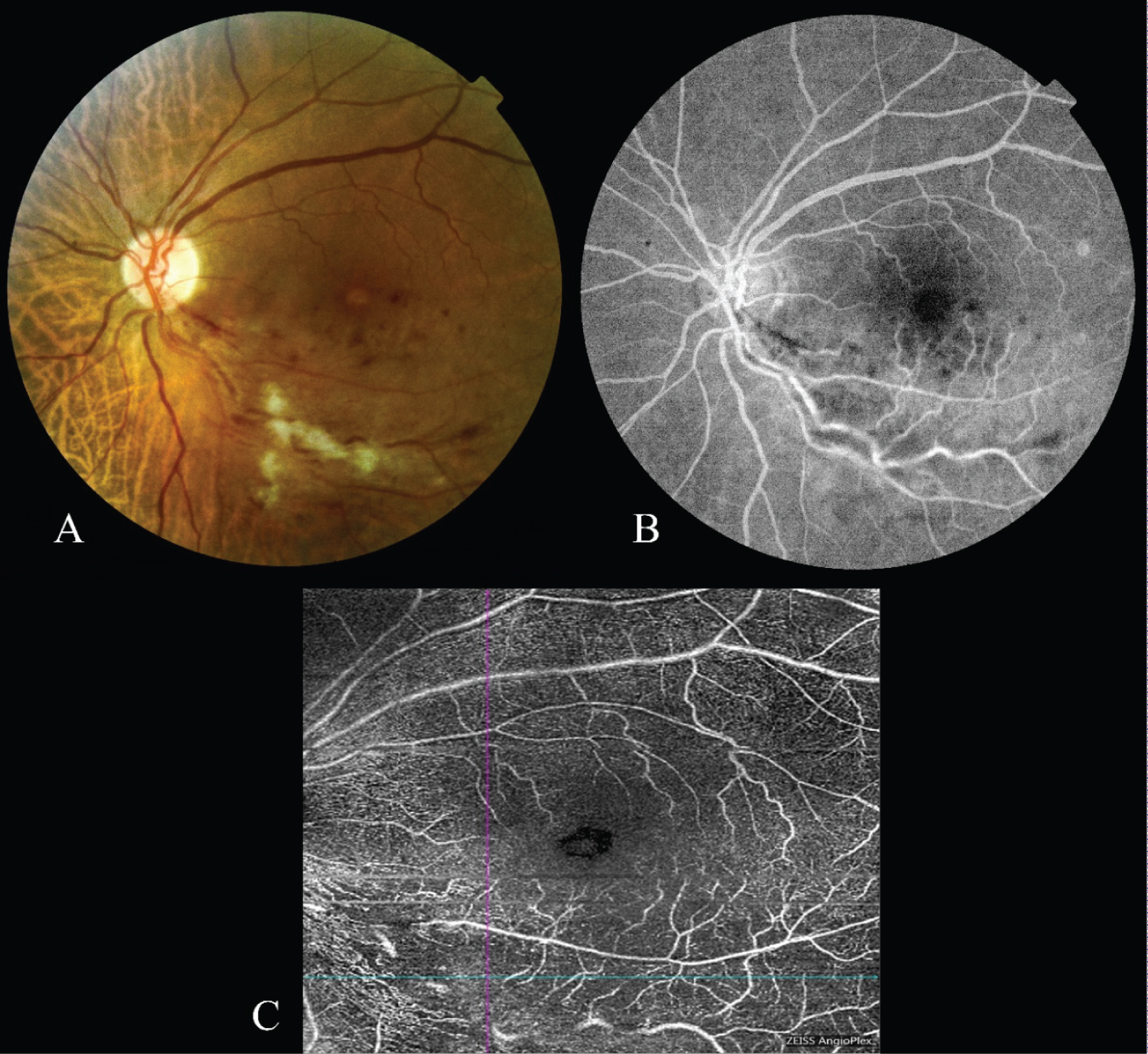
A 67-year-old female patient was admitted to our hospital with the complaint of low vision in the left eye. From the history of the patient, it was learned that she had amplyopia in the right eye and decreased vision in the left eye for a week. On the ophthalmological examination, the best corrected visual acuity in the right eye was counting fingers from one meter and 0.05 decimal in the left eye. In the fundoscopic examination of the patient whose biomicroscopic examination and intraocular pressures were normal, a tilted disc was observed in the right eye; Flame hemorrhages, soft exudates and macular edema were observed in the lower temporal retinal region of the left eye (Figure 1). A delay in venous filling, a blockage in bleeding areas, and a leakage in the late period was observed in FFA. Any ischemia was not observed in the lower temporal branch vein region. In OCT-A (Cirrus 5000 with AngioPlex, Carl Zeiss, Germany), no ischemia was observed in the lower temporal branch vein area in the superficial plexus (Figure 1). A single dose of aflibercept was administered to the patient as a treatment. At the first month follow-up, the vision was 0.4 decimal in the left eye.
Case 2
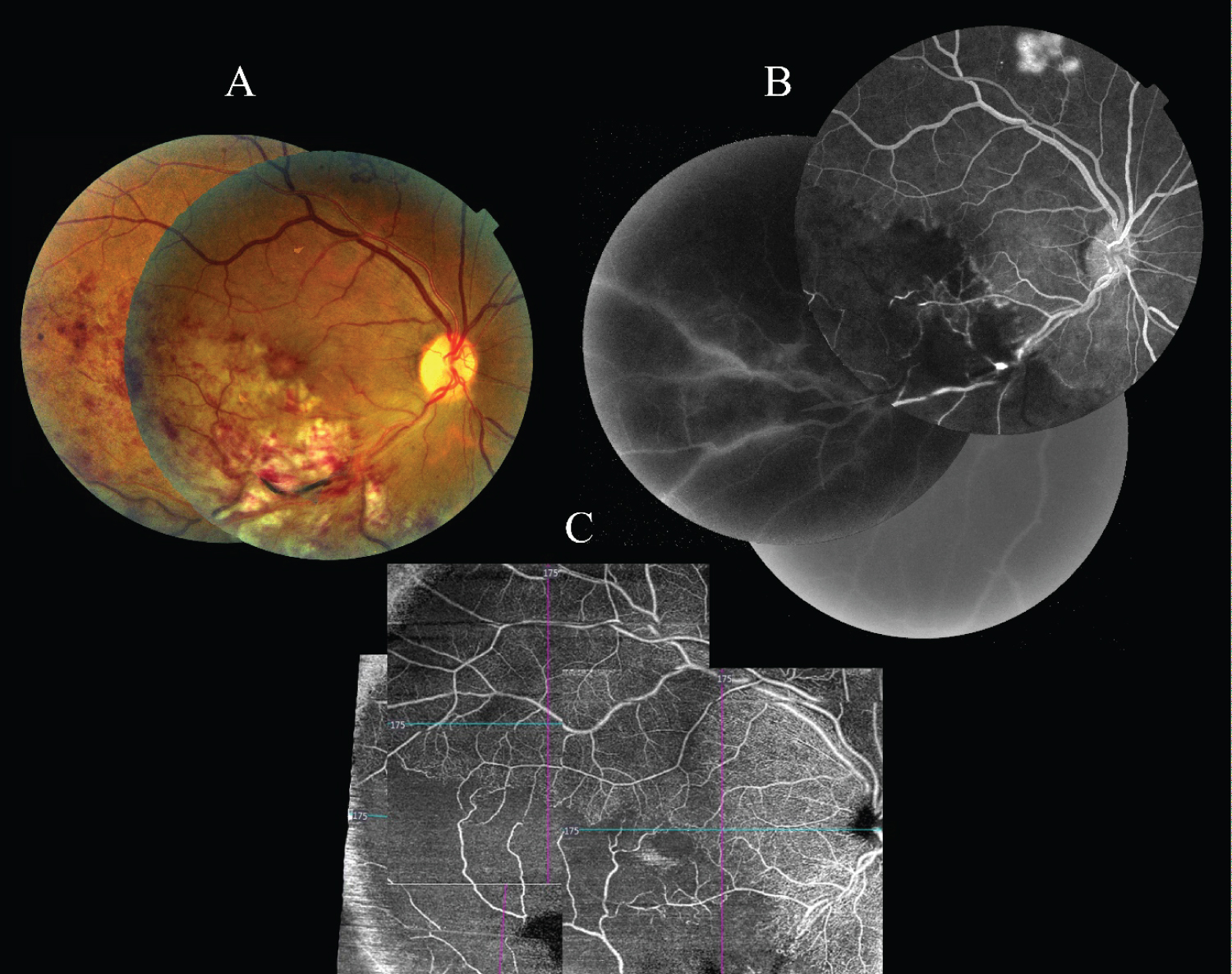
A 39-year-old female patient was admitted to our hospital with the complaint of low vision in the right eye. On the ophthalmological examination, the best corrected visual acuity was counting fingers from one meter in the right eye and 0.9 decimal in the left eye. Biomicroscopic examination and intraocular pressures were normal. Fundus examination revealed the flame hemorrhages, soft exudates and macular edema in the lower temporal region of the right eye (Figure 2). In FFA, a delay in venous filling, a blockage in hemorrhage areas, a decrease in the capillary perfusion, and an ischemia in the lower temporal branch vein region were observed. In OCT-A images (Cirrus 5000 with AngioPlex, Carl Zeiss, Germany), ischemic areas were also observed in the lower temporal branch vein region in the superficial plexus. Comparing the FFA and OCT-A images; It was observed that the ischemic areas were exactly comparable (Figure 2). Anti-VEGF injection was recommended to the patient as a treatment.
Discussion
Retinal ischemia can be observed in many retinal vascular diseases such as the diabetic retinopathy, retinal vein occlusion, retinal artery occlusion, and hypertensive retinopathy [11]. Demonstrating the presence of ischemia is crucial in determining the treatment protocols. FFA is the gold standard method currently used to demonstrate the ischemia. However, serious side effects such as anaphylaxis and mortality can occur due to both being time-consuming and the contrast agent used [6]. In this case report, we compared the findings of FFA and OCT-A in two patients with ischemic and non-ischemic BRVO. We found out that ischemia developing in the BRVO can be detected by using OCT-A.
Angiography is part of the retinal vein occlusions' (RVO) medical work-up. The key objective of these tests is to show the ischemic retinal region. The normal procedure, FFA, gold standard method, will display ischemic regions in each central and peripheral retina, and the proposed images have been utilized in both prognostication and treatment decisions.
OCT-A is a new method that is obtained by combining the successive vertical plan Optical Coherence Tomography images of the retina, visualizing the retinal vascular network using the intravascular erythrocyte movements. OCT-A is rapid, non-invasive as compared to FFA and facilitates better detailed simulation of microvascular transitions. OCT-A facilitates improved visualization of microvascular anomalies in RVO, including neovascular fronds, FAZ, and other microvascular abnormalities, owing to the lack of leakage and tissue staining and the better penetration of the longer wavelengths used in OCT-A [7,12-19]. OCT-A's capability to define both superficial capillary plexus (SCP) and deep capillary plexus (DCP) microvascular changes and ischemia is very significant because alot of vascular changes in RVO occur in DCP, which can not be interpreted by FFA [20,21]. OCT-A can be used to describe both the retinal and choroidal microvasculature, while FFA is used to observe the retinal vessels.
Despite many advantages, small scan areas, segmentation errors occurs due to differences in macular morphology, failure to assess the existence of leakage, susceptibility to image artifacts induced by patient motion and shadowing from retinal pathologies such as cystoid macular edema, and restricted capacity to visualize blood circulation from the observable flow limit are the short comings of OCT-A [20,22]. Microaneurysms images with FFA can not always be visible on OCT-A since they could have flow rates below the OCT-A detection threshold. There are also some obstacles to its use because during image acquisition, OCT-A requires the patient to accurately focus on a light (approximately 3 seconds), which can be challenging to do for patients with poor visual acuity [23]. OCT-A is susceptible to diverse objects, such as shadowing artifacts consistent with intraretinal/subretinal hemorrhage, superficial retinal vessel displacement artifacts across the lower retinal layers, and artifacts of motion. The normal field of view for the better image quality typically measures 3 × 3 mm, and extending the field of scanning in OCT-A decreases flow data.
In conclusion, as considered from the two patients we presented in this report, OCT-A images can show whether there is a perfusion in the region of branch vein occlusion. In addition to this information, the amount of edema in the macula can be observed simultaneously. The OCT-A method is promising in distinguishing non-ischemic and ischemic situations and in determining the clinical decisions in the branch vein occlusions. Though FFA has been the gold standard procedure, OCT-A will not only help us to appreciate improvements in the intricate macula microvasculature after vein occlusion, but will definitely play an undeniable role in future RVO patient care.
References
- Klein R, Moss SE, Meuer SM, et al. (2008) The 15-year cumulative incidence of retinal vein occlusion: The beaver dam eye study. Arch Ophthalmol 126: 513-518.
- Rogers SL, McIntosh RL, Lim L, et al. (2010) Natural history of branch retinal vein occlusion: An evidence-based systematic review. Ophthalmology 117: 1094.e5-1101.e5.
- McIntosh RL, Rogers SL, Lim L, et al. (2010) Natural history of central retinal vein occlusion: An evidence-based systematic review. Ophthalmology 117: 1113.e15-1123.e15.
- Rehak J, Rehak M (2008) Branch retinal vein occlusion: Pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res 33: 111-131.
- Shilling J, Jones C (1984) Retinal branch vein occlusion: A study of argon laser photocoagulation in the treatment of macular oedema. Br J Ophthalmol 68: 196-198.
- Yannuzzi LA, Rohrer KT, Tindel LJ, et al. (1986) Fluorescein angiography complication survey. Ophthalmology 93: 611-617.
- Spaide RF, Klancnik JM, Cooney MJ (2015) Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol 133: 45-50.
- Wylęgała A, Teper S, Dobrowolski D, et al. (2016) Optical coherence angiography: A review. Medicine (Baltimore) 95: e4907.
- Kim DY, Fingler J, Zawadzki RJ, et al. (2013) Optical imaging of the chorioretinal vasculature in the living human eye. Proc Natl Acad Sci 110: 14354-14359.
- Coscas G, Lupidi M, Coscas F (2016) Heidelberg spectralis optical coherence tomography angiography: Technical aspects. In: Bandello F, Souied EH, Querques G, OCT angiography in retinal and macular diseases. DevOphthalmol, Basel, Karger, 1-5.
- Lim HB, Kim MS, Yo YJ, et al. (2015) Prediction of retinal ischemia in branch retinal vein occlusion: Spectral-domain optical cohorence tomography study. Invest Ophthalmol Vis Sci 56: 6622-6629.
- Tsai G, Banaee T, Conti FF, et al. (2018) Optical coherence tomography angiography in eyes with retinal vein occlusion. J Ophthalmic Vis Res 13: 315-3
- Waheed NK, De Carlo TE, Chin AT, et al. (2015) OCT angiography in retinal diagnosis and treatment. Retinal Phys 12: 26-
- Baumal CR (2016) Optical coherence tomography angiography of retinal artery occlusion. In: Bandello F, Souied E, Querques G, OCT angiography in retinal and macular dseases. Basel, Karger, Switzerland.
- Kimura M, Nozaki M, Yoshida M, et al. (2016) Wide-field optical coherence tomography angiography using extended field imaging technique to evaluate the nonperfusion area in retinal vein occlusion. Clin Ophthalmol 10: 1291-
- de Carlo TE, Bonini Filho MA, Baumal CR, et al. (2016) Evaluation of preretinal neovascularization in proliferative diabetic retinopathy using optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina 47: 115-
- Rispoli M, Antonio L, Mastropasqua L, et al. (2016) Angiography Version 2.0. Retina Today 2016, 74-82.
- Novais EA, Adhi M, Moult EM, et al. (2016) Choroidal neovascularization analyzed on ultrahigh-speed swept-source optical coherence tomography angiography compared to spectral-domain optical coherence tomography angiography. Am J Ophthalmol 164: 80-
- Salz DA, de Carlo TE, Adhi M, et al. (2016) Select features of diabeticretinopathy on swept-source optical coherence tomographic angiography compared with fluorescein angiography and normal eyes. JAMA Ophthalmol 134: 644-
- de Carlo TE, Romano A, Waheed NK, et al. (2015) A review of optical coherence tomography angiography (OCTA). Int J RetinaVitreous.
- Savastano MC, Lumbroso B, Rispoli M (2015) In vivo characterization of retinal vascularization morphology using optical coherence tomography angiography. Retina 35: 2196-
- Cole ED, Novais EA, Louzada RN, et al. (2016) Visualization of changes in thechoriocapillaris, choroidalvessels, andretinal morphology after focal laser photocoagulation using OCT angiography. Invest Ophthalmol Vis Sci 57: OCT356-OCT3
- Nobre-Cardoso J, Keane PA, Sim DA, et al. (2016) Systematic evaluation of optical coherence tomography angiography in retinal vein occlusion. Am J Ophthalmol 163: 93.e6-e6.
Corresponding Author
Huri Sabur, Department of Ophthalmology, Bergama State Hospital, Islamsaray District Adnan Menderes Avenue, 35700, Bergama/Izmir, Turkey, Tel: +90-444 3516, Fax: +90 232 631 2896
Copyright
© 2020 Sabur H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.