Anti-Myelin Oligodendrocyte Glycoprotein (MOG) in a Patient with Optic Neuritis
Abstract
Background
The myelin oligodendrocyte glycoprotein (MOG) is a membrane protein expressed on the outmost surface of the myelin sheath in the central nervous system (CNS).
The MOG-antibodies (Abs) are detected in variable proportions of patients with different diseases such as Multiple Sclerosis (MS), Neuromyelitis Optica/Neuromyelitis Optica Spectrum Disorder, bilateral optic neuritis, acute disseminated encephalomyelitis and anti-NMDA-receptor-antibody-associated encephalitis. The role of MOG-Abs in the clinical setting is unclear.
Patient
We present a case report of a 15-year-old Caucasian schoolgirl thoroughly examined with a single episode of right-sided Optic Neuritis (ON) who tested positive for MOG-Abs. Magnetic resonance (contrast enhanced) revealed a thickened right optic nerve and a T2-hyperintense lesion in the periventricular white matter. The patient did not fulfil the diagnostic criteria for the above-mentioned diseases.
The patient was treated with a course of high-dose methylprednisolone. Conventional cell-based immunoassays revealed that she was seropositive for MOG-Abs and seronegative for AQP4-Abs.
Conclusion
The clinical implications of MOG-Abs are not yet clear, but MOG-Abs may detect a subclass of otherwise idiopathic ON patients negative for AQP4 Abs. This may have implications for treatment and prognosis of these patients.
Keywords
Neuro-Ophthalmology, Myelin oligodendrocyte glycoprotein (MOG), Neuromyelitis optica, Optic Neuritis, Multiple sclerosis
Introduction
The MOG-Abs are detected in variable proportions of patients with different CNS diseases such as bilateral ON, Neuromyelitis Optica (NMO)/Neuromyelitis Optica Spectrum Disorder (NMOSD), multiple sclerosis (MS), acute disseminated encephalomyelitis (ADEM), and anti-NMDA-receptor (NMDAR)-antibody-associated encephalitis [1].
Antibodies against native MOG are present in 20-40% of children with ADEM or MS [1]. The AQP4-Abs are known to be highly specific biomarkers for NMO, but some studies [2-4] have also found antibodies against myelin oligodendrocyte glycoprotein (MOG-Abs) in clinically defined AQP4 negative NMO/NMOSD patients.
Several studies (with MOG-Abs positive ON patients) suggest that these patients do have a more favourable outcome compared to AQP4 positive patients [5-7] (e.g. NMOSD patients with MOG antibodies have shown distinct clinical features, fewer attacks, and better recovery than patients with AQP4 antibodies or patients seronegative for both antibodies [7]).
In general, the clinical implications of MOG-Abs for diagnosis, treatment, and prognosis in several demyelinating autoimmune CNS diseases remain unexplained.
Here we describe a case report of a Caucasian female with otherwise idiopathic unilateral ON who tested negative for AQP4-Abs and positive for MOG-Abs.
Case Presentation
A 15-year-old Caucasian schoolgirl referred to Clinic of Optic Neuritis and Clinic of Multiple Sclerosis, Rigshospitalet-Glostrup, Copenhagen. The patient presented with bilateral visual blurring and bilateral retrobulbar pain.
Her medical and family history was unremarkable, and no infection or vaccinations were reported in the period prior to ON symptom onset.
A primary concern was juvenile MS, but differential diagnoses considered were ADEM and bilateral ON, especially taken the age of the patient into account, as well as NMOSD.
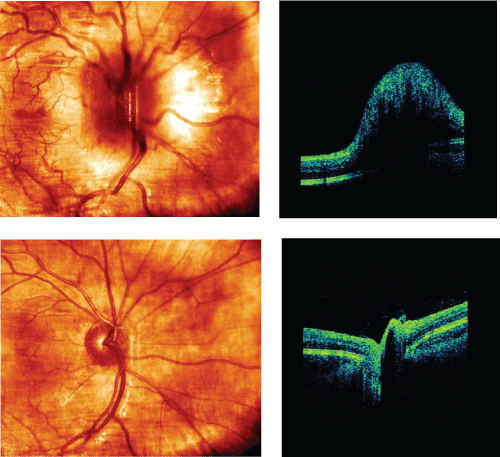
Ophthalmic examination revealed a positive relative afferent papillary defect on the right eye. Visual acuity (VA) was 0.04 (right eye) and 1.25 (left eye) and on right eye the patient was not able to see boards intended for low-contrast VA test and colour vision test. On auto perimetry (using the Humphrey central 30-2 program), a central scotoma was detected corresponding to all four quadrants on right eye. Full field visual evoked potential (ff-VEP) recorded no response (right eye) and 101 ms in latency and 17.2 µm in amplitude (left eye). Optical coherence tomography (OCT, Cirrus 4000) revealed a swollen disc on right eye (Figure 1) with an averaging retinal nerve fibre layer thickness (RNFLt) of 231 µm (right eye) and 102 µm (left eye). The 3 Tesla (3 T) magnetic resonance imaging (MRI) exposed a T2-hyperintense periventricular lesion, signs of ON (right eye) and medulla was normal.
Treatment with methylprednisolone was considered, but as the visual prognosis following ON in children is usually good and pursuant to local protocol, the initial spontaneous course was awaited.
Then 10 days later, an orbital MRI sequence (axial coronal fluid-attenuated inversion recovery (FLAIR)-sequence with fat suppression) clearly showed a high signal involving the entire right optic nerve anterior from the optic canal.
An extensive examination of blood (all the following tests within normal range) was made to exclude differential diagnoses (e.g. full blood count, liver count, platelet count, glucose, folate, cobalamin, vitamin D thyroid stimulating hormone (TSH), immunoglobulins, P-ANA, P-ANCA, Ig-M Rheum factor, Sjogren syndrome B-antibody, serum-ACE test to exclude sarcoidosis (sensitivity and specificity of ACE is 60% and 70% respectively) and Syphilis testing). Treponema pallidum was tested for by a routine method used at the laboratory at Rigshospitalet. If the enzyme immunoassay (Ortho and Abott) is positive, an RPR is performed to check for unspecific antibodies and a line immune assay (Beckton Dickinson) is checking for previous antibodies against Treponema Pallidum.
The patient tested seronegative for AQP4, but seropositive for MOG-Abs (analyses performed in Oxford Clinical Immunological Lab). The technique indirectly detects immunofluorescence on live transfected cell-based assay (CBA). MOG-Abs were determined by visualisation of binding to human embryonic kidney cells transiently transfected with the extracellular and transmembrane domains of human MOG.
Due to insufficient spontaneous remission and high signal in the entire right optic nerve, the patient was treated with a course of oral methylprednisolone (500 mg/day for 5 days until tapering off during another 10 days). Lumbar puncture revealed normal cerebrospinal fluid analysis (incl. cell count, protein, glucose, IgG index, oligoclonal bands, borrelia and herpes virus). Chest X-ray was normal. OCT was repeated and showed an averaging RNFLt of 85 µm (right eye) and 101 µm (left eye). GCLt 56 µm (right eye) and 88 µm (left eye). The VA was 0.1 (right eye) and 1.25 (left eye). Nine months later, MRI was repeated to exclude eventually subclinical lesions, but no such new lesions were found. No signs of ON on MRI in CNS. The VA on right eye was 0.8. RNFLt was 60 µm (right eye) and 101 µm (left eye). GCLt was 52 µm (right eye) and 89 µm (left eye) (Table 1).
Discussion
This patient presented with a monophasic disease course of right-sided papillitis. Though extensive examination program suggested idiopathic ON, the pathogenesis showed to be mediated by MOG-Abs.
As earlier mentioned MOG-Abs are present in several autoimmune CNS diseases. It can be difficult to differentiate between these diseases because of partly overlapping symptoms. According to the International Paediatric MS Study Group (IPMSSG), this patient could neither be diagnosed with ADEM, acute myelitis, MS, Anti-NMDAR encephalitis, bilateral ON nor NMO/NMOSD.
Initially, orbital MRI sequence clearly showed severe swelling in the right optic nerve. Nine months later, MRI demonstrated no signs of ON and VA improved from 0.04 to 0.8 on the right eye, but on OCT RNFLt showed a repeatedly thinning.
One study [8] found that a significant proportion of MOG-Abs positive patients (detected by CBA) with isolated ON typically experienced better VA recovery than anti-AQP4+ ON patients after having received a course of methylprednisolone treatment. Based on a comparison of OCT parameters (CIPL and circumpapillary RNFLt) between MOG-Abs and AQP4 positive ON eyes, this study suggested that MOG-Abs positive ON patients experienced less retinal neuronal loss compared to AQP4 positive ON patients despite initially severe optic nerve swelling seen on MRI. Also in other studies, MOG-Abs associated ON has been thought to show a more favourable outcome compared to AQP4 positive patients [5-7].
On the other hand, a recent multicenter study of up to 50 patients [9] found no significant differences in peripapillary RNFLt, ganglion cell and inner plexiform layer volume (GCIP) and visual function impairment between MOG-IgG-positive and AQP4-IgG-positive patients with ON. Indeed, these are important findings, but results were based on long-term monitoring of ON courses, i.e., when patients with relapsing rather than monophasic ON were taken into account.
Due to this, long term immunosuppressive treatment with methylprednisolone and azathioprine of the patient was considered but was not prescribed to this patient in puberty. Furthermore, there are no double-blinded trials supporting long-term treatment, as the presence of MOG Abs is seldom and the importance of this analysis in the clinical setting of ON only recently has begun to receive further attention.
Early diagnosis and appropriate initiation of immunosuppressive treatment (depending on findings on extensive evaluations) are important in ON because many inflammatory CNS diseases (20% in MS [10]) present with ON as the initial attack.
In summary MOG analysis should be considered in (a) Idiopathic ON, (b) Mono and/or relapsing ON courses (c) Especially in cases of AQP4 negative ON patients and (d) In patients harbouring disease courses less likely for MS (as in this case report).
This should be done to draw attention to the wide-ranging diversity of MOG-Abs associated CNS disorders, but also MOG-Abs positive ON patients may identify a subgroup of isolated ON patients with a distinct prognosis from the AQP4 positive patients [6].
Further case reports and long-term prospective multicentre studies with longer clinical follow-up are needed in future to understand these potential implications of MOG-Abs in ON patients, and if and how to treat these patients.
References
- Rostasy K, Mader S, Schanda K, et al. (2012) Anti-myelin oligodendrocyte glycoprotein antibodies in pediatric patients with optic neuritis. Arch Neurol 69: 752-756.
- Mader S, Gredler V, Schanda K, et al. (2011) Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation 8: 184.
- Kitley J, Woodhall M, Waters P, et al. (2012) Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology 79: 1273-1277.
- Reinhard Hohlfeld, Dornmair K, Meinl E, et al. (2016) The search for the target antigens of multiple sclerosis, part 2: CD8+ T cells, B cells, and antibodies in the focus of reverse-translational research. Lancet Neurol 15: 317-331.
- Sato DK, Callegaro D, Lana-Peixoto MA, et al. (2014) Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 82: 474-481.
- Akaishi T, Sato DK, Nakashima I, et al. (2016) MRI and retinal abnormalities in isolated optic neuritis with myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies: a comparative study. J Neurol Neurosurg Psychiatry 87: 446-448.
- Nakajima H, Motomura M, Tanaka K, et al. (2015) Antibodies to myelin oligodendrocyte glycoprotein in idiopathic optic neuritis. BMJ Open 5: e007766.
- Akaishi T, Nakashima I, Takeshita T, et al. (2016) Lesion length of optic neuritis impacts visual prognosis in neuromyelitis optica. J Neuroimmunol 293: 28-33.
- Florence Pache, Hanna Zimmermann, Janine Mikolajczak, et al. (2016) MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: Afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. Journal of Neuroinflammation 13: 282.
- Frederiksen JL (2000) A prospective study of optic neuritis: Clinical, MRI, CSF, neurophysiological and HLA findings. Acta Ophthalmologica Scandinavica 78: 490-491.
Corresponding Author
Mathias Falck Schmidt, Clinic of Optic Neuritis and Clinic of Multiple Sclerosis, Department of Neurology, Rigshospitalet - Glostrup, University of Copenhagen, Valdemar Hansens Vej 13, 2600 Glostrup, Denmark.
Copyright
© 2018 Schmidt MF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.