Resection Methods of Cervical Medullary Tumors with Mixed Histological Characteristics
Abstract
The Subependymoma is derived from subependymal astrocytic cells. In the present work we report 3 patients: 1 patient with data of endocranial hypertension, and two with neurological focus and spinal involvement. MRI (Magnetic Resonance Imagen) showed heterogeneous images, solid and cystic components, from different locations. Ependimo-Astrocytomas have neoplastic ependymal cells and gliofibrillar formation. Embryologically, the ventricle is lined by ependymal cells, below which is a layer of glial fibers and a layer of subependymal cells. When these layers of differentiation do not exist in development, there is high mitosis and these lesions with mixed characteristics can be found. Different tips and suggestions are provided in its management for resection in the surgical treatment of these cervical tumors.
Keywords
Ependymoma, Glia, Neoplasms, Astrocytoma, Cervical myelopathy
Introduction
Ependymomas (PEs) are slow-growing tumors. They have a higher frequency of presentation in children or young adults and have a worse prognosis in children. They represent 3.9% of all neuroepithelial tumors. In adulthood, it will represent 34.5% of all central nervous system (CNS) ependymomas (EP) and approximately 60% of all intramedullary tumors [1-3]. Men and women are equally affected [4]. Approximately 65% of cases have associated syringomyelia, particularly when they are present in cervical locations [5]. PEs are classified as: 1) Subependymomma, 2) Myxopapillary ependymoma (WHO grade I), 3) Classic ependymoma (OMS grade II), 4) RELA-fusion positive ependymoma, and 5) Anaplastic ependymoma (WHO grade III). Classic ependymomas have three histological variants: I) Papillary, II) Tanicytic and III) Clear cell [6-8]. The entity Subependymoma (SE) was described for the first time by Scheinker as a distinctive tumor that occurs in the ventricular wall and arises from the subependymal matrix with maturation in glial cells [9]. This lesion was designated from the histological analysis of French and Bucy. As "Subependymal Astrocytoma", however, SE will be considered as a variant of EP [8,9]. Below we present three representative clinical cases of histologically mixed tumor lesions and the management of cervical spinal cord resection.
Clinical Case 1
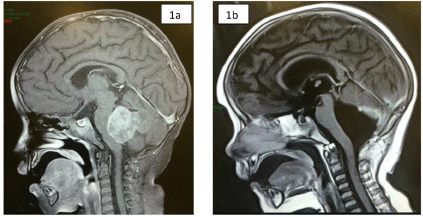
5-year-old female with headache and left hemiparesis of 2 weeks evolution. The MRI (Nuclear Magnetic Resonance) image showed (Figure 1A) an intraventricular, heterogeneous, solid image with cystic lesions. In this image, it was not possible to define whether the origin of the lesion was the floor or the ceiling of the IV ventricle. The patient was treated surgically and a suboccipital craniectomy and total resection of the lesion were performed, with a fragment of the tumorous lesion being sent to the neuropathology service of the General Hospital of Mexico. The histological diagnosis was Ependymal-Astrocytoma (E-A) (Figure 1B). The patient had a good evolution, and a 48-month follow-up was performed, without observing recurrence of the tumor lesion.
Clinical Case 2
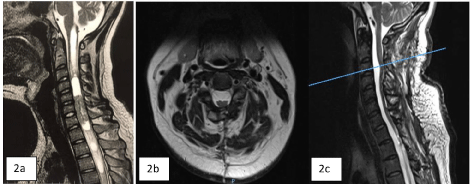
34-year-old male with hypoesthesia in the right hemibody and posteriorly in the left hemibody, with a sensory level at C5, progressive paraparesis on the right side of the body, occipital headache and cervicalgia of 2 months of evolution, with a McCormick scale of 3 The MRI (Figure 2) showed a well-defined lesion at the C5-C6-C7 levels, hypo-intense in T1, intramedullary, with defined borders, in T2 a caudal lesion with a cystic and solid appearance with a slight component Rostral and caudal and rostral parenchyma edema, with a good cleavage plane, caudal syringomyelia was also observed up to T4 and rostral presenting syringobulbia. The patient was treated surgically, and a posterior cervical approach was performed with laminoplasty with microplates and micro screws, monitoring with somatosensory evoked potentials and drug management with anti-spinal edema measures. Posterior myelotomy was performed through the posterior median sulcus, placement of anchor points in the dura mater with 4/0 prolene thread, arachnoid and pia mater with 6/0 prolene thread, and a total resection of the tumor lesion was performed identifying the cleavage medium. In this case, closure of the spinal cord (pia mater, arachnoid) and hermetic closure of the dura mater are performed. The histopathological result obtained was E-A. After the surgical procedure, the patient regained sensitivity at 48 hours, later the strength in both pelvic limbs and thoracic limbs with remission of symptoms in 80%, reaching a McCormick scale of 1. The patient had good evolution and a 24-month follow-up was performed, presenting resolution of syringomyelia and syringobulbia with thinning of the spinal cord without observing recurrence of the tumor lesion.
Clinical Case 3
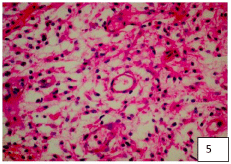
42-year-old male with a 4-month history of holocranial headache, nausea and vomiting on several occasions, gait disturbances, progressive decrease in strength in the right extremities beginning in the upper extremity that progressed to an ipsilateral lower limb, and subsequently affected contralateral limbs. He also presented hypoesthesia of C2-T3, alterations in pain sensitivity and temperature, with McCormick functional scale 4. In the MRI, a heterogeneous cervical tumor lesion of C4-C7 was observed, with small hyperdense images in T1, and in T2 cystic components, with a cleavage plane. The patient is scheduled for surgery and a posterior cervical approach is performed with laminoplasty with microplates and micro screws and total resection of the tumor lesion with myelotomy, leaving the spinal cord open without retinalization of the spinal cord layers (arachnoid, pia mater) and only with closure. airtight dura mater with 4/0 prolenne. In the histopathological analysis of the lesion, the diagnosis of E-A was obtained. A 36-month follow-up was performed, observing clinical improvement with the McCormick scale 2, without recurrence of the tumor lesion and with histology images of the tumor lesion (Figure 2C).
Discussion
The initial symptoms of EP are nonspecific. The classic clinical picture they present is a central spinal cord syndrome, accompanied by an upper and lower motor neuron syndrome [10,11], in addition the lower extremities may be spastic bilaterally, but this is less frequently [12,13]. The sensory symptoms that they will present are dysesthesia, in more than 70% of patients [11-16], localized pain at the level of the affected cervical segment, and it is rarely of a radicular characteristic; an early progression of the symptoms may also occur [10]. Urogenital and anorectal dysfunction. if they do appear, they do so early. Differential diagnoses are with primary glial tumors [11-16], they include astrocytomas, hemangioblastomas, schwannomas and to a lesser extent spinal metastases and arterio-venous malformations. About 3% of spinal tumor lesions are astrocytomas [17]. The most frequent place of presentation of tumors is the cervical region in 22.5%, followed in 12% of cervical-thoracic location, 20% of these tumor lesions are associated with syringomyelia [5]. Histopathologically, until 1950 there were no reports of mixed tumors, the year in which neuronal, glial, and mixed tumors were described [8,18]. PEs frequently arise from the ependyma of the floor of the IV ventricle, from the foramen of Magendie, and can occasionally arise from the foramina of Luschka [19]. Regarding the astrocytic nature of SE in an electron microscopy study carried out by Duffel, et al. It was detected that there are no differences between astrocytoma (AS) and SP [19,20]. However, Russell and Rubinstein and Rubinstein consider SP as a variant of PD, because it has a frequency within the typical ependymoma [19,20]. EPs in immunohistochemistry are generally positive for GFAP, vimentin, S100, CD99, nestin, and NCAM [18,21]. Both EP and SP are unequivocally identified with ependymal cells and also with fibrous astrocytes. These form rosettes that, seen in electron microscopy, consist of terminal bars, microvilli, and cilia. Less frequently, there is the presence of extensive cytoplasmic degeneration, the presence of more astrocytes, and in SP the ependymal cells contain much more abundant glial filaments [19,20]. Due to the different histological characteristics of ES, some of these tumors are interpreted as Mixed Glial tumors, with Ependymal cells or predominantly with astrocytes.
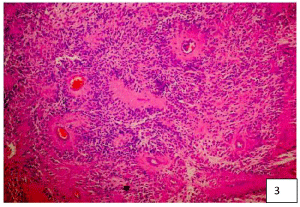
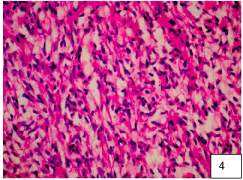
To exemplify how these lesions present mixed characteristics with a predominance of EP characteristic cells with a background of AS, we show the representative histopathology images of our three patients in (Figure 3, Figure 4 and Figure 5). The greater number of fibrous astrocytes and the increase glial filaments in the ependymal cells of the subependymal areas, are responsible for the sclerotic and fibrillar appearance seen in microscopy [19,20]. This formation can be better explained, from the embryological point of view, the IV ventricle is lined by a continuous layer of ependymal cells [22,23]. Below this is a layer of glial fibers, and more deeply a layer of subependymal glial cells. Therefore, there is no membrane that separates these two layers [23,24]. The subependymal layer contains undifferentiated cells, with high mitosis, which play a very important role in the formation of the cerebral cortex. This is why these cells can persist into adult life, and continue with mitosis [19,20]. EP growth patterns have been described as an epithelial and glial pavement pattern with a Rosette formation system [20,25]. The basement membrane of these ependymal tumors reported in the literature were only found in the ependymal cysts of myxopapillary tumors, mainly in the layers of the vessels, these called the Rosenthal fibers [20,21,26]. Several authors do not recognize these fibers as characteristics of this tumor, since they are found in various tumor lesions [6,20]. Although there are EP with areas of infiltration, which present diffuse lesions and non-EP lesions with focal infiltrating tissue, perivascular pseudo-rushes characteristic of EP were not confirmed. They also reported extensive regions, where the perivascular tumor cells were longitudinally or circumferentially aligned in formations that are not typical of EP. Even in compact areas with perivascular formations, they presented lesions that did not bear a great resemblance to the classic cellular EP [18,19]. It is worth wondering if these tumor lesions, in their histological variant, could be variants of astrocytoma. Since the original description in 1930, this term has been applied in a variable way to the infiltration of the SA with a tendency to perivascular orientation " Astroblastic Formation '' and well circumscribed, often superficial lesions with characteristics of EP [6,19]. Regarding prognosis, the 5-year progression rate for pediatric patients with EP is around 50%. The ten-year survival rates for pediatric and adult patients are 57% and 45%, respectively. Complete surgical resection is the only reliable and effective prognostic factor to predict a longer survival time [4-8,20,27]. In terms of surgical technique for complete resection, the position of the patient for resection of these tumors is important. The head must be attached to the Mayfield head, with the neck flexed and the head elevated, which reduces spinal curvature at these levels and significantly reduces trans-operative bleeding. Sensory and motor control is carried out by means of somatosensory evoked potentials that are used throughout the surgical procedure [26,28]. If somato-sensory evoked potentials are lost after median myelotomy, they will not be a premonitory of postoperative motor function [27]. This control is carried out by means of subdural somato-sensory potentials that will be reliable in 60% of cases. A 50% decrease in the response amplitude of the potentials at the end of the surgical procedure may be an indication of permanent motor sequelae [20,25-28]. In most cases, standard laminectomy can be performed for small lesions less than three levels. The laminectomy must extend at least 1 segment above and one segment below the solid tumor lesion, and the facets must be preserved to avoid causing instability and alterations in sagittal balance. Another option is to perform laminoplasty (there are different techniques with microplates and micro screws, etc.), which may be a very reasonable option [13,15], to preserve the cervical sagittal balance to the best extent, but in the case of extensive tumor lesions greater than 3 levels, it is recommended to perform posterior cervical fusion plus arthrodesis to prevent and preserve lordosis as physiologically as possible to avoid cervical kyphosis in the future. Anesthetic management is very important so that anesthetic medications do not interfere with the monitoring of somatosensory potentials, a total intravenous anesthesia must be performed. In our experience, we administer medications to prevent spinal edema as spinal protection measures, to avoid post-operative injuries due to manipulation, administering infusions of methylprednisolone 30-50 mg / kg / weight one hour prior to performing the procedure. Surgery will guarantee better post-surgical results. It is recommended to continue with the same steroid measures for three days after surgery 500 mg IV every 8 hours, to avoid posterior fossa syndrome and reperfusion syndrome, or spinal edema [6,22], and window syndrome (spinal herniation due to tumor resection through a small 1-2 level laminectomy). Medullary reconstruction by myelotomy performed for tumor resection can be with simple points of approximation of the pia mater and arachnoid, and then a hermetic closure of the dura mater should be performed and corroborated with valsalva maneuvers to avoid complications of fistulas, especially of cerebrospinal fluid. On the other hand, we also recommend not performing spinal reconstruction (closure by the arachnoid and pia mater planes with simple approximation points) and only performing hermetic closure of the dura mater in cases of extensive spinal manipulation and spinal edema observed during the intraoperative period. This is recommended because in our results we obtained a slight difference in terms of improvement in patients with very extensive tumor lesions. It should be taken into account that the majority of patients will experience sensory loss in the early postoperative period, probably as a result of mid-posterior myelotomy, transitory edema or vascular compromise [4,8] the loss lasting 3-6 months as presented in our cases. The functional prognosis is carried out by means of the Mc Cormick scale. In this functional prognosis, the strongest predictor is the pre-operative one, that is, if you enter surgery with a greatly diminished functional capacity, the prognosis is reserved, compared to a scale of optimal functional capacity [4,6,7,8,11]. In cases of recurrence of EP, especially in children, the use of MRI-guided laser-induced thermal therapy is highlighted in patients especially with recurrent intracranial Anaplastic Ependymoma. Obtaining good results in the medium term [8,20].
Conclusion
EA is characterized by presenting neoplastic ependymal cells, which not only will identify the formation of rosettes, with terminal bars, microvilli, and cilia, but also the gliofibrillar formation will be observed. Obtaining in this way the characteristic of rosettes, in a fibrillar mesh bottom, and with astrocytic cells, which give the characteristic of a mixed lesion. Complete resection of cervical spinal tumor lesions is a favorable prognostic factor. Also having a low Mc Cornick scale prior to surgery, transoperative monitoring, and pre, intra, and post-surgical spinal protection measures will lead to satisfactory functional results.
Financing
The following work was carried out with the authors' own resources, without funding from private or federal institutions or companies.
Conflict of Interests
The authors do not present any conflict of interest with the following work presented.
References
- Helseth A, Mørk SJ, (1989) Primary intraspinal neoplasms in Norway, 1955-1986. A population-based survey of 467 patients. J Neurosurg 71: 842-845.
- Mørk SJ, Løken AC (1977) Ependymoma. A follow-up study of 101 cases. Cancer 40: 907-915.
- Sonneland PRL, Scheithauer BW, Onofrio BM (1985) Myxopapillary ependymoma. A clinicopathologic and immunocytochemical study of 77 cases. Cancer 56: 883-893.
- McCormick PC, Torres R, Post KD, et al. (1990) Intramedullary ependymoma of the spinal cord. J Neurosurg 72: 523-533.
- Samii M, Klekamp J (1994) Surgical results of 100 intramedullary tumors in relation to accompanying syringomyelia. Neurosurgery 35: 865-873.
- Zhiguo Liu, Jing Li, Zhiyan Liu, et al. (2014) Supratentorial cortical ependymoma: Case series and review of the literature. Neuropathology 34: 243-252.
- David N Louis, Arie Perry,Guido Reifenberger et al. (2016) The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol 131: 803-820.
- Norman L. Lehman (2008) Central nervous system tumors with ependymal features: A broadened spectrum of primarily ependymal differentiation? J Neuropathol Exp Neurol 67: 177-188.
- Yao-Shif U, Andrewt . l. Chenp, Hd,saulk a T, et al.( 1974) Is subependymoma (subependymal glomerate astrocytoma) an astrocytoma or ependymoma? A comparative ultraestuctural and tissue culture study. Cancer 34:1992-2008.
- McCormick PC, Stein BM (1990) Intramedullary tumors in adults. Neurosurg Clin North Am 1: 609-630.
- Cooper PR (1989) Outcome after operative treatment of intramedullary spinal cord tumors in adults: Intermediate and long-term results in 51 patients. Neurosurgery 25: 855-859.
- Epstein FJ, Farmer J-P, Freed D (1993) Adult intramedullary spinal cord ependymomas: The result of surgery in 38 patients. J Neurosurg 79: 204-209.
- McCormick PC, Post KD, Stein BM (1990) Intradural extramedullary tumors in adults. Neurosurg Clin North Am 1: 591-608.
- Hoshimaru M, Koyama T, Hashimmoto N, et al. (1999) Results of microsurgical treatment for intramedullary spinal cord ependymomas: Analysis of 36 cases. Neurosurgery 44: 264-269.
- Sloof JL, Kernohan JW, McCarthy CS (1964) Primary intramedullary tumors of the spinal cord and filum terminale. WB Saunders, Philadelphia.
- Steven J Millen, Bruce H Campbell, Glenn A Meyer, et al. (1985) Mixed glioma of the cerebellopontine angle. Am J Otol 6: 503-507.
- Mark M. Souweidane (2015) Laser ablation for recurrent intracranial ependymoma. J Neurosurg Pediatr 15: 361-362.
- Malte Ottenhausen, Georgios Ntoulias, Imithri Bodhinayake, et al. (2018) Intradural spinal tumors in adults-update on managemente and outcome. Neurosurg Rev 42: 371-388
- Randy S D'Amico, Moshe Praver, Zanazzi GJ, et al. (2017) Subependymomas are low-grade heterogeneous glial neoplasms defind by Subventricular zone lineage markers. World Neurosurgery 107: 451-463.
- K Egger, M Hohenhaus, V.Van Velthoven et al. (2016) Spinal diffusion tensor tractography for diferentiation of intramedullary tumor-suspected lesions. Eur J Radiol 85: 2275-2280.
- Kazoyoshi Kobayashi, Shihiro Imagama, Fumihiko Kato, et al. (2017) MRI characteristics of spinal ependymoma in WHO Grade II: A review of 59 cases. Spine (Phila Pa 1976) 43: E525-E530.
- Azam Basheer, Richard Rammo, Steven Kalkanis, et al. (2017) Multifocal intradural extramedullary pilocytic astrocytomas of the spinal cord: A case report and review of the literature. Neurosurgery 80: 179-182.
- Tamir Ailon, Christopher Dunham, Anne-Sophie Carret, et al. (2015) The role of resection alone in select children with intracranial ependymoma: The canadian pediatric brain tumour consortium experience. Childs Nerv Syst 31:57-65.
- Bedjan Behmanesh, Florian Gessler (2017) Natural history of intramedullary spinal cord ependymoma yn patients preferring noneoperative treatment. J Neurooncol 135: 93-98.
- Woon Tak Tuh, Chun Kee Chung (2017) Spinal cord subependymoma surgery: A multi-institutional experience. J Korean Neurosurg Soc 61: 233-242.
- Kieron J Sweeney, Matt Reynolds (2016) Gross total resection rates of grade II/III intramedullary ependymomas using the surgical strategy of en-bloc resection without intra operative neurophysiological monitoring. Br J Neurosurg 31: 2-5.
- Morota N, Deletis V, Constantini S, et al. (1997) The role of motor evoked potentials during surgery for intramedullary spinal cord tumors. Neurosurgery 41: 1327-1336.
- Adams DC, Emerson RG. Heyer EJ (1993) Intraoperative evoked potential monitoring with controlled neuromuscular blockade. Anesth Anal 77: 913-918.
Corresponding Author
Erick Ariñez Barahona MD, Department of Neurocirugía, Hospital General de México "Dr. Eduardo Liceaga", Hospital san angel inn universidad, Servicio de Neurología y Neurocirugía, Avenida Cuauhtémoc 403. Edificio 7. Interior 503. Colonia Roma Sur, Consultorio 1001, México,Tel: +( 55 )48 13 82 25.
Copyright
© 2021 Barahona EA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.