SGLT2 Inhibitor Use in Patients with Advanced Chronic Kidney Disease; A Systematic Review and Meta-Analysis
Abstract
This paper examines the impact of sodium-glucose cotransporter 2 (SGLT2) inhibitors on patients with stage 4 and stage 5 CKD, including dialysis patients. Conducting a systematic review and meta-analysis allows us to compare results from different published studies on SGLT2 inhibitor use in patients with eGFR < 20 mL/min/1.73 m2. The objective of this paper is to determine if SGLT2 inhibitors preserve residual kidney function and decrease mortality in patients with eGFR < 20 mL/min/1.73 m2 including those on dialysis Four studies published on academic databases were found and used in this analysis. The meta-analyses showed that SGLT2 inhibitors slowed the decline of eGFRs in patients with eGFR < 20 mL/min/1.73 m2 and prevented ESKD leading to permanent dialysis in patients who were not already on dialysis.
Keywords
SGLT2 Inhibitors, Stage 4 CKD, Stage 5 CKD, Dialysis, Residual kidney function
Introduction
Diabetes is the most common cause of chronic kidney disease (CKD) and end-stage kidney disease (ESKD) in the United States. Kidney damage occurs when elevated levels of glucose damage small blood vessels and other cells within the kidneys. There are many medications used to protect the kidneys from damage [1]. The first-line medications for slowing damage to the kidneys are angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs). ACEi and ARBs are often used in combination with other medications to protect the kidneys and manage blood pressure or blood sugar. One type of medication commonly used with ACEi/ARBs is sodium-glucose cotransporter 2 (SGLT2) inhibitors. SGLT2 inhibitors work on the proximal tubule to block the reabsorption of glucose and sodium. SGLT2 inhibitors are used to lower blood sugar levels via excreting glucose in the urine [2]. The FDA approves SGLT2 inhibitor use in adults with type 2 diabetes but requires an estimated glomerular filtration rate (eGFR) of at least 20-30 mL/min/1.73 m2. SGLT2 inhibitors are not approved for patients with eGFR less than 20 mL/min/1.73 m2 or in kidney failure [3].
In recent research, SGLT2 inhibitors have been found to slow the progression of CKD. SGLT2 inhibitors decrease renal filtration in the glomeruli by keeping glucose and sodium in the urine. This change in renal filtration lowers the pressure within the glomeruli and protects them from damage. SGLT2 inhibitors have been found to initially decrease the eGFR, especially when patients are dehydrated, but they have been found to preserve eGFR long-term when compared to other medications [4].
The objective of this paper is to use meta-analysis to determine if SGLT2 inhibitors preserve residual kidney function in patients with stage 4 and stage 5 CKD, including dialysis patients. A literature review was conducted to determine the available studies on SGLT2 inhibitor use in patients with eGFR < 20 mL/min/1.73 m2, including patients on dialysis. The review found 4 studies published between 2021 and 2025 that researched the impact of SGLT2 inhibitors on patients with stage 4 or stage 5 CKD and patients on different forms of dialysis. The SGLT2 inhibitors used in these studies were dapagliflozin (Farxiga), canagliflozin (Invokana), and empagliflozin (Jardiance). The results of this paper and the discussion about the future use of SGLT2 inhibitors in patients with eGFR < 20 mL/min/1.73 m2, including those on dialysis, are included later in this paper.
Methods
Literature review 4.1
A comprehensive search was conducted using academic databases and search engines including UpToDate, PubMed, and Google Scholar. The following keywords were used in the search process:
Keywords = “SGLT2 inhibitor(s)” AND “dialysis”, “eskd”, “stage 4 CKD”, “stage 5 CKD”
The references in the resulting articles and studies were reviewed to include the articles that did not contain the keywords but still studied the impact of SGLT2 inhibitors on stage 4 and stage 5 CKD patients, including dialysis patients.
Source inclusion and exclusion criteria 4.2
Studies included in this meta-analysis were selected based on the following criteria:
1. Written in English
2. Available to the public via online databases
3. Studies include patients with stage 4 CKD, stage 5 CKD, and/or those on dialysis
4. The studies included quantitative clinical data, separated by CKD stage.
Studies were excluded if they lacked published quantitative data, enrolled pediatric populations, or involved combination therapies where the independent effect of SGLT2 inhibition could not be isolated.
Assembly of data 4.3
The quantitative data and demographic information from all patients with stage 4 and/or stage 5 CKD, including those on dialysis, were recorded in an Excel Sheet. The table below shows the demographic information of each study included in this paper (Table 1).
Two studies focused on patients receiving dialysis, the data reported in the studies was used to calculate the eGFR over time (Study 1 and Study 2). The 2021 CKD-EPI Creatinine Equation was used to calculate the average eGFR at each timepoint. The CKD-EPI formula is shown below [5].
Where Scr is serum creatinine in mg/dL, k is 0.7 for females or 0.9 for males, a is -0.241 for females or -0.302 for males, min is the minimum Scr/k or 1, and max is the maximum Scr/k or 1. Since the studies only published data on the average serum creatinine at each timepoint, the formula was adjusted for the number of females and males included in the studies by using average k, a, and formula multiplier values. One study focused on patients with stage 4 CKD, included graphs showing the eGFR over time (Study 3). These graphs and slopes were uploaded to AI to estimate the point values within each of the graphs. The values were estimated three times using ChatGPT and the average of all three estimates was used for the meta-analysis. The calculated and AI determined eGFRs were recorded together over time. The slopes of the eGFRs were calculated using the following formula.
The final 12 months of data from the study 3 was used to estimate the change in eGFR per month when less than 20 mL/min/1.73m2. Once the slopes were calculated, the standard errors were calculated by taking the average standard deviation of the calculated eGFRs and dividing it by the study time in months (Studie 1, Studie 2, and Studie 3). The adverse events that occurred in all studies were recorded.
Analytical Approach 4.4
Several types of meta-analyses were used to evaluate the data from the studies. The first meta-analysis was conducted on the eGFRs using the random-effects model (Study 1, Study 2, and Study 3). The second and third meta-analyses were conducted on the number of adverse events that occurred in patients taking SGLT2 inhibitors compared to controls (Study 3, and Study 4). The final meta-analysis was conducted on the all-cause mortality in patients taking SGLT2 inhibitors in all the studies. The results of the meta-analyses were considered statistically significant if p-value < 0.05.
Software and reproducibility 4.5
The version of ChatGPT used was based on the GPT-5.1 model.
All meta-analyses were performed using RStudio (version 2025.09.02) and R (version 4.5.2) with the meta package.
Results
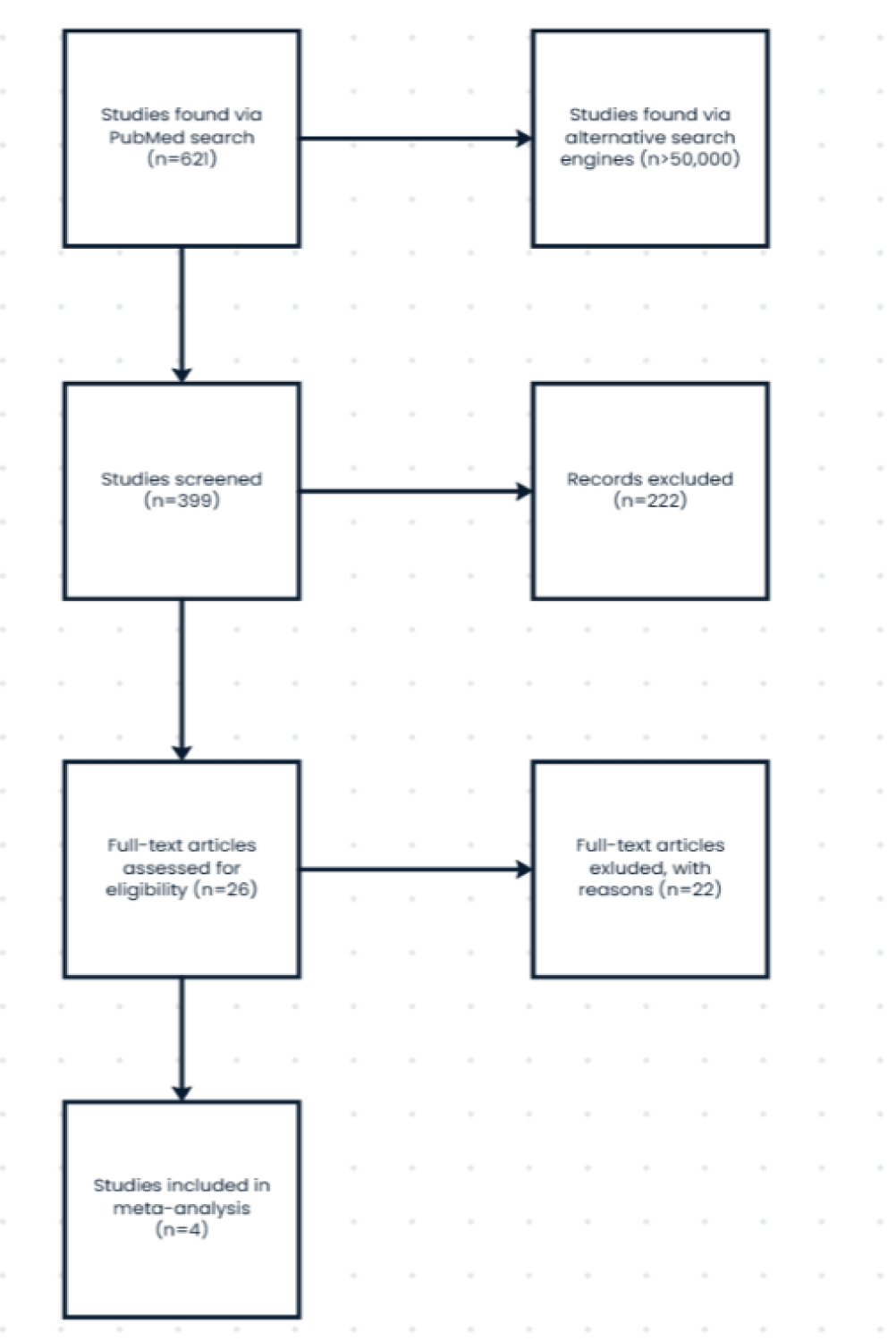
The studies included in this analysis were determined based off the following flow diagram.
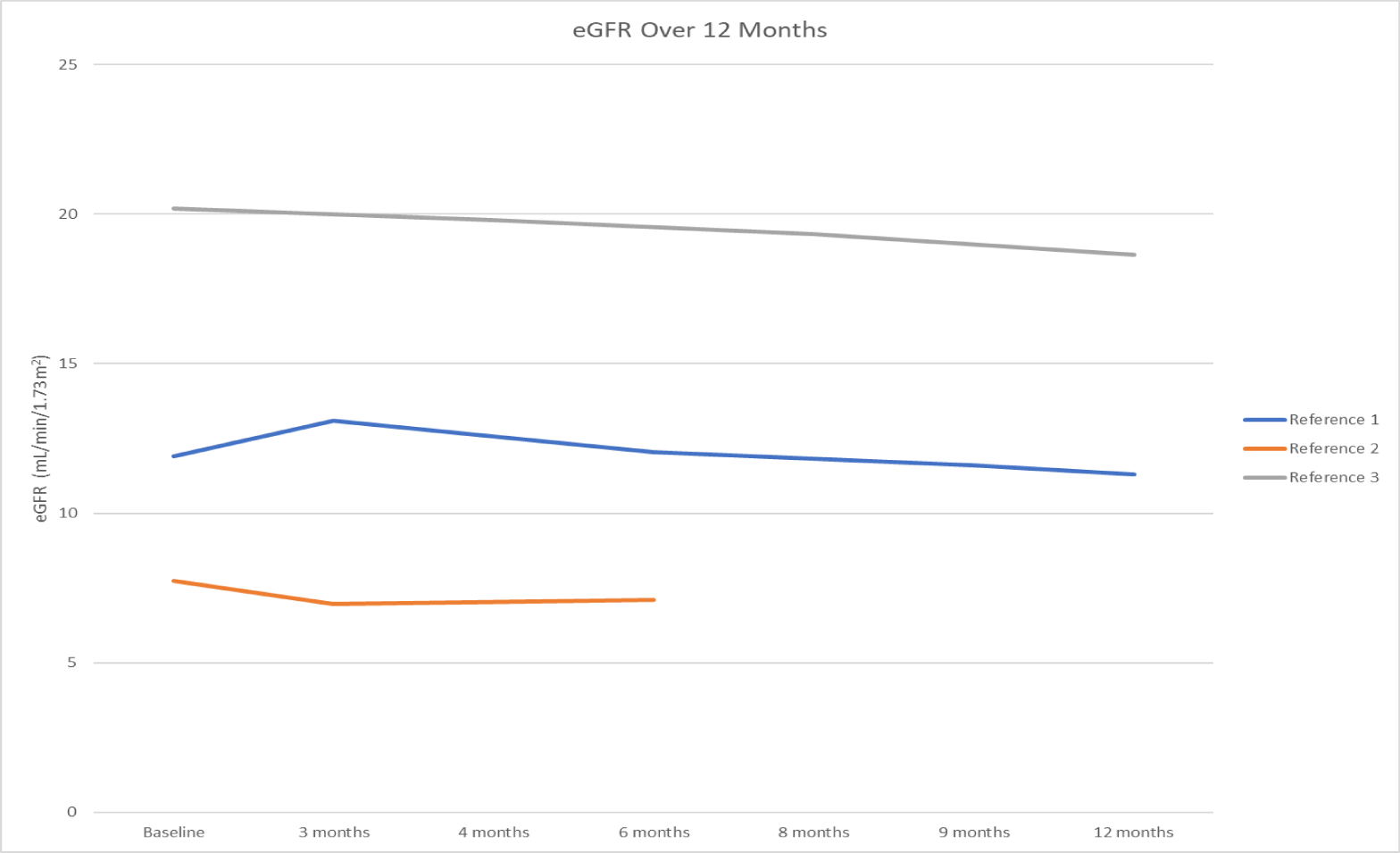
The included studies were required to present quantitative data that could be used to estimate kidney function over time and/or outcomes based on CKD stages. All of the studies included in this analysis were required to include patients on SGLT2 inhibitors with eGFR < 20 mL/min/1.73 m2. Once the eGFRs were calculated, the eGFRs were graphed over 12 months to see the overall trends (Figure 1).
A meta-analysis was then conducted on the slopes of the eGFRs over time from Study 1, Study 2, and Study 3 using the following data. Since Study 1 and Study 2 did not have control groups, the meta-analysis was conducted without control values (Table 1).
The outputs of the meta-analysis are shown below.
Random-Effects Model (k = 3; tau^2 estimator: REML)
logLik deviance AIC BIC AICc
0.5993 -1.1985 2.8015 0.1878 14.8015
tau^2 (estimated amount of total heterogeneity): 0 (SE = 0.0672)
tau (square root of estimated tau^2 value): 0
I^2 (total heterogeneity / total variability): 0.00%
H^2 (total variability / sampling variability): 1.00
Test for Heterogeneity
Q(df = 2) = 0.0429, p-val = 0.9788
Model Results
estimate se zval pval ci.lb ci.ub
-0.1279 0.0632 -2.0242 0.0430 -0.2517 -0.0041* ---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘’ 1
These results are explained and interpreted in the discussion section of this paper.
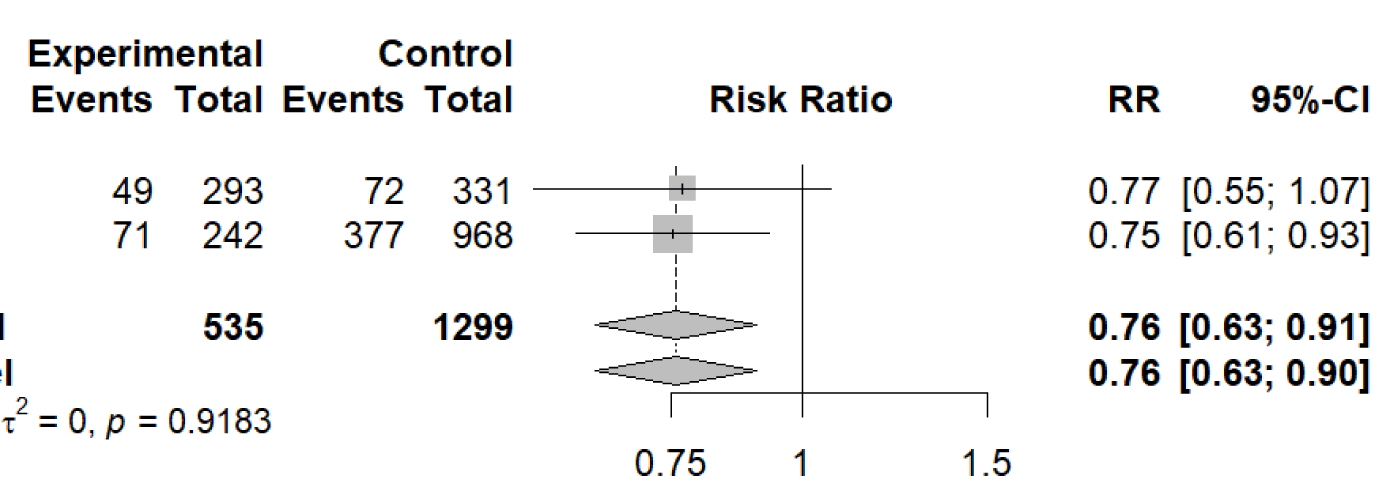
Figure 2 A meta-analysis was then conducted on the renal and cardiovascular adverse events that occurred in the experimental groups compared to the control groups in Study 3 and Study 4. The adverse events that were compared were “ESKD”, and “CV death or hospitalization for heart failure”/”CV event”. Where ESKD was defined as when the patient required permanent dialysis. CV event was defined as percutaneous coronary intervention, coronary artery bypass surgery, thrombolysis therapy, cardiogenic shock, heart failure, malignant dysrhythmia, myocardial infarction, or stroke (Table 2 and Table 3).
The outputs of the meta-analyses are shown below in the same order as the tables above.
Number of studies: k = 2
Number of observations: o = 1834 (o.e = 535, o.c = 1299)
Number of events: e = 569
RR 95%-CI z
Common effect model 0.7581 [0.6349; 0.9052] -3.06
Random effects model 0.7578 [0.6348; 0.9048] -3.07
p-value
Common effect model 0.0022
Random effects model 0.0022
Quantifying heterogeneity
tau^2 = 0; tau = 0; I^2 = 0.0%; H = 1.00
Test of heterogeneity
Q d.f. p-value
0.01 1 0.9183
Details of meta-analysis methods
- Mantel-Haenszel method (common effect model)
- Inverse variance method (random effects model)
- Restricted maximum-likelihood estimator for tau^2
- Calculation of I^2 based on Q (Figure 3)
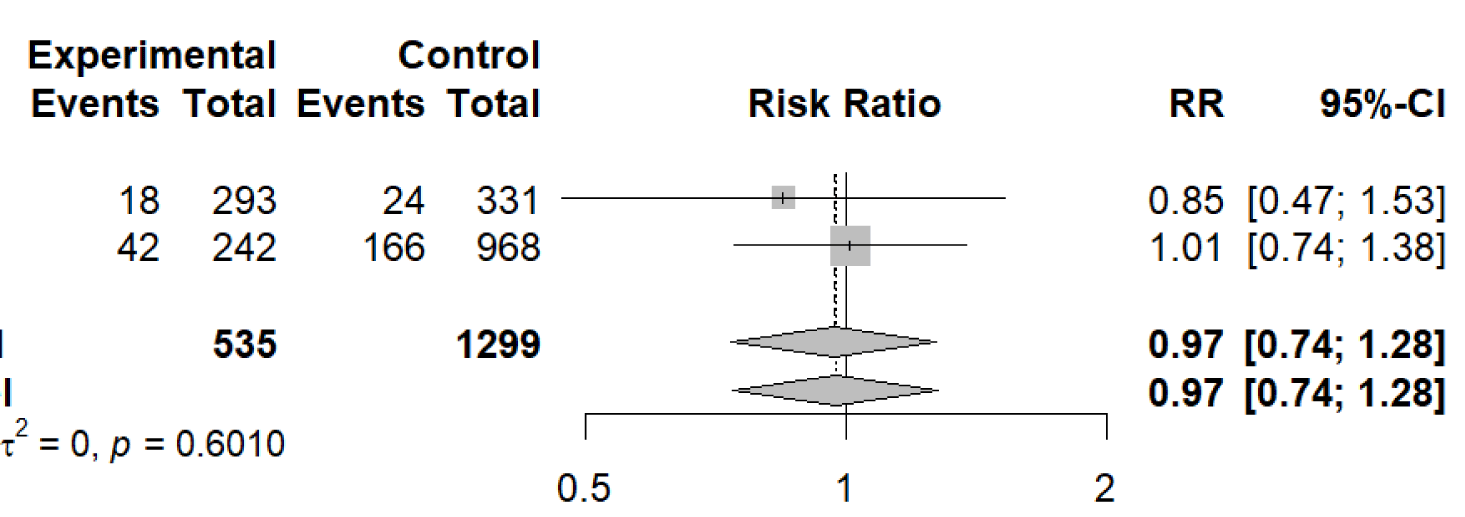
Number of studies: k = 2
Number of observations: o = 1834 (o.e = 535, o.c = 1299)
Number of events: e = 250
RR 95%-CI z
Common effect model 0.9703 [0.7383; 1.2751] -0.22
Random effects model 0.9743 [0.7416; 1.2802] -0.19
p-value
Common effect model 0.8287
Random effects model 0.8519
Quantifying heterogeneity
tau^2 = 0; tau = 0; I^2 = 0.0%; H = 1.00
Test of heterogeneity
Q d.f. p-value
0.27 1 0.6010
Details of meta-analysis methods
- Mantel-Haenszel method (common effect model)
- Inverse variance method (random effects model)
- Restricted maximum-likelihood estimator for tau^2
- Calculation of I^2 based on Q
These results are explained and interpreted in the discussion section of this paper (Figure 4).
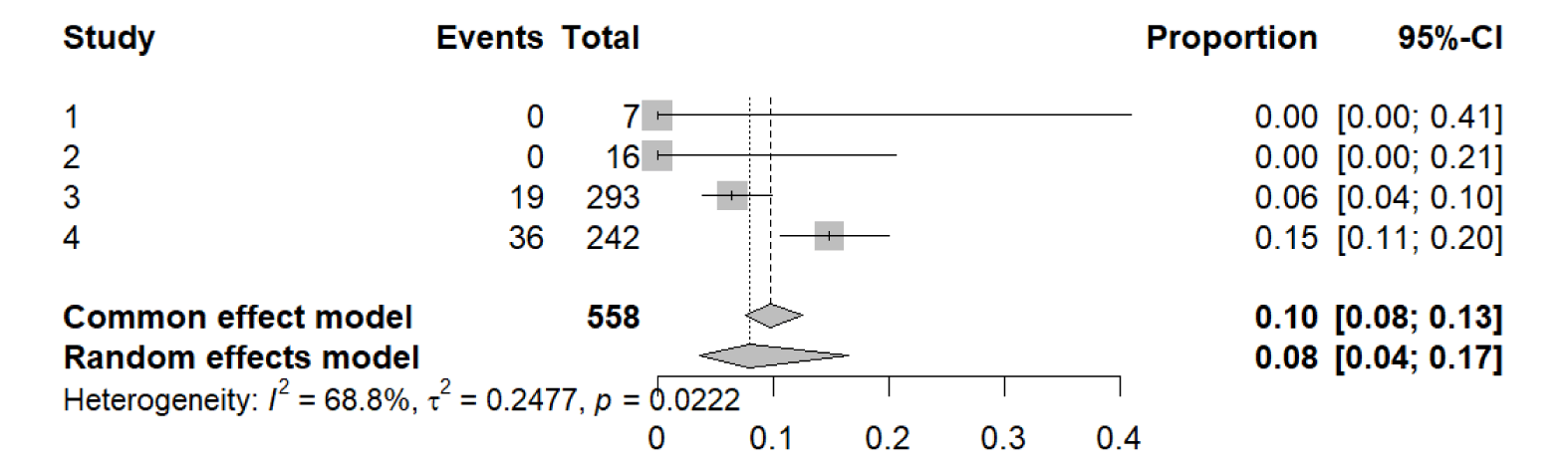
The final meta-analysis was conducted using the number of all-cause mortalities from each study to determine the rate of mortality in patients with eGFR < 20 mL/min/1.73 m2, including patients on dialysis. The following data was used to determine the rate of all-cause mortality that occurred in these patients taking SGLT2 inhibitors (Table 4).
The outputs of the meta-analysis are shown below.
Number of studies: k = 4
Number of observations: o = 558
Number of events: e = 55
proportion 95%-CI
Common effect model 0.0986 [0.0764; 0.1262]
Random effects model 0.0801 [0.0368; 0.1654]
Quantifying heterogeneity (with 95%-CIs):
tau^2 = 0.2477; tau = 0.4977
I^2 = 68.8% [9.7%; 89.2%]; H = 1.79 [1.05; 3.04]
Test of heterogeneity
Q d.f. p-value
Wald 9.61 3 0.0222
LRT 15.02 3 0.0018
Details of meta-analysis methods
- Random intercept logistic regression model
- Maximum-likelihood estimator for tau^2
- Calculation of I^2 based on Q
- Logit transformation
This data was obtained from the FDA Adverse Event Reporting System (FAERS) Public Dashboard and used to determine the rate of all-cause mortality in patients taking SGLT2 inhibitors with eGFRs > 30 mL/min/1.73 m2 [6].
The percentage of all-cause mortality reported by the FDA was then compared to the value calculated from the studies analyzed in this paper (Figure 5) (Table 5).
Discussion
The first meta-analysis was performed using the random-effects model on the eGFRs over time. The results of the meta-analysis showed that there is a statistically significant overall decline in eGFR across the studies. The heterogeneity assessment shows that there was almost no difference between the studies and the decline of eGFRs were consistent across all three references included. These results indicate that SGLT2 inhibitors caused eGFRs to decline slowly in patients with advanced CKD (eGFR < 20 mL/min/1.73 m2) and patients on dialysis. Therefore, the SGLT2 inhibitors slow the decline of eGFRs and preserve residual kidney function in patients with advanced CKD including those on dialysis.
The next two meta-analyses were conducted on adverse outcomes to determine if the SGLT2 inhibitors prevent those outcomes in patients with eGFR < 20 mL/min/1.73 m2 not including patients on dialysis. The outcomes analyzed in this paper were “ESKD”, and “CV death or hospitalization for heart failure”/”CV event”. The meta-analyses were conducted using a meta-analysis of binary outcome data to compare the number of adverse outcomes that occurred in patients taking SGLT2 inhibitors to the control patients in references 3 and 4. The result of the meta-analysis on the “ESKD” outcomes showed that there was a statistically significant decrease in the number of patients who developed ESKD when taking SGLT2 inhibitors. SGLT2 inhibitor treatment reduced the risk of ESKD and progression to permanent dialysis by 24% relative to the controls. The result of the meta-analysis on the “CV death or hospitalization for heart failure”/”CV event” outcomes showed that there was not a statistically significant decrease in the number of patients who were hospitalized for heart failure/cardiovascular death, or had a cardiovascular event. SGLT2 inhibitor treatment reduced the risk of hospitalization for heart failure/cardiovascular death or cardiovascular events by only 3% relative to the controls. Although these studies do not show a statistically significant decrease in cardiovascular events that occurred, there are other studies that indicate that SGLT2 inhibitors decrease the risk of cardiovascular events that occur in patients [4].
Throughout these studies, several patients had side effects due to the SGLT2 inhibitors. The side effects present in these studies were most commonly UTIs, AKI, and genital infections. However, SGLT2 inhibitors have been found to cause other side effects like hyperkalemia, DKA, or limb amputation. The occurrence of each side effect can be found in the individual studies. Overall, the side effects that occurred in the patients were able to be treated appropriately without stopping the SGLT2 inhibitors. The final meta-analysis was conducted on the all-cause mortalities in patients taking the SGLT2 inhibitors in all the studies. The meta-analysis found that the rate of all-cause mortality in patients with eGFR < 20 mL/min/1.73 m2, including those on dialysis, was about 8%. According to the FDA Adverse Event Reporting System (FAERS) Public Dashboard, of the patients reported taking SGLT2 inhibitors there was a reported rate of all-cause mortality of about 7% [6]. These results show that the rate of all-cause mortality in the studies analyzed is comparable to the FDA reported rate of all-cause mortality. Therefore, the use of SGLT2 inhibitors in patients with eGFR < 20 mL/min/1.73 m2 and those on dialysis does not impact the all-cause mortality when compared to patients with eGFR > 30 mL/min/1.73 m2.
In addition to the meta-analyses, study 1 showed that 2 patients out of the 7-patient study were able to discontinue hemodialysis due to their improvement in eGFRs. Reference 2 did not include any information about patients discontinuing hemodialysis.
Since there were only four studies that met the criteria to be included in this meta-analysis, the number of studies used in each of the meta-analyses was limited. There is currently very limited data on the use of SGLT2 inhibitors in patients with eGFR < 20 mL/min/1.73 m2 and those on dialysis. There is currently one randomized controlled trial being conducted on patients with eGFR < 25 mL/min/1.73 m2 and patients on dialysis called the RENAL LIFECYCLE [7]. This controlled trial will give more information about the use of SGLT2 inhibitors in patients with advanced CKD, including those on dialysis. More randomized controlled trials should be conducted on SGLT2 inhibitor use in patients with eGFR < 20 mL/min/1.73 m2 including those on dialysis to fully understand the long-term effects of these medications.
Conclusion
The outcomes of the meta-analyses performed in this paper showed that SGLT2 inhibitors slowed the decline of eGFRs when eGFR < 20 mL/min/1.73 m2 and prevent ESKD leading to dialysis in patients not already on dialysis. The first meta-analysis found that SGLT2 inhibitors slowed the decline of eGFRs in patients with eGFR < 20 mL/min/1.73 m2 including those on dialysis. The meta-analyses showed that SGLT2 inhibitors decreased the number of patients who progressed from stage 5 CKD to ESKD leading to permanent dialysis when compared to controls. The studies analyzed showed that the all-cause mortality rate in patients taking SGLT2 inhibitors with eGFR < 20 mL/min/1.73 m2, including those on dialysis, was comparable to the FDA reported percentage of all-cause mortality in patients with eGFR > 30 mL/min/1.73 m2 taking SGLT2 inhibitors. The data used in this paper was limited due to the limited amount of available data on SGLT2 inhibitor use in patients with advanced CKD and patients on dialysis. According to the data used in this analysis, SGLT2 inhibitors should be used in patients with eGFR < 20 mL/min/1.73 m2 to preserve renal kidney function and decrease mortality due to ESKD leading to dialysis.
References
- Andrew S Levey, Lesley A Inker (2025) Definition and staging of chronic kidney disease in adults. UpToDate.
- Mark Rosenberg (2025) Overview of the management of chronic kidney disease in adults. UpToDate.
- Center for drug evaluation and research. (n.d.), Sodium-glucose cotransporter-2 (SGLT2) Inhibitors. U.S. Food and drug administration.
- Dapagliflozin: Drug information. UpToDate.
- CKD-Epi creatinine equation (2021) National kidney foundation.
- FDA Adverse event reporting system (FAERS) Public Dashboard. Qlik sense.
- (2025) University Medical Center Groningen (n.d.) The renal lifecycle trial: A rct to assess the effect of dapagliflozin on renal and cardiovascular outcomes in patients with severe CKD. Clinicaltrials.gov.
Corresponding Author
Katherine Darrigrand, DeBusk College of Osteopathic Medicine, Lincoln Memorial University, 301 Martin St, Apt 11, London KY 40741, USA
Copyright
© 2025 Hasni K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.