Association Between Blood Flow Rate of Arteriovenous Access and Mortality in Prevalent Haemodialysis Patients: A Five-Year Prospective Study
Abstract
Background: An arteriovenous (AV) access with high blood flow (QA) value (>2 L/min) or low QA value (< 0.4 L/min) due to a significant stenosis can increase the risk of death in haemodialysis (HD) patients through high-output heart failure and underdialysis development, respectively. But, outside these extreme values, little is known regarding the significance of QA levels in AV accesses without stenosis on the mortality risk of HD patients in the absence of haemodynamic disturbance. The aim of this study is to analyse all-cause mortality of prevalent HD patients and its relationship with AV access function (QA value).
Methods: We prospectively measured QA of AV accesses in prevalent HD patients under routine QA surveillance for stenosis over a 5-year period.
Results: We performed 950 measurements of QA in 145 AV accesses of 131 HD patients for 2624 months of overall follow-up. The mortality rate was 24.4% (n=32), cardiovascular being the main cause of death (62.5%). Patients who died showed lower baseline (907.6±398.1) and overall (933.9±393.5) QA (ml/min) compared with survivors (1150.8±432 and 1207.3±476.6, respectively) (p=0.005 and 0.002, respectively). The adjusted hazard ratio for all-cause mortality was 1.280 (95% confidence interval CI, 1.057-1.551, p=0.012) for patient's age (for a 5-year increase) and 1.143 (95% CI, 1.009-1.294, p=0.035) for QA at baseline (for a decrease of 100 ml/min). Receiver operating characteristic curve analysis identified 948 ml/min as the best baseline cut-off QA value to predict all-cause mortality. The comparative analysis of Kaplan-Meier survival curves showed the lowest survival probability for HD patients with baseline QA value of AV access < 948 ml/min (λ2 = 16.4, p < 0.001, log-rank test).
Conclusions: In addition to patient's age, baseline QA of AV access was an independent predictor for all- cause mortality and could possibly represent a quantitative indicator of systemic vascular health in HD patients.
Keywords
All-cause mortality, Arteriovenous dialysis access, Blood flow rate
Introduction
Although a recent analysis of data from the European Registry showed an improvement in survival of European incident haemodialysis (HD) patients over time more than in the general population [1], mortality rate of HD patients remains a cause for concern. As shocking as the data, it has been demonstrated that survival of incident dialysis patients is lower than patients with several different solid-organ cancers [2]. In this regard, the vascular access (VA) for HD is considered as one potentially modifiable risk factor of death in HD patients [3,4].
The central venous catheter (CVC) for HD has been associated with an increased mortality risk in both incident and prevalent HD patients [3-5]. Compared to using an arteriovenous fistula (AVF) in the first HD session, there has been reported a hazard ratio (HR) of death for all-causes over time of 1.55 and 1.43 for incident patients starting HD through a tunneled and non-tunneled CVC, respectively [3].
Although to a lesser extent than CVC, the arteriovenous (AV) access also contributes to the excess of mortality linked with VA in HD patients [4-8]. The arteriovenous graft (AVG) has an intermediate mortality risk between CVC (the highest risk) and AVF (the lowest risk) [4,5]. AVF can also have an impact on HD patient survival [6-8]. In this respect, the negative effects of high-flow (QA) AVF have been previously described [6,9,10]. High-QA (>2 L/min) AVF can cause high-output heart failure development in some HD patients and, therefore, can increase the risk of death [6,11-13]. A low QA level (< 0.4 L/min) in an AV access with a significant stenosis can be associated with an increased mortality risk in the setting of under-dialysis [14-16]. In fact, the QA value and HD adequacy improves after successful elective AV access treatment for stenosis [17] and, therefore, the risk of death can return to baseline level. But, outside these extreme values, we have little information regarding the significance of QA levels in AV accesses without stenosis on the mortality risk of HD patients in a stable clinical condition and absence of haemodynamic disturbance [14,18-20]. The aim of this study is to analyze all-cause mortality of prevalent HD patients and its relationship with AV access function (QA value).
Methods
Patients
We conducted a survival sub-analysis from prevalent HD patients dialysed three times a week at the Hospital Universitari Mollet in Barcelona (Catalonia, Spain) and enrolled in a five-year single-centre prospective AV access stenosis surveillance study using QA measurements [17]. The patients' inclusion criteria were the following: 1) 18 years of age or older. 2) Clinical and haemodynamic stability. 3) HD treatment performed through AV access (either AVF or AVG). 4) AV access needling though two needles for at least 12 consecutive HD sessions. 5) reaching a pump flow higher than 250 ml/min in at least 12 consecutive HD sessions. 6) AV access without any signs of infection or suspicion of stenosis. 7) obtaining the informed consent form from patients.
The following demographic and clinical variables of patients and their AV accesses were assessed: gender, age, primary kidney disease, cardiovascular disease (either coronary artery disease, cerebrovascular disease and peripheral vascular disease), time on HD, single-pool Kt/V index, mean arterial pressure, type of AV access (AVF or AVG), history of previous AV accesses, ratio number AV access/patient, duration of current AV access, QA of AV access and successful elective AV access intervention for stenosis.
The primary outcome was all-cause mortality. The causes of death were obtained from patient reports and classified into seven categories as previously established [3]. The protocol of the study was approved by the local Clinical Research Committee and each patient signed the informed consent for its participation in the study.
Assessment of AV access function
The AV access function was evaluated at least every 4 months through QA determination by using the Delta- H method (Crit Line III monitor, ABF-mode, HemaMetrics, Kaysville, UT, USA). The baseline QA value was calculated from the QA values obtained in two consecutive HD sessions. The overall QA value was obtained after averaging all QA measurements recorded during the study period. In addition, the last QA obtained in a stable systemic haemodynamic period before the patient's death was also registered. The mean arterial pressure (diastolic plus 1/3 pulse pressure) and HD adequacy (single-pool Kt/V index) were measured at the same time as each QA determination.
Surveillance protocol for AV access stenosis
All AV accesses showing an absolute QA < 700 ml/min or a fall in QA>20% over time from baseline were referred for angiography. A subsequent elective intervention through percutaneous transluminal angioplasty or revision surgery was performed if a significant AV access stenosis was confirmed, that is, a luminal narrowing greater than 50% compared with the adjacent vessel segment.
Statistical analysis
Data analysis was performed using SPSS for Windows, version 20.0 (SPSS Inc., Chicago, IL, USA). The values were expressed as mean±standard deviation, percentages and 95% confidence interval (CI) as appropriate.
Differences between means of groups were assessed by two-sample independent t test. To assess the relationship between the last QA obtained before the patient's death and the continuous variables considered, a Pearson's correlation test was applied. A multivariate Cox regression analysis was performed to calculate the adjusted HR for all-cause mortality.
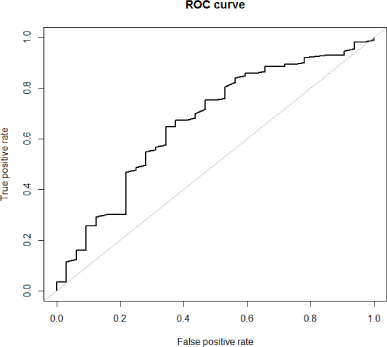
A time-dependent receiver operating characteristic (ROC) curve analysis was performed to determine the optimal threshold value for baseline QA of AV access to predict all-cause mortality.
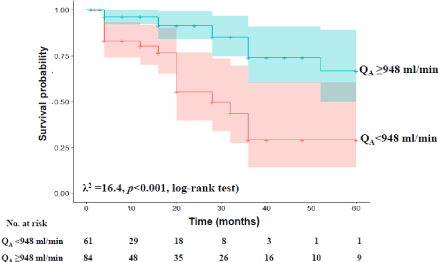
The survival probability of HD patients was analysed using Kaplan-Meier survival curves performed from the cut-off baseline QA value of AV access obtained and these were compared by log-rank test. Statistical significance was defined as p < 0.05.
Results
Overall characteristics of the study population
One hundred and thirty-one patients aged 62.6±13.5 years and dialysed through 145 AV accesses (84.1% AVF) met the inclusion criteria and were enrolled in the prospective study; ten patients participated in this study with two AV accesses and two patients with three AV accesses. Of them, 950 QA measurements were performed for 2624 months of overall follow-up. The mean baseline and overall QA (ml/min) obtained from these 145 AV accesses were 1097.1±435.3 and 1166.6±473.1, respectively.
Mortality analysis
Thirty-two patients (24.4%) died during follow-up. The leading cause of death was cardiovascular (62.5%, n=20) followed by infection (18.8%, n=6), unknown (12.5%, n=4), liver disease (3.1%, n=1) and cancer (3.1%, n=1). Cardiovascular causes were the following: congestive heart failure (n=6), myocardial infarction (n=6), stroke (n=5), sudden death (n=2) and arrhythmia (n=1).
Comparative characteristics of the patients who died and who survived
Table 1 shows the comparative analysis of both groups of patients. At baseline, patients who died were significantly older and tended to have a greater percentage of at least one cardiovascular comorbidity compared with survivors. No differences in the distribution of AVF and AVG between both groups of patients were recorded.
Functional evaluation of AV accesses in patients who died and who survived
Patients who died had significantly lower baseline and overall mean QA compared with survivors. The proportion of patients who underwent successful VA intervention for stenosis during follow-up did not differ between both groups of patients (Table 1). The last mean QA obtained before the patients' death, but within the period when they were still clinically stable, was 883.8±435.9 ml/min.
Correlations with the last QA obtained before the patient's death
Among the continuous variables included at baseline in Table 1, only baseline QA were positively correlated with the last QA obtained before the patient's death (Pearson' correlation coefficient 0.908, p < 0.001).
Independent predictors for all-cause mortality
Patient's age [for a 5-year increase, HR 1.280 (95% CI, 1.057-1.551), p=0.012] and QA of AV access at baseline [for a decrease of 100 ml/min, HR 1.143 (95% CI, 1.009-1.294), p=0.035] were independently associated with all-cause mortality.
Time-dependent ROC curve analysis of baseline QA for all-cause mortality
The ROC curve analysis identified the baseline QA of 948 ml/min as the best threshold value to predict all- cause mortality (sensitivity 65.6%, specificity 64.6%, positive predictive value 34.4%, negative predictive value 86.9%). The area under the ROC curve was 0.668 (95% CI: 0.558-0.778, p < 0.001) (Figure 1).
Survival probability of HD patients according to baseline cut-off QA value of AV access
Kaplan–Meier survival curves were different according to the optimal cut-off QA value of AV access for all-cause mortality obtained at baseline. As seen in Figure 2, survival probability of HD patients was significantly lower in patients with a baseline QA value of their AV accesses lower than 948 ml/min compared with the remaining patients (λ2 =16.4, p < 0.001, log-rank test).
Discussion
In the current study, patient's age was an independent predictor for all-cause mortality and the risk of mortality rose by 28% for every 5 years of increase in patient's age. In addition, patients who died tended to have a greater incidence of cardiovascular disease compared with survivors. In this regard, data of the Dialysis Outcomes and Practice Patterns Study, from 16,720 HD patients across seven countries followed up to 5 years, showed that most cardiovascular comorbidities and patient's age were associated with increased risk of death, with this risk increasing by 3% to 4% per year [21]. From data of the Catalan registry (period 1997-2019, n=22,404), considering four age groups of HD patients (< 44, 45-64, 65-74 and > 74 years-old), the risk of death for all-causes over time rose when increasing the age bracket and it was 3.92-fold higher for HD patients older than 74 years (n=7324) than those younger than 44 years-old (reference group, n=2228) [22].
In our study, the baseline QA of AV access was an independent predictor for all-cause mortality in prevalent HD patients. There are few published studies on the association between the QA of AV access and mortality in HD patients [14,18-20]. Wu et al. conducted a retrospective observational cohort study including 378 prevalent HD patients and showed that a lower QA level (< 1000 ml/min) of AV access (85% AVF) was an independent predictor of both short-term and long-term all-cause mortality; in addition, they identified the QA value of 1020 ml/min as the most discriminatory cut-off point for all-cause mortality [18]. A recent retrospective cohort study of 165 HD dialysed through an AV access (88.5 % AVF) identified a baseline QA of 900 mL/min as the optimal cut-off value for cardiovascular mortality and showed that HD patients having an initial QA < 900mL/min were almost 4 times more likely to die from a cardiovascular event in the first 4 years after starting HD compared with the remaining patients [20].
In the present study, the risk of mortality rose by 14.3% for every 100 ml decrease in QA at baseline. How could we explain this inverse relationship between baseline QA of AV access and mortality risk of HD patients in a stable clinical condition? A reduced QA value recorded in an AV access without stenosis can be indicative of structural vessel wall alterations, such as the presence of microcalcifications on the media layer of the afferent artery involved in the AV anastomosis [23], which can represent the iceberg tip of systemic vascular calcification including coronary arteries [24]. The clinical translation of this scenario could be an increase in the incidence of cardiovascular events and, thus, an increase in the mortality rate [24-26]. Therefore, a low baseline QA in stenosis-free AV accesses could be a local marker of generalised vascular calcification in HD patients. In this regard, microcalcifications on the media layer in the afferent artery, diagnosed by preoperative histological analysis of arterial specimens obtained just before surgery, have been proved to be independently associated, on the one hand, with a limitation of the final AV access function (low baseline QA) attained at the end of the AV access maturation process (23) and, on the other hand, with coronary artery calcification and/or increased cardiovascular mortality risk [24-26]. Interestingly, it has been shown that a deficiency in fetuin-A, a circulating glycoprotein potent inhibitor of vascular calcification, is associated with low QA levels of AV access [27] and, in addition, with an increase in all-cause and cardiovascular mortality risk of HD patients [28,29].
The main weakness of the present study is related to the limited number of patients included. In addition, our study shows the experience of a single centre and, therefore, the results obtained might not be extended to other centres.
The main strength was the attempt to find a baseline QA level of AV access as a predictor for all-cause mortality through an accurate assessment of AV access function by using almost a thousand QA determinations over a 5-year period. The type of VA at HD initiation has been included in most of predictive models of patient' survival [30,31]. But, in addition to this qualitative predictor for mortality, such as catheter yes/no, it could be important to have quantitative predictive variables on the mortality risk from the VA. In this regard, if our results are confirmed in further studies, the baseline threshold QA level of AV access, which determines a higher risk of mortality, could also be added in these predictive models.
In summary, in addition to patient's age, baseline QA of AV access was an independent predictor for all- cause mortality and could possibly represent a quantitative indicator of vascular health in HD patients.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval
Ethical approval was given by the Clinical Research Committee.
Contributorship
The paper was revised critically for important intellectual content and given final approval for publication by all the authors. RR-T made substantial contributions to the conception and design of the work, acquisition, analysis and interpretation of data, wrote the article and reviewed it.
References
- Rianne Boenink, Vianda S Stel, Bård E Waldum-Grevbo, et al. (2020) Data from the ERA-EDTA Registry were examined for trends in excess mortality in European adults on kidney replacement therapy. Kidney Int 98: 999-1008.
- Naylor KL, Kim SJ, McArthur E, et al. (2019) Mortality in Incident Maintenance Dialysis Patients Versus Incident Solid Organ Cancer Patients: A Population-Based Cohort. Am J Kidney Dis 73: 765-776.
- Roca-Tey R, Arcos E, Comas J, et al. (2016) Starting hemodialysis with catheter and mortality risk: persistent association in a competing risk analysis. J Vasc Access 17: 20-28.
- Foley RN (2017) Epidemiology and Risk Factors for Early Mortality After Dialysis Initiation. Semin Nephrol 37: 114-119.
- Pastan S, Soucie JM, McClellan WM (2002) Vascular access and increased risk of death among hemodialysis patients. Kidney Int 62: 620-626.
- Amerling R, Ronco C, Kuhlman M, et al. (2011) Arteriovenous fistula toxicity. Blood Purif 31: 113-120.
- Faull R, Rao N, Worthley M (2018) Do arteriovenous fistulas increase cardiac risk? Semin Dial 31: 357-361.
- Dikow R, Schwenger V, Zeier M, et al. (2002) Do AV fistulas contribute to cardiac mortality in hemodialysis patients? Semin Dial 15: 14-17.
- Vaes RH, Tordoir JH, Scheltinga MR (2014) Systemic effects of a high-flow arteriovenous fistula for hemodialysis. J Vasc Access 15: 163-168.
- MacRae JM, Levin A, Belenkie I (2006) The cardiovascular effects of arteriovenous fistulas in chronic kidney disease: a cause for concern? Semin Dial 19: 349-352.
- MacRae JM, Pandeya S, Humen DP, et al. (2004) Arteriovenous fistula-associated high-output cardiac failure: a review of mechanisms. Am J Kidney Dis 43: e17-22.
- Basile C, Lomonte C, Vernaglione L, et al. (2008) The relationship between the flow of arteriovenous fistula and cardiac output in haemodialysis patients. Nephrol Dial Transplant 23: 282-287.
- Roca-Tey R (2016) Permanent arteriovenous fistula or catheter dialysis for heart failure patients. J Vasc Access 17: S23-S29.
- Al-Ghonaim M, Manns BJ, Hirsch DJ, et al. (2008) Relation between access blood flow and mortality in chronic hemodialysis patients. Clin J Am Soc Nephrol 3: 387-391.
- Amerling R (2012) Con: On cardiovascular outcomes and the arteriovenous fistula: lesser of evils. Nephrol Dial Transplant 27: 3756-3757.
- Held PJ, Port FK, Wolfe RA, et al. (1996) The dose of hemodialysis and patient mortality. Kidney Int 50: 550-556.
- Roca-Tey R, Samon R, Ibrik O, et al. (2012) Five years of vascular access stenosis surveillance by blood flow rate measurements during hemodialysis using the Delta-H method. J Vasc Access 13: 321-328.
- Wu CK, Wu CL, Lin CH, et al. (2017) Association of vascular access flow with short-term and long-term mortality in chronic haemodialysis patients: a retrospective cohort study. BMJ Open 7: e017035.
- Hu Z, Zhu F, Zhang N, et al. (2017) Impact of arteriovenous fistula blood flow on serum il-6, cardiovascular events and death: An ambispective cohort analysis of 64 Chinese hemodialysis patients. PLoS One 12: e0172490.
- Yadav R, Gerrickens MWM, van Kuijk SMJ, et al. (2021) Access flow volume (Qa) and survival in a haemodialysis population: an analysis of 5208 Qa measurements over a 9-year period. Nephrology Dialysis Transplantation: gfab242.
- Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. (2003) Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 14: 3270-3277.
- Organització Catalana de Trasplantaments (OCATT). Registre de malalts renals de Catalunya, informe estadístic 2019 (nº 35). Barcelona. Departament de Salut, Generalitat de Catalunya, April 2021.
- Roca-Tey R, Bordes R, Martínez-Cercós R, et al. (2019) The impact of pre-existing radial artery pathology by histological assessment on the maturation, function and patency of the radiocephalic fistula for hemodialysis. Int Angiol 38: 239-249.
- Lee YB, Choi BM, Hwang HS, et al. (2017) The Impact of Arterial Micro- calcifcation of the Vascular Access on Coronary Artery Calcifcation and Cardiovascular Mortality in Incident Hemodialysis Patients. Korean J Med 92: 269-276.
- Yun YS, Choi SJ, Lee JY, et al. (2014) Impact of arterial microcalcification of the vascular access on cardiovascular mortality in hemodialysis patients. Hemodial Int 18: 54-61.
- Lee JY, Kim YO (2017) Pre-existing arterial pathologic changes affecting arteriovenous fistula patency and cardiovascular mortality in hemodialysis patients. Korean J Intern Med 32: 790- 797.
- Roca-Tey R, Ramírez de Arellano M, González-Oliva JC, et al. (2021) Is fetuin-A a biomarker of dialysis access dysfunction? J Vasc Access 11297298211035846.
- Ketteler M, Bongartz P, Westenfeld R, et al. (2003) Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet 361: 827-833.
- Stenvinkel P, Wang K, Qureshi AR, et al. (2005) Low fetuin-A levels are associated with cardiovascular death: Impact of variations in the gene encoding fetuin. Kidney Int 67: 2383-2392.
- Mauri JM, Clèries M, Vela E, et al. (2008) Design and validation of a model to predict early mortality in haemodialysis patients. Nephrol Dial Transplant 23: 1690-1696.
- Siga MM, Ducher M, Florens N, et al. (2020) Prediction of all- cause mortality in haemodialysis patients using a Bayesian network. Nephrol Dial Transplant 35: 1420-1425.
Corresponding Author
Ramon Roca-Tey, Tamarit 144-146, 08015-Barcelona, Spain, Tel: 00-34-690352100, Fax: 00-34-93-5760304
Copyright
© 2022 Roca-Tey R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.