Effects of Different Doses of Oral Essential Amino Acid Supplementation in the Patients Aged over 80 Years on Chronic Hemodialysis
Abstract
Background: The prevalence of malnutrition especially of essential amino acid (EAA) is a significant concern in end-stage renal disease (ESRD) and hemodialysis (HD) patients. The present study evaluates the efficacy of Amiyu® Granule (AG), a pharmaceutically manufactured oral EAA and histidine supplement, in the improvement of the nutritional indices of the ESRD and HD elderly patients (> 80-year-old).
Methods: Hypoalbuminemia (2.7-3.4 g/dL) was observed in nine of the 175 patients on HD. We examined the nutritional indices, anemia, and renal function markers for 12 months in the chronic HD octogenarian patients given a low dose (five cases, 2.5 g/day) or a moderate dose (four cases, 5 g/day) of daily oral AG.
Results: The serum albumin (Alb) levels increased significantly in the octogenarian ESRD patients treated with 2.5 g vs. 5 g of AG (3rd month after treatment: 3.2 ± 0.1 g/dL vs. 3.6 ± 0.2 g/dL, P < 0.05; 6th month: 3.3 ± 0.2 g/dL vs. 3.6 ± 0.3 g/dL, not significant [ns]; one year: 3.3 ± 0.4 g/dL vs. 3.5 ± 0.3 g/dL, ns). The geriatric nutritional risk index was higher with 5 g than with 2.5 g AG, however, the difference was not significant. There was no significant difference in the hemoglobin, hematocrit, serum creatinine, blood urea nitrogen, corrected calcium, and phosphate levels with 2.5 g vs. 5g AG at 3rd month-one year.
Conclusion: AG, at a moderate dose (5.0 g/day) or a low dose (2.5 g/day), provides oral EAA and histidine supplementation and is a potentially effective therapy for treating malnutrition, serum Alb levels, in the chronic HD octogenarian patients at the 6th month-one year.
Keywords
Geriatric nutritional risk index (GNRI), Hemodialysis (HD), Hypoalbuminemia, Octogenarian patients, Oral essential amino acid, Histidine supplementation (Amiyu granule®).
Introduction
The advent of hemodialysis (HD) has greatly improved survival in end-stage renal disease (ESRD) patients [1]. However, the progression of cardiovascular disease remains a major clinical concern in these patients. Additionally, the incidence of malnutrition especially essential amino acid (EAA) is also frequently encountered in the ESRD patients undergoing HD.
Eight different amino acids are considered essential-threonine, valine, methionine, isoleucine, leucine, phenylalanine, tryptophan, and lysine. Previously, oral EAA and histidine supplementation have been described for ESRD and chronic HD patients [2-5]. In Japan, the oral EAA and histidine supplementation (Amiyu® Granule [AG], 7.5 g/day or three powders/day [one after every meal], usual dosage) has been used for chronic HD patients (age 16-75 years) for several decades [6-8].
In this study, we evaluated the beneficial and adverse effects of EAA and histidine supplementation (AG 2.5 g/day, one powder, a low dosage or AG 5.0 g/day, two powders, a moderate dosage) in the octogenarian ESRD patients (> 80-year-old; yo) with hypoalbuminemia on maintenance HD. Furthermore, the effect of nutritional supplementation with AG on serum calcium (Ca) and phosphate (P) levels homeostasis was also assessed.
Methods
Patients
A total of 175 patients with ESRD on maintenance HD at Fukumitsu Clinic and Toma Clinic were examined. The effect of the pharmaceutical preparation of oral EAA and histidine was evaluated in the only five or four ESRD octogenarian patients (> 80 yo) undergoing chronic HD (AG 2.5 g/day; one powder, three cases male, two cases female; mean age: 83.6 ± 1.1 years [standard deviation (SD)]; age range: 82-85 years with a mean duration of HD of 20.6 ± 19.1 months, or AG 5.0 g/day; two powders, two cases male, two cases female; mean age: 87.3 ± 2.8 years; age range: 84-90 years with a mean duration of HD of 26.8 ± 37.6 months) and the presence of hypoalbuminemia (serum albumin [Alb] levels: AG 2.5 g/day; 3.1 ± 0.2 [2.9-3.3] g/dL or AG 5.0 g/day; 3.1 ± 0.3 [2.7-3.4] g/dL).
Patients with serum Alb > 3.5 g/dL were excluded from the study because the serum Alb levels cannot be increased above baseline by the use of oral EAA and histidine supplementation (AG) in those with levels greater than the 3.5 g/dL threshold in Japan [6,9,10]. Thus, the very few ESRD patients (> 80 yo and serum Alb levels: 2.7-3.4 g/dL) were selected and examined from the 175 HD pateints.
Oral EAA and histidine supplementation (AG 2.5 g/day, one powder/day, one powder at breakfast, low dosage; or AG 5.0 g/day, two powders/day, one powder at breakfast and one at supper, moderate dosage; 2.12 g amino acid per powder) was performed for a period of twelve months between the overall study period of February 2016 and June 2020. The serum nutriotinal indices, renal and hapatic functional markers, Ca, P, and the presence of anemia were assessed in these patients.
The present study was performed using a non-randomized analysis at the two centers sites with a prospective and non-controlled design. The study procedures were carried out in accordance with the ethical standards of the Human Investigation Committee at Fukumitsu Clinic and Toma Clinic (Fukumitsu - 4 - 2021). All patients gave their oral informed consent.
All procedures performed in the study involving human participants were in accordance with the ethical standard of the institutional and/or national research committee with the 1964 Helsinki declaration and its later amendments or comparable ethical standard.
Dialysis schedule
The patients underwent 4-5 hour sessions of maintenance HD therapy thrice a week. We used a standard bicarbonate hollow-fiber dialyzer with the following buffer composition: 140 mEq/L sodium, 2.0 mEq/L potassium, 2.75 mEq/L calcium (Ca++), 1.0 mEq/L magnesium, 112 mEq/L chloride, 8 mEq/L CH3COO-, 27.5 mEq/L HCO3-, and 125 mg/dL glucose in the dialysate. The blood flow rate was 200 mL/min and the dialysate flow rate was kept constant at 500 mL/min.
Blood sampling
The serum total protein (TP), Alb, hemoglobin (Hb) hematocrit (Ht), serum creatinine (Cr), blood urea nitrogen (BUN), uric acid, corrected Ca (c-Ca), P, aspartate aminotransferase (AST), alanine aminotransferase (ALT), Alb/Globulin (Glb) ratio (A/G = Alb/Glb = Alb/[TP-Alb]), and geriatric nutritional risk index (GNRI) were measured in the non-fasted blood samples drawn immediately before HD and before treatment, in 3rd month, 6th month, and one year after treatment.
The GNRI was developed by modifying the nutritional risk index for elderly patients [11]. This index is calculated from the serum Alb levels and body weight (dry weight [DW] in the case of HD patients) using the following equation:
GNRI = 14.89 × Alb (g/dL) + 41.7 DW/ideal body weight (IBW)
DW/IBW was set to 1 when the HD patient's DW exceeded the IBW. The IBW in the present study was defined as the value calculated from the height and a body mass index of 22, because of its validity [12].
The target Hb level was based on the current recommended guidelines for the treatment of anemia in CKD as established by the National Kidney Foundation [13]. These guidelines suggest Hb in the range of 11 g/L to 12 g/L (Ht 33% to 36%) before HD in most patients treated with recombinant human erythropoietin [14].
The management of serum c-Ca and P concentrations was performed based on previously published recommendations [15]. Serum c-Ca and P levels were calibrated to 8.4-10.0 mg/dL and 3.5-6.0 mg/dL, respectively. The following medicines were administered to titrate the serum levels of Ca and P: Vitamin D receptor activators, calcium-sensing receptor, phosphate binders, and 2.75 mEq/L Ca++ in the dialysate.
Statistical analyses
The data are expressed as mean ± SD. Statistical differences were computed using the Mann-Whitney's U test and the Friedman test for non-parametoric data. A P value of < 0.05 was considered statistically significant.
Results
The treatment with AG did not result in significantly increased DW in the octogenarian patients undergoing chronic HD (Table 1). Significant increase in serum TP in the one powder vs. the two powders of AG (3rd month after treatmen: 6.2 ± 0.5 g/dL vs. 6.5 ± 0.3 g/dL, not significant [ns]; 6th month: 6.2 ± 0.5 g/dL vs. 6.5 ± 0.7 g/dL, ns; one year: 6.2 ± 0.3 g/dL vs. 6.5 ± 0.4 g/dL, ns), Alb (3rd month after treatment: 3.2 ± 0.1 g/dL vs. 3.6 ± 0.2 g/dL, P < 0.05; 6th month: 3.3 ± 0.2 g/dL vs. 3.6 ± 0.3 g/dL, ns; one year: 3.3 ± 0.4 g/dL vs. 3.5 ± 0.3 g/dL, ns), and A/G levels (3rd month after treatment: 1.1 ± 0.3 g/dL vs. 1.2 ± 0.2, ns; 6th month: 1.2 ± 0.4 g/dL vs. 1.2 ± 0.3; ns; one year: 1.2 ± 0.3 g/dL vs. 1.2 ± 0.1, ns).
The GNRI values were lower upon treatment with the one powder than with the two powders of AG (3rd month after treatment: 88.2 ± 3.9 g/dL vs. 93.0 ± 7.4, ns; 6th month: 90.3 ± 4.2 g/dL vs. 93.4 ± 8.7, ns; one year: 91.1 ± 8.4 g/dL vs. 92.4 ± 8.7, ns), however, the difference was not significant (Table 1).
As shown in the Table 2, the observed more increased or decreased in the Hb and Ht levels in the one than in the two powders groups at the 3rd month after treatment, 6th month, or one year were not statistically significant in the chronic HD octogenarian patients treated with the AG. However, the observed significantly more increased in the Hb and Ht levels in the one powder group than in the two powders group before treatment (P < 0.05).
The serum Cr, uric acid, c-Ca, P and BUN levels were not significantly altered by AG treatment (Table 3).
However, significant decrease were noted in serum AST levels in the one powder vs. the two powders groups (before treatment: 11 ± 1 mg/dL vs. 17 ± 6 mg/dL, P < 0.05; 3rd month after treatment: 10 ± 2 mg/dL vs. 20 ± 6 mg/dL, P < 0.05; 6th month: 10 ± 2 mg/dL vs. 18 ± 3 mg/dL, P < 0.05; one year: 10 ± 1 mg/dL vs. 17 ± 3 mg/dL, P < 0.05) (Table 3). Significant decrease were also noted in serum ALT levels in the one powder vs. the two powders groups (before treatment: 6 ± 2 mg/dL vs. 10 ± 2 mg/dL, P < 0.05; 3rd month after treatment: 7 ± 3 mg/dL vs. 11 ± 1 mg/dL, P < 0.05; 6th month: 6 ± 2 mg/dL vs. 11 ± 2 mg/dL, P < 0.05; one year: 5 ± 2 mg/dL vs. 10 ± 3 mg/dL, P < 0.05) with AG treatment (Table 3).
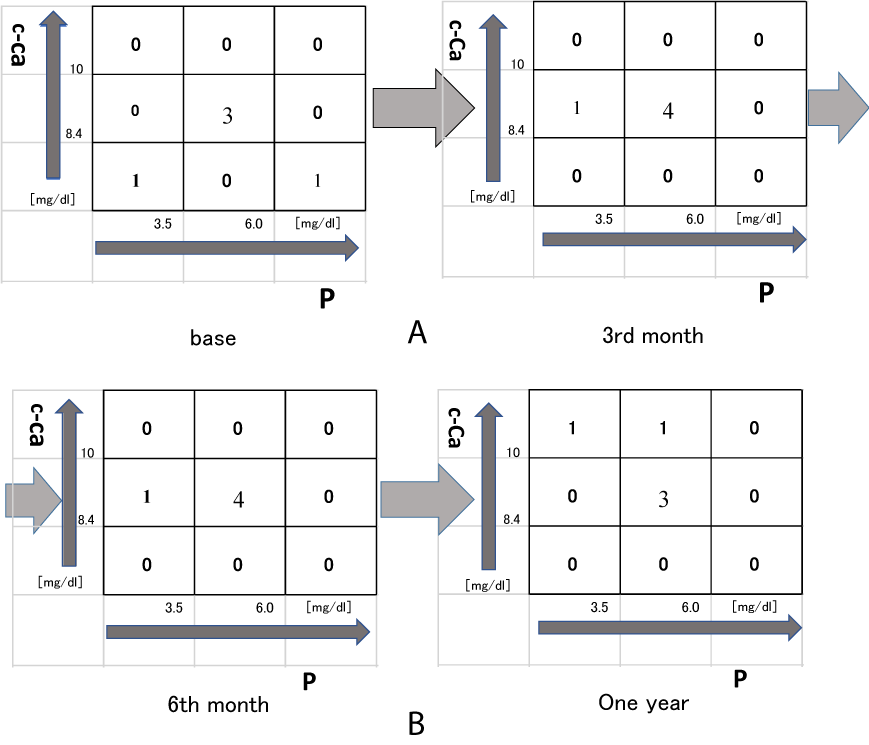
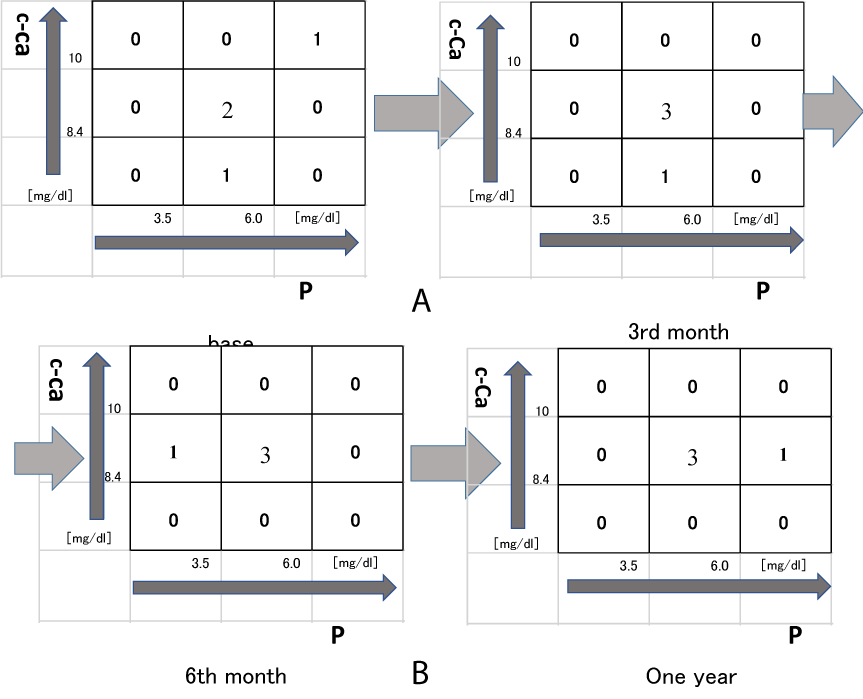
Serum c-Ca and P levels were tightly controlled during treatment with the moderate dose of AG 5.0 g/day or the low dose of AG 2.5 g/day. As previously reported, the nine-section chart was utilized as a tool to guide the achievement of optimal serum c-Ca and P levels [15]. The target ranges seleted for serum c-Ca and P levels were 8.4-10.0 mg/dL and 3.5-6.0 mg/dL, respectively. The target for serum c-Ca and P levels were achieved in 3 (60%), 4 (80%), 4 (80%), or 3 of 5 cases (60%) at the before treatment, 3rd month, 6th month or one year after treatment (Figure 1a and Figure 1b) in the one powder of AG. The target for serum c-Ca and P levels were achieved in 2 (50%), 3 (75%), 3 (75%), or 3 of 4 cases (75%) at the before treatment, 3rd month, 6th month or one year after treatment (Figure 2a and Figure 2b) in the two powders of AG. However, there were not significant in the chronic HD octogenarian patients (the nine-section chart) treated with the AG 2.5 g/day or 5.0 g/day.
No acute or chronic adverse effects associated with the moderate dose or the low doses AG in the HD octogenarian pateints were observed during the treatment and follow-up period.
Discussion
The present findings clearly indicate that AG, an oral EAA and histidine pharmaceutical preparation, is an effective and well tolerated nutritional supplement in the octogenarian ESRD and chronic HD patients. This study also demonstrates that while 7.5 g/day is the usual dose of AG in clinical practice, favorable effects are produced even with the low dosage AG (2.5 g/day) or the moderate dosage AG (5.0 g/day).
The serum Alb values were significantly higher in the moderate AG treated elderly-ESRD patients undergoing chronic HD at the 3rd month after treatment compared to the low AG (Table 1). We firmly believe that the oral EAA and histidine supplementation in the low or moderate dose can help to correct hypoalbuminemia [10] since the 6th month before treatment, and reduce mortality in the octogenarian ESRD patients.
The serum Alb, Hb, Ht, Cr, and BUN levels in low dose vs. moderate dose of AG were 3.2-3.4 g/dL vs. 3.5-3.6 g/dL, 11.0-11.6 g/L vs. 10.9-11.6 g/L, 34-35% vs. 34-36%, 7.8-8.2 mg/dL vs. 7.8-8.1 mg/dL, and 53-54 mg/dL vs. 54-59 mg/dL, respectively (Table 1, Table 2 and Table 3). The serum Alb and BUN levels were higher in the two powders of AG than in the one powder of AG, however, the difference was not significant, except for the serum Alb levels at the 3rd month after treatment.
Various nutritional screening tools have been described in the literature for the ESRD patients and those on maintenance HD [16]. These include the GNRI developed by modifying the nutritional risk index for elderly patients [11], the malnutrition-inflammation score (MIS) [17], the nutritional screening tools objective score of nutrition on dialysis (OSND) [18], the malnutrition screening tool (MST) [19], the malnutrition universal screening tool (MUST) [20], the nutritional screening tools nutritional risk screening 2002 (NRS-2002) [21], and the mini nutritional assessment (MNA-SF) [22]. However, only three of the above screening tools (GNRI, MIS, and OSND) are designed to assess the serum Alb levels in the chronic HD elderly patients.
The AST and ALT levels were significantly higher in the two powders than in the one powder before, at the 3rd month, the 6th month, and one year (Table 3). However, they were significantly higher in the two powders group than in the one powder group before treatment and such patients didn't have liver diseases.
The use of the nine-section chart as a clinical decision-making in the selection of appropriate therapeutic approaches for the correction of serum c-Ca and P levels has previously been described [15]. This approach entails the classification of the serum levels of c-Ca and P into one of the nine possible patterns followed by the selection of the appropriate treatment. A significant majority of patients achieved optimum levels of serum P and to a greater extent serum c-Ca, at both the 6th month and one year after treatment with the moderate dose of AG [9]. The present study implies that the supplementation with 2.5 g/day or 5.0 g/day AG may not control serum c-Ca and P levels within the target range during treatment in the chronic HD octogenarian patients with small sample size (Figure 1b and Figure 2b). Future prospective studies with larger sample size are needed to establish the validity of these findings.
This study is associated with several potential limitations. A limitation of the present study was the observational nature of the study performed in a prospective and non-controlled design with small sample size. Thus, this study was an observational study of daily clinical practice with 2.5 g/day or 5.0 g/day AG. The need for the oral EAA and histidine may partially reflect the physiological senile changes that are commonly found in the octogenarian individuals.
In conclusion, the oral administration of EAA and histidine supplementation in form of a pharmaceutical preparation named AG (5.0 g/day, moderate dose or 2.5 g/day, low dose) improved the serum Alb levels at the 6th months and one year in the octogenarian ESRD and chronic HD patients and might be an effective therapy for treating malnutrition and hypoalbuminemia in such patients. Future prospective studies with larger sample size are needed to establish the validity of these findings.
Acknowledgments
We are grateful to the staff members, especially, the hospital nurses, the clinical engineers, the medical clerks, at Fukumitsu Clinic and Toma Clinic for their valuable technical advice and critical reading of the manuscript.
Statement of Authorship
Study concept and design: T. Sanai and T. Onoue, analysis and interpretation of data: T. Sanai and M. Nagata, draft of the manuscript and approval of the final version: T. Sanai, T. Onoue, and T. Ono, and revision of manuscript for important intellectual content and approval of the final version: all authors.
Conflict of Interact (COI)
The authors declare no competing financial interests exit.
Funding
The authors received no direct funding for this research.
References
- Nitta K, Masakane I, Hanafusa N, et al. (2020) 2019 Annual dialysis data report, JSDT renal data registry. J Jpn Soc Dial Ther 53: 579-632.
- Rose WC (1957) The amino and requirements of adult man. Nutr Abst Rev 27: 631-647.
- Rose WC, Wixom RL, Lockhart HB, et al. (1955) The amino acid requirements of man. XV. The valine requirement; summary and final observation. J Biol Chem 217: 987-995.
- Fűst P (1972) 15N-studies in severe renal failure. II. Evidence for the essentiality of histidine. Scand J Clin Lab Invest 30: 307-312.
- Giordano C (1963) Use of exogenous and endogenous urea for protein synthesis in normal and uremic subjects. J Lab Clin Med 62: 231-246.
- Hirano N, Nakano T, Okada N (1979) Effect of oral essential amino acid in hemodialysis patients. Gendai Shinryo 21: 1037-1040.
- Magara E, Osawa G, Kinoshita Y, et al. (1979) Essential amino acid (AMI-UG) treatment for anemia in hemodialysis patients. Gendai Shinryo 21: 1041-1045.
- Teraoka S, Kawai T, Fujita S, et al. (1987) Adverse effectts of essential amino acid hyperalimentation in chronic uremic subjects. Jpn J Prent Ent Nutr 9: 195-202.
- Sanai T, Onoue T, Ono T, et al. (2021) Beneficial effects of oral essential amino acid supplementation in the elderly patients on chronic hemodialysis. J Nutr Sci Vetaminol, in revised.
- Kumagai E, Furumachi K, Miyata T, et al. (2016) Oral essential amino acid supplementation improves the serum amino acid profiles and albumin levels of hemodialysis patients with hypoalbuminemia. J Jpn Soc Dial Ther 49: 225-231a.
- Bouilanne O, Morineau G, Dupont C, et al. (2005) Geriatric nutritional risk index: A new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 82: 777-783.
- Shah B, Sucher K, Hollenbeck CB (2006) Comparison of ideal body weight equations and published height-weight tables with body mass index tables for healthy adults in the United States. Nutr Clin Pract 21: 312-319.
- Parfrey PS, Lauve M, Latremouille-Viau D, et al. (2009) Erythropoietin therapy and left ventricular mass index in CKD and ESRD patients: A meta-analysis. Clin J Am Soc Nephrol 4: 755-762.
- KDOQI (2007) Clinical practice guideline and clinical practice recommendation for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis 50: 471-530.
- Fukagawa M, Yokoyama K, Koiwa F, et al. (2013) Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial 17: 247-288.
- Yamada K, Furuya R, Takita T, et al. (2008) Simplified nutritional screening tool for patients on maintenance hemodialysis. Am J Clin Nutr 87: 106-113.
- Kalantar-Zadeh K, Kopple JD, Block G, et al. (2001) A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38: 1251-1263.
- Beberashvili I, Azar A, Sinuani I, et al. (2010) Objective score of nutrition on dialysis (OSND) as an alternative for the malnutrition-inflammation score in assessment of nutritional risk of haemodialysis patients. Nephrol Dial Transplant 25: 2662-2671.
- Ferguson M, Capra S, Bauer J, et al. (1999) Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 15: 458-464.
- Stratton RJ, Hackston A, Longmore D, et al. (2004) Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool' (‘MUST') for adults. Br J Nutr 92: 799-808.
- Reilly HM, Martineau JK, Moran K, et al. (1995) Nutritional screening-evaluation and implementation of a simple Nutrition Risk Score. Clin Nutr 14: 269-273.
- Kondrup J, Allison SP, Elia M, et al. (2003) ESPEN guidelines for nutritional screening 2002. Clin Nutr 22: 415-421.
Corresponding Author
Toru Sanai, MD, Division of Nephrology, Department of Internal Medicine, Fukumitsu Clinic, 4-10-1 Kashiihama, Higashi-Ku, Fukuoka-City, 813-0016 Fukuoka, Japan, Tel: +81-92-681-3331, Fax: +81-92-672-5154
Copyright
© 2021 Sanai T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.