A Novel Technique for Safe Laparoscopic-Assisted Subtotal Splenectomy in Children with Hereditary Spherocytosis
Abstract
Purpose
Partial splenectomy has emerged as a surgical option for children with hereditary spherocytosis. The goal of reducing anemia and hypersplenism while preserving splenic immunologic function to avoid potential Overwhelming Post-Splenectomy Infection (OPSI) is logical. This report evaluates the feasibility and safety of a novel technique that utilizes the advantages of laparoscopy to mobilize the spleen and division of short gastric arteries and veins followed by evisceration of the spleen to utilize the advantages of open surgical technique and ease of hemostasis during subtotal resection of the spleen. This technique of Laparoscopic-Assisted Subtotal Splenectomy (LASS) has not been reported.
methods
As part of a retrospective IRB-approved study, data were collected from all the children who were either attempted to or underwent LASS in our institution from January 2013 to July 2016. Data retrieval included age, gender, weight, operative time, complications, postoperative transfusion requirements, and follow-up examination.
Results
Six children were included. Five children underwent LASS successfully. One child required a total splenectomy during the procedure due to massive splenomegaly. Mean operative time was 105 minutes. There were no complications following the procedure and the average Length of Stay (LOS) was 2 days. Postoperatively, all children have had a sustained increase in hemoglobin and decrease in reticulocyte count and bilirubin levels. During a mean follow-up of 1.5 years (range 1 to 3 years) none of the children have required transfusion and they have been free from recurrent anemia and abdominal pain.
Conclusions
This report indicates that partial splenectomy for hereditary spherocytosis utilizing LASS is feasible and safe and may lead to a decrease in operative time and LOS. Long term follow-up is necessary, but our results support LASS as an alternative for children with hereditary spherocytosis who may otherwise require total splenectomy. Splenic preservation may provide an immunologic advantage for these children, with a potential reduction in OPSI.
Introduction
Hereditary Spherocytosis (HS) is a red blood cell membrane disorder characterized by hemolytic anemia and splenomegaly. Because the spleen serves as the main site of retention and hemolysis of abnormal erythrocytes, splenectomy is considered as an effective treatment for patients with moderate to severe disease [1]. Unfortunately, asplenia results in potential long-term morbidity that can lead to vascular thrombosis, pulmonary hypertension, and an immunocompromised state. For children, the immunocompromised state can be serious and may result in Overwhelming Post-Splenectomy Infection (OPSI), sepsis, and even death. OPSI risk following splenectomy in children is as high as 15%, with splenectomy for HS being the most significant predictor [2].
Partial or subtotal splenectomy has emerged as an alternative surgical option in HS. The objective of reducing the spherocytosis related hypersplenism and anemia while preserving splenic function to reduce the risk of OPSI is logical. While large studies have shown laparoscopic splenectomy is safe and efficacious, the technical aspects of laparoscopic partial splenectomy remain challenging [3-5]. The intracorporeal splenic resection can result in splenosis and significant hemorrhage. This pilot study reports the feasibility and safety of a novel technique in Laparoscopic-Assisted Subtotal Splenectomy (LASS).
Methods
Patients and clinical information
As part of our approved IRB protocol for evaluating innovative surgical technologies in children, data were collected retrospectively from all the children who were either attempted to or underwent LASS for HS in our institution from January 2013 to July 2016. All children received standard immunizations 2 weeks prior to the operations to prepare for potential total splenectomy. Data retrieval included age, gender, weight, blood tests (complete blood count and reticulocyte count), operative time, complications, perioperative transfusion requirements, length of stay, and follow-up time. They were all followed for at least six months with complete blood counts and reticulocyte counts.
Surgical technique
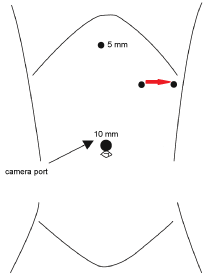
The approach to laparoscopic splenectomy is well reported. We utilized a standard four-port technique (Figure 1). Initial access was gained through a 10 mm umbilical port. In the left upper quadrant, 2 cm below the costal margin, two working ports were placed. These port sites were 6 cm apart and would be the medial and lateral margins of a small incision which would be used to eviscerate the fully mobilized spleen. A fourth subxiphoid working port was also placed.
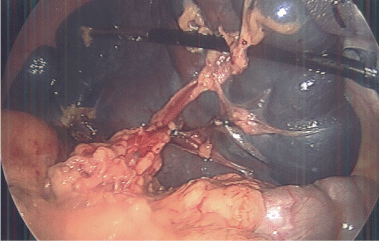
In our technique, we emphasize the selection of the lower pole of the spleen and its concomitant vascular pedicle as our preserved segment. Therefore, the spleen is mobilized first by the division of the gastrosplenic ligament and short gastric arteries and veins. This optimizes exposure of the splenic hilum and main splenic artery and vein. Dissection was carried out in the splenic hilum to expose the branches of the splenic vessels extending to the lower pole. With a combination of vascular staplers and clips, all branches except those to the lower pole were ligated (Figure 2). All visceral and retroperitoneal attachments to the spleen were divided.
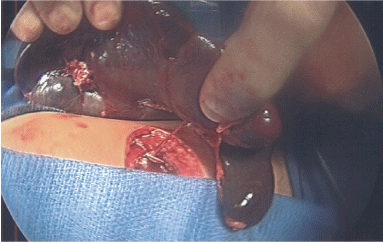
The critical component of our technique was the intracorporeal rotation of the spleen so as to keep the superior tip inferior to and below the costal margin. The superior tip of the spleen is then grasped via the lateral port in the left upper quadrant. The abdomen was opened at that site by connecting the two left upper quadrant port sites, and the spleen is eviscerated on the remaining vascular pedicle (Figure 3). The spleen was then inspected. If the inferior pole of the spleen is viable and well vascularized, the spleen was transected. The eviscerated spleen permits direct and controlled intermittent vascular occlusion to allow for transection with minimal hemorrhage.
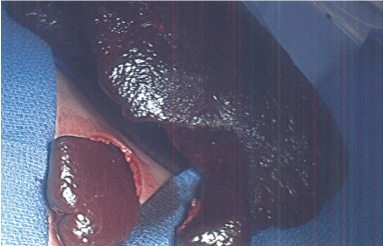
After transection, we utilized the argon beam coagulator and topical hemostatic agents to ensure hemostasis on the cut edge before the remnant is returned to the intracorporeal position. Our preserved remnant was approximately four cubic centimeters in all cases (Figure 4).
Results
Six children were included in our study. Five children underwent successful LASS based on a lower pole artery and vein branch. One child required laparoscopic total splenectomy during the procedure due to massive splenomegaly and inability to safely dissect the hilar vessels. The children ranged in age from 4 to 9 years. Four boys and 2 girls. Mean operative time was 105 minutes (range 75 to 135 minutes). There were no complications in the group and the average Length of Stay (LOS) was two days (range 1 to 3 days). On follow-up, all children have had a sustained increase in hemoglobin > 11 g/dL and none required transfusion. They have all had a decrease in reticulocyte count and bilirubin level. There have been no episodes of acute abdominal pain to suggest torsion of the vascular pedicle. None of the children with successful LASS have returned for completion splenectomy. Mean follow up was 1.5 years (range 1 to 3 years) (Table 1 and Table 2).
Discussion
Spleen surgery is the recommended treatment of children with HS who suffer from hypersplenism, anemia, transfusion requirements and failure to thrive [1]. Historically, splenectomy via an open or minimally invasive approach was the only surgical option for these children. While splenectomy is the gold standard for severe HS, the potential for future infectious and vascular morbidity, especially in children, can be significant Partial splenectomy has emerged as a reasonable alternative with the objective to remove enough spleen to minimize the risk of the hematologic consequences of HS but preserve enough spleen to maintain optimal immune function [4]. The minimally invasive approach to partial splenectomy is technically challenging, and reports are limited to case series from high-volume centers [4,6-10]. Our results indicate that LASS is feasible, safe, and may lead to a decrease in operative time, LOS and bleeding compared to traditional splenectomy.
Advances in Minimally Invasive Surgery (MIS) have resulted in an optimal approach to splenectomy associated with less pain, decreased adhesion formation, improved cosmesis and faster recovery when compared to the open approach [11-13]. Although our technique requires lengthening one of the laparoscopic port sites to permit evisceration, the size of the specimen retrieval incision is significantly smaller than what would be required to achieve splenectomy via a traditional open laparotomy. Our approach takes advantage of improved laparoscopic exposure, magnification and focused dissection. In addition, we are able to avoid bleeding and loss of visualization by performing the partial resection in the extracorporeal position. We believe this is an excellent compromise that mitigates many of the current limitations associated with a traditional laparoscopic partial splenectomy without committing the patient to a large open operation.
Our approach to preserve a four-cubic centimeter of the spleen is controversial. Data regarding the optimal splenic remnant after partial splenectomy is limited. Animal studies have shown a spleen remnant of 25% of the normal spleen volume will retain immunologic function [6]. Reported remnant volume in humans undergoing partial splenectomy is variable. Most studies report preservation of 10-30% of normal spleen volume, but some have argued for a more extensive resection with a remnant of 5% [10,14,15]. In our series, the extracorporeal resection allowed for a standardized resection and a remnant volume of four cubic centimeters. In all cases, we were also able to observe a line of demarcation that clearly demonstrated a well-perfused volume of the remnant spleen. Our evisceration strategy allowed for precise measurements and division of a four-cubic centimeter of spleen that was well vascularized. None of the children in this report have suffered infectious complications and it appears we have eliminated the metabolic consequences of their HS. However, follow-up time in our series was short and we did not assess splenic perfusion or function postoperatively. These factors will need to be incorporated into future prospective studies and could be measured with spleen scan and opsonin levels respectively.
Our decision to preserve the inferior vascular supply to the spleen is controversial as well. Preservation of the upper pole of the spleen is facilitated by the preservation of the short gastric vessels, which will provide adequate perfusion and outflow as has been demonstrated in postoperative imaging [6]. Theoretically, the broad short gastric pedicle decreases the risk of torsion. With a laparoscopic approach, preservation of the upper pole is the more commonly performed technique and dissection of the hilum may be technically challenging [6-9]. In contrast, preservation of the lower pole is performed routinely in open partial splenectomy and may allow for more accurate evaluation of the residual splenic mass [5,10]. Our LASS technique begins with the ligation of the short gastric vessels. This allows for safe inspection of the hilum and ease of evisceration. We also divide all splenic ligaments, which improves exposure and visualization of the hilum. Although none of the children in this report have suffered symptoms of splenic torsion, the narrow lower pole vascular pedicle poses a theoretical risk. Further follow-up is indicated.
One consideration of subtotal splenectomy that deserves mention is regrowth of the splenic remnant and recurrence of symptomatic HS. The studies that have followed patients after partial splenectomy with imaging demonstrate splenic regrowth to 15-30% of original size at two years and 40% at four years [1,14,16,17]. Despite regrowth, completion splenectomy is rarely required [1,4,11,17]. Our report is limited by lack of dedicated imaging at follow up to determine the degree of splenic regrowth. None the children in our series required completion splenectomy. However, our follow up is short. We believe that future prospective analysis will be necessary to consider this issue and long term follow-up in these patients is warranted.
Conclusion
In conclusion, our report indicates that our novel LASS technique that utilizes the advantages of laparoscopy to mobilize the spleen and division of short gastric arteries and veins followed by evisceration of the spleen to utilize the advantages of open surgical technique and ease of hemostasis during resection is feasible and safe. LASS may lead to a decreased operative time and length of stay in these children with hereditary spherocytosis. Partial splenectomy provides sustained laboratory and clinical improvement in most children. Prospective studies and long-term follow-up are needed to determine the role for partial splenectomy and the optimal splenic remnant to preserve immunologic function. However, our results support LASS as an alternative for children who may otherwise require total splenectomy for HS.
References
- Casale M, Perrotta S (2011) Splenectomy for hereditary spherocytosis: Complete, partial or not at all? Expert Rev Hematol 6: 627-635.
- Barsness K, Reynolds M (2012) The Spleen. In: Coran, Pediatric Surgery. Elsevier, Philadelphia, USA, 1390-1392.
- de Buys Roessingh AS, de Lagausie P, Rohrlich P, et al. (2002) Follow-up of partial splenectomy in children with hereditary spherocytosis. J Pediatr Surg 37: 1459-1463.
- Buesing KL, Tracy ET, Kiernan C, et al. (2011) Partial splenectomy for hereditary spherocytosis: A multi-institutional review. J Pediatr Surg 46: 178-183.
- Bader-Meunier B, Gauthier F, Archambaud F, et al. (2001) Long-term evaluation of the beneficial effect of subtotal splenectomy for management of hereditary spherocytosis. Blood 97: 399-403.
- Dutta S, Price VE, Blanchette V, et al. (2006) A laparoscopic approach to partial splenectomy for children with hereditary spherocytosis. Surg Endosc 20: 1719-1724.
- Héry G, Becmeur F, Méfat L, et al. (2008) Laparoscopic partial splenectomy: Indications and results of a multicenter retrospective study. Surg Endosc 22: 45-49.
- Morinis J, Dutta S, Blanchette V, et al. (2008) Laparoscopic partial vs. total splenectomy in children with hereditary spherocytosis. J Pediatr Surg 43: 1649-1652.
- Slater BJ, Chan FP, Davis K, et al. (2010) Institutional experience with laparoscopic partial splenectomy for hereditary spherocytosis. J Pediatr Surg 45: 1682-1686.
- Vasilescu C, Stanciulea O, Tudor S, et al. (2006) Laparoscopic subtotal splenectomy in hereditary spherocytosis: To preserve the upper or the lower pole of the spleen? Surg Endosc 20: 748-752.
- Brunt LM, Langer JC, Quasebarth MA, et al. (1996) Comparative analysis of laparoscopic versus open splenectomy. Am J Surg 172: 596-599.
- Katkhouda N, Mavor E (2000) Laparoscopic splenectomy. Surg Clin North Am 80: 1285-1297.
- Minkes RK, Lagzdins M, Langer JC (2000) Laparoscopic versus open splenectomy in children. J Pediatr Surg 35: 699-701.
- Hollingsworth CL, Rice HE (2010) Hereditary spherocytosis and partial splenectomy in children: Review of surgical technique and the role of imaging. Pediatr Radiol 40: 1177-1183.
- Stoehr GA, Stauffer UG, Eber SW (2005) Near-total splenectomy: A new technique for the management of hereditary spherocytosis. Ann Surg 241: 40-47.
- Tchernia G, Bader-Meunier B, Berterottiere P, et al. (1997) Effectiveness of partial splenectomy in hereditary spherocytosis. Curr Opin Hematol 4: 136-141.
- Rice HE, Oldham KT, Hillery CA, et al. (2003) Clinical and hematologic benefits of partial splenectomy for congenital hemolytic anemias in children. Ann Surg 237: 281-288.
Corresponding Author
Jeffrey R Lukish, MD, Associate Professor of Surgery, Division of Pediatric Surgery, Department of Surgery, Johns Hopkins University, Bloomberg Children's Center Suite 7353, 1800 Orleans Street, Baltimore, Maryland 21287-0005, USA, Tel: 410-955-5628, Fax: 410-502-5314.
Copyright
© 2017 Kovler M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.