Simultaneous Bilateral Surgery as a Model for Learning Video Endoscopic Inguinal Lymphadenectomy (VEIL)
Abstract
Purpose
Video Endoscopic Inguinal Lymphadenectomy (VEIL) is feasible and safe in selected patient with groin metastases. Simultaneous bilateral performance seems to improve morbidity, however it still requires standardization. This article aims to describe a standardized and planned training model for VEIL.
Materials and methods
This model comprises a standardized training as follows: 1) Oral and video presentation; 2) Two groins operated by the teacher with camera manipulated by trainees; 3) Simultaneous VEIL performed by trainees. Three medical centers tested this model between January and December 2014. Perioperative, functional and oncological data are evaluated. Statistical analysis was done by using arithmetic average and Student T-test. Data were compared among expert, trainees and trained surgeons.
Results
Ten groins were operated by trainees and twenty by the same trained surgeons. Demographic characteristics are in accordance with most of the reported studies. Surgical performance and oncological results of trainees and trained surgeons didn't differ significantly from the expert surgeon.
Conclusions
Despite the limited number of patients, the proposed model is effective as an option for training in large scale. Potential advantages include feasibility and optimization of operative time.
Keywords
Training, Lymph node excision, Penile cancer
Abbreviations
VEIL: Video Endoscopic Inguinal Lymphadenectomy; HPV: Human Papillomavirus
Introduction
Penile cancer is a rare neoplasm in developed countries [1,2]. It affects more often uncircumcised individuals, with phimosis, bad hygiene or nutrition. Infection by Human Papillomavirus (HPV) has also been implicated [3].
Patient presents unsightly, smelly and exudative penile lesion that interferes with quality of life. Involvement of inguinal lymph nodes is seen in 10 to 30%, and only in 1 to 3% is accompanied by visceral metastases [1,2]. The best method for evaluation of inguinal lymph nodes is still contradictory [4,5].
Treatment for penile cancer must be individualized and depends on primary lesion and lymph node involvement. Treatment of primary lesion aims to complete tumor excision with a safety margin of 1 to 2 cm [6-10]. Lymph node involvement is the most important predictor of survival. There are many controversies about lymphadenectomy, related to its technique and time, laterality, extension and complications. This surgery has considerable morbidity and eventual adjuvant treatment is necessary in cases of deep involvement [4,6,11].
Video Endoscopic Inguinal Lymphadenectomy (VEIL) is seen as feasible and safe in selected patients [12-17]. Despite the encouraging results, this surgery is not still widespread and is currently carried out mainly in referral centers. Bilateral VEIL lymphadenectomy is feasible, with reduced morbidity and maintaining the oncological principles [18]. However it is a technique in evolution and still requires more spread. A standardized training model is advisable for disseminate VEIL among surgeons performing groin lymphadenectomy [19].
These evidences have motivated us to develop new methods for qualification and dissemination of this new technique. This article aimed to describe and evaluate the results of a new standardized training model for learning simultaneous VEIL.
Materials and Methods
The study is a retrospective observational case series and has been approved by ethic committees of all centers where surgeries were performed. Data were obtained from patient files only. All financial support of the activities and expenses related to this study has been done by the authors.
VEIL lymphadenectomy follows standardized steps. The leg is folded over the thigh in a way to put in evidence the femoral triangle that is marked with ink over the skin. After the marking, the leg is extended and fixed to the table with abduction and light external rotation of the thigh. The video monitor is positioned at the contralateral side to the operated one at the patient's pelvic waist. At 2 cm of the femoral triangle vertex in a distal sense an incision of 1.5 cm in the skin and in the subcutaneous tissue until the Scarpa's fascia is performed, being developed a subcutaneous plan with scissors and later with a digital maneuver in the largest possible extension. A second incision of 1 cm, at around 2 cm above and 6 cm medially to the first incision, to the introduction of a 10 mm port. It is possible to identify the trajectory of the saphenous vein through this access. A laterally symmetric position 5 mm port is introduced for graspers, dissection tweezers and scissors. At the initial access, a 10 mm hasson trocar is preferably introduced. All the ports are fixed to the skin through a purse-string suture with cotton 0. At the initial port, we introduce a 0-degree optic, and at the medial port, we introduce the tweezers of the harmonic scalpel and the clipper. The surgeon and the camera operator are positioned laterally to the operated member. The creation of a working space is completed through the initial insufflation of CO2 with a 15-mmHg pressure, with its fast diffusion, being able to keep the pressure at 5-10 mmHg during the procedure. Transillumination allows a good orientation regarding the progression of the dissection area. Retrograde separation of the skin flap is done. This step is fundamental to the success of the procedure and is performed with a harmonic scalpel. Initially we perform the separation between the skin and the fibroareolar tissue that contains the superficial lymph nodes until the external oblique muscle fascia on the superior part. Afterwards we proceed to the dissection of the fundamental parameters, having as a limit the long adductor muscle and its fascia medially, the sartorius muscle and its fascia laterally, and the inguinal ligament superiorly. It is possible to identify branches of the femoral nerve that should be preserved. Identification and cranial dissection of long saphenous vein until the oval fossa are done. After the identification of the femoral artery and the opening of the femoral vein sheath we define the lateral limit of the dissection, allowing the access to the deep cervical lymph nodes. At this moment it can be necessary to control with 1 or 2 branch clips coming from the femoral artery that run anteriorly to the femoral vein. The fibroareolar tissue is dissected with a harmonic scalpel and the control of the final section at the femoral triangle vertex is obtained with clips. During this operative time, the use of the harmonic scalpel and a careful manipulation of the specimen in areas near the veins are necessary to avoid vascular lesion. As in the conventional technique, the aim is to skeletonize the femoral veins, resecting all local lymphatic tissue. Distal ligature of the long saphenous vein with clips is done. Most part of the branches of the long saphenous vein is controlled only by the harmonic scalpel. Branches larger than 4 mm need clips for the ligature. The entrance of the long saphenous vein in the femoral vein should be well dissected and controlled preferably with polymer clips. Final liberation of the specimen medially to the long saphenous vein, ligating the proximal portion of the lymph nodes at the deep region of the femoral channel with clips. After completing the liberation of the specimen, the endoscope view attests that all the tissue of the region was completely resected. Then removal of the surgical specimen by the 15 mm incision. In case the specimen is of large dimensions, it can be put inside a bag and latter removed. Vacuum drainage through the 5 mm orifice are used in all procedures and they should be removed when drainage is smaller than 50 ml. Suture of the larger incisions is done. Use of antibiotics was restricted to prophilaxis.
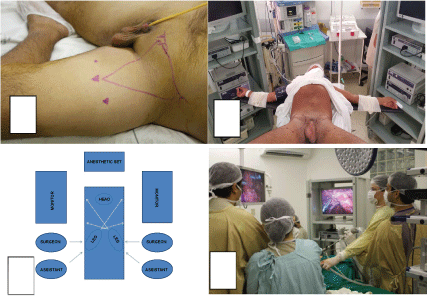
The first step of the model is an oral and video presentation by an expert surgeon. All tips and tricks are discussed. The second step is a demonstrative non-simultaneous bilateral VEIL done by expert surgeon (Figure 1A). Two groins are operated by expert with camera operated by trainees. Frozen biopsy is performed during surgery to evaluate the need for deep lymphadenectomy.
The third step is simultaneous bilateral VEIL with trainees performing the operation and camera operated by other trainees. The expert stays out of surgical field but instructs both teams concurrently. Surgery is done with the same steps for VEIL, but simultaneously (Figure 1B, Figure 1C and Figure 1D).
This training model was put to practice with patients with penile cancer who had previously undergone partial or total penectomy, with high risk for inguinal metastasis but without palpable lymph nodes, between January and December 2014. All surgeries were done at least four weeks after prior surgery and they were done in three centers around the world. After that, between January and December 2015, twenty groins were operated by the same trained surgeons, without tutor.
Data were evaluated including demographic, vital signs, operative time, bleeding, intraoperative complications, count and presence of positive lymph nodes on frozen biopsy, conversion, global complications, drainage time, cellulitis, lymphoceles, necrosis and hospitalization. Performances were compared among expert surgeon (ten years of experience previously reported in literature), trainees and the same surgeons after training.
Statistical analysis was done by using arithmetic average and Student T-test, with confidence interval 95% (p < 0.05).
Results
Seventy groins were operated by expert surgeon, reported in literature and data were reviewed. Eight patients were included in this model. Three patients were operated by the expert surgeon (non-simultaneous bilateral VEIL) and five by trainees (simultaneous bilateral VEIL). The main social and demographic characteristics of sample were evaluated (Table 1). After that, ten patients were operated by the same trained surgeons (seven by simultaneous bilateral VEIL).
Surgical performance was evaluated and compared. All patients remained hemodynamically stable. Average operative bleeding in milliliters for VEIL executed by expert surgeon was 45 (30-60) per side, by trainees was 55 (40-70) per side and by the same trained surgeons was 50 (40-70). There were no intraoperative complications. Average number of lymph nodes for VEIL executed by expert surgeon was 7 (6-8) per side, by trainees was 7 (5-8) per side and by the same trained surgeons was 6 (4-7). There was no conversion.
Perioperative data were evaluated. Global complications (pain, fever, hypothermia, atelectasis, thrombosis, pulmonary embolism and pneumonia) were seen in one patient operated by the expert surgeon (pain) and one patient operated by trainees (fever). Specific perioperative data (operative time, drainage time, cellulitis, lymphoceles, skin necrosis, hospital stay) were evaluated and compared (Table 2).
Discussion
The most frequent age group in the sample was between 50 and 70 years (60%) and once again penile cancer was more common in impoverished men, according to other evidences [1,2,6]. The most important risk factors were phimosis (37.5%) and smoke (25%). In literature the evidences are similar [1-3,6,20].
Few patients had lymph node disease at diagnosis (37.5%). Lymph node disease at diagnosis are seen in 10 to 30% of cases [1,21]. There is no consensus about the best indication and time for lymphadenectomy in penile cancer. In this series, we adopted some agreed criteria of worse prognosis, including patients with primary lesion T2-T4 stages, cell differentiation G2-G3 and, vascular or perineural invasion. All surgeries were done at least four weeks after prior surgery and antibiotic therapy and only in patients without lymph node disease.
Conventional surgery still remains the favored choice, despite its limitations [11]. This approach has the disadvantage of large incision. Moreover, there is substantial incidence of complications such as long drainage time, cellulitis, lymphoceles, skin necrosis, myocutaneous necrosis and long hospital stay. VEIL surgery was introduced in 2006 [22,23]. The advantages are fast recovery with preliminary good results [14,17,24]. The refinement of this technique extending indications to patients with palpable lymph nodes was also described with good results [25]. In the last few years, innovations have been proposed to improve VEIL surgery and outcomes. One of them is simultaneous bilateral VEIL [18]. The potential advantages include feasibility, maintenance of oncological templates, improvement in operative and anesthesia time and low morbidity.
Some procedures such as VEIL are uncommon and more challenging to teach. In 10 years our tutor will go to several global centers to demonstrate the technique but there were few opportunities to transfer skill and proficiency. In this paper we show our improved method to implement this new surgery in some centers around the world. In this schedule, we follow the adaptation of classic principle of see one, do one and after proficiency teach one.
The proposed model is based on concepts seen in other models used for training of many other surgeries and their successful experiences [26-31]. The main concept of this model is the standardization of surgical procedures as well as educational training programs in order to shorten individual learning curves and generate common quality standards. The model overcomes obstacles and allows optimize the learning and results of trainees, which are generally similar to those of expert's surgeons. Model can offer training to more than one surgeon at a time with few cases, so this can be an interesting and efficient method of teaching.
Selection of right patients is important to successful training model application. The skills of trainees must be respected and we offer them patients with good clinical and physical condition. Indication for VEIL was made based on pathological criteria. Patients with palpable lymph nodes were excluded from the study because only one series reporting data and surgery seems to be challenging [25].
In our series there was no significant difference in average operative time among expert, trainees and trained surgeons. We believe that the training model allowed standardization and optimized surgical time. This data is similar to other evidences in literature [14,18,25]. There was no significant difference in average operative bleeding among them. There were no intraoperative complications and no conversion, according to literature [14,17,18,25]. There was no significant difference in average number of lymph nodes among then. Once again data are similar to other available series, which present an average number of lymph nodes between 6 and 12.
One patient operated by the expert surgeon had pain (Clavien degree I) and one patient operated by trainees had fever (Clavien I). These data follow the low incidence of complications seen in other studies [11,17]. There was no significant difference in average drainage time among expert, trainees and trained surgeons. Data are similar to other available series, which present average drainage time between 5 and 7 days. There were no statistically significant differences among them in specific postoperative complications like cellulitis (Clavien II), lymphoceles (Clavien IIIA) and necrosis (Clavien IIIB). Besides, data showed low incidence and are similar to other series. We believe that use of clips and harmonic scalpel promote less local injury and they may be responsible for the low incidence of all these complications. Hospital stay in our report was similar among then and other series which reinforces the potential of training model in decreasing the morbidity [14,18,25]. In general, it seems that standardized learning is able to transfer ability to learners. It decreases complications and approximates results of trainees and expert surgeons. The most accentuated limitation of this study is the small number of cases. As an experimental model to teach we have a subjective excellent impression considering the satisfaction of trainees and operative results themselves. More experience and validation for other centers will confirm if our educational model is really useful.
Conclusions
The proposed model to training surgeons in VEIL is effective and can be offered as option for training in large scale. The potential advantages include feasibility, maintenance of oncological templates, improvement in operative and anesthesia time and low morbidity. The standardization shortens the individual learning curves. The model overcomes obstacles and results of trainees and trained surgeons are generally similar to those of experts.
Conflict of Interest
None declared.
References
- Bleeker MC, Heideman DA, Snijders PJ, et al. (2009) Penile cancer: epidemiology, pathogenesis and prevention. World J Urol 27: 141-150.
- Wollina U, Steinbach F, Verma S, et al. (2014) Penile tumours: A review. J Eur Acad Dermatol Venereol 28: 1267-1276.
- de Sanjosé S, Bruni L, Alemany L (2014) HPV in genital cancers (at the exception of cervical cancer) and anal cancers. Presse Med 43: e423-e428.
- Heyns CF, Mendoza-Valdés A, Pompeo AC (2010) Diagnosis and staging of penile cancer. Urology 76: S15-S23.
- Hughes B, Leijte J, Shabbir M, et al. (2009) Non-invasive and minimally invasive staging of regional lymph nodes in penile cancer. World J Urol 27: 197-203.
- Hakenberg OW, Compérat EM, Minhas S, et al. (2015) EAU guidelines on penile cancer: 2014 update. Eur Urol 67: 142-150.
- Pereira N, Cabral AR, Vieira R, et al. (2013) Conservative treatment of penile carcinoma - A retrospective study of 10 years. An Bras Dermatol 88: 844-846.
- Hegarty PK, Shabbir M, Hughes B, et al. (2009) Penile preserving surgery and surgical strategies to maximize penile form and function in penile cancer: Recommendations from the United Kingdom experience. World J Urol 27: 179-187.
- Minhas S, Kayes O, Hegarty P, et al. (2005) What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int 96: 1040-1043.
- Ficarra V, Maffei N, Piacentini I, et al. (2002) Local treatment of penile squamous cell carcinoma. Urol Int 69: 169-173.
- Protzel C, Alcaraz A, Horenblas S, et al. (2009) Lymphadenectomy in the surgical management of penile cancer. Eur Urol 55: 1075-1088.
- Kharadjian TB, Matin SF, Pettaway CA (2014) Early experience of robotic-assisted inguinal lymphadenectomy: review of surgical outcomes relative to alternative approaches. Curr Urol Rep 15: 412.
- Martin SF, Cormier JN, Ward JF, et al. (2013) Phase 1 prospective evaluation of the oncological adequacy of robotic assisted video-endoscopic inguinal lymphadenectomy in patients with penile carcinoma. BJU Int 111: 1068-1074.
- Pahwa HS, Misra S, Kumar A, et al. (2013) Video Endoscopic Inguinal Lymphadenectomy (VEIL)--a prospective critical perioperative assessment of feasibility and morbidity with points of technique in penile carcinoma. World J Surg Oncol 11: 42.
- Xue-Lu Zhou, Ji-Feng Zhang, Jian-Feng Zhang, et al. (2013) Endoscopic inguinal lymphadenectomy for penile carcinoma and genital malignancy: A preliminary report. J Endourol 27: 657-661.
- Sotelo R, Sanchez-Salas R, Clavijo R (2009) Endoscopic inguinal lymph node dissection for penile carcinoma: The developing of a novel technique. World J Urol 27: 213-219.
- Tobias-Machado M, Tavares A, Ornellas AA, et al. (2007) Video endoscopic inguinal lymphadenectomy: a new minimally invasive procedure for radical management of inguinal nodes in patients with penile squamous cell carcinoma. J Urol 177: 953-957.
- Pompeo A, Tobias-Machado M, Molina WR, et al. (2013) Extending boundaries in minimally invasive procedures with simultaneous bilateral video endoscopic inguinal lymphadenectomy (veil) for penile cancer: initial Denver health medical center and ABC school of medicine experience and surgical considerations. Int Braz J Urol 39: 587-592.
- Jakub JW, Terando AM, Sarnaik A, et al. (2017) Safety and Feasibility of Minimally Invasive Inguinal Lymph Node Dissection in Patients With Melanoma (SAFE-MILND): Report of a Prospective Multi-institutional Trial. Ann Surg 265: 192-196.
- Buechner SA (2002) Common skin disorders of the penis. BJU Int 90: 498-506.
- Heyns CF, Fleshner N, Sangar V, et al. (2010) Management of the lymph nodes in penile cancer. Urology 76: S43-S57.
- Tobias-Machado M, Tavares A, Molina WR Jr, et al. (2006) Video endoscopic inguinal lymphadenectomy (VEIL): Initial Case report and comparison with open radical procedure. Arch Esp Urol 59: 849-852.
- Tobias-Machado M, Tavares A, Molina WR Jr, et al. (2006) Video endoscopic inguinal lymphadenectomy (VEIL): Minimally invasive resection of inguinal lymph nodes. Int Braz J Urol 32: 316-321.
- obias-Machado M, Tavares A, Silva MN, et al. (2008) Can video endoscopic inguinal lymphadenectomy achieve a lower morbidity than open lymph node dissection in penile cancer patients? J Endourol 22: 1687-1691.
- Carlos AS, Romanelli P, Nishimoto R, et al. (2013) Expanded criteria for video endoscopic inguinal lymphadenectomy (VEIL) in penile cancer: Palpable lymph nodes. Int Braz J Urol 39: 893.
- Perez-Duarte FJ, Fernandez-Tome B, Diaz-Guemes I, et al. (2014) Development and initial assessment of a training program for laparoscopic radical prostatectomy. First module: The urethrovesical anastomosis. J Endourol 28: 854-860.
- Klein J, Teber D, Frede T, et al. (2013) Development, validation and operating room-transfer of a six-step laparoscopic training program for the vesicourethral anastomosis. J Endourol 27: 349-354.
- Torricelli FC, Guglielmetti G, Duarte RJ, et al. (2011) Laparoscopic skill laboratory in urological surgery: tools and methods for resident training. Int Braz J Urol 37: 108-111.
- Ramírez Backhaus M, Uwe Stolzenburg J, Do M, et al. (2009) Learning laparoscopic radical prostatectomy with the Leipzig program. Analysis of the training module program. Actas Urol Esp 33: 290-295.
- Rabenalt R, Minh D, Dietel A, et al. (2006) Laparoscopic surgery in urology: Taining and education. Urologe A 45: 1155-1162.
- Stolzenburg JU, Rabenalt R, Do M, et al. (2006) Modular training for residents with no prior experience with open pelvic surgery in endoscopic extraperitoneal radical prostatectomy. Eur Urol 49: 491-498.
Corresponding Author
Pablo Aloisio Lima Mattos, MD, MSc, Department of Urology, Sao Marcos Hospital, Teresina, Brazil, Tel: +55-86 999212678.
Copyright
© 2017 Machado MT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.