Enthalpy - Entropy Compensation in the Hydrolysis of Four Tetracycline Antibiotics
An article recently published analyzed the hydrolytic transformation of four tetracycline antibiotics - tetracycline, chlortetracycline, oxytetracycline, and doxycycline - under various environmental settings and determined their parents and transformation products in the wastewater treatment plants [1]. In the article, the authors found that the hydrolytic behavior of the four tetracycline antibiotics followed first-order reaction kinetics and that the rates of the acid-catalyzed hydrolysis were significantly lower than the rates of the base-catalyzed and neutral pH hydrolysis. By applying the Arrhenius approach, the authors identified activation energy, which measures the effect of temperature on tetracycline hydrolysis, to range from 42 kJ/mol to 77 kJ/mol at pH 7. Hydrolytic degradation rates for all four tetracycline antibiotics increased with elevating temperatures, providing a clear indication of temperature-dependent kinetics.
While the authors displayed the hydrolytic stability of the four tetracycline antibiotics under different environmental conditions based on their measurements of activation energy, we found the kinetic results could be further analyzed in terms of thermodynamics using the transition-state theory [2]. We also included an enthalpy-entropy compensation [3] to facilitate a deeper understanding to the phenomenon of tetracycline hydrolysis.
We first obtained the Gibbs free energy of activation (ΔG°‡) at absolute temperature T (in Kelvin) using the rearranged version of the Eyring equation (Eq. 1):
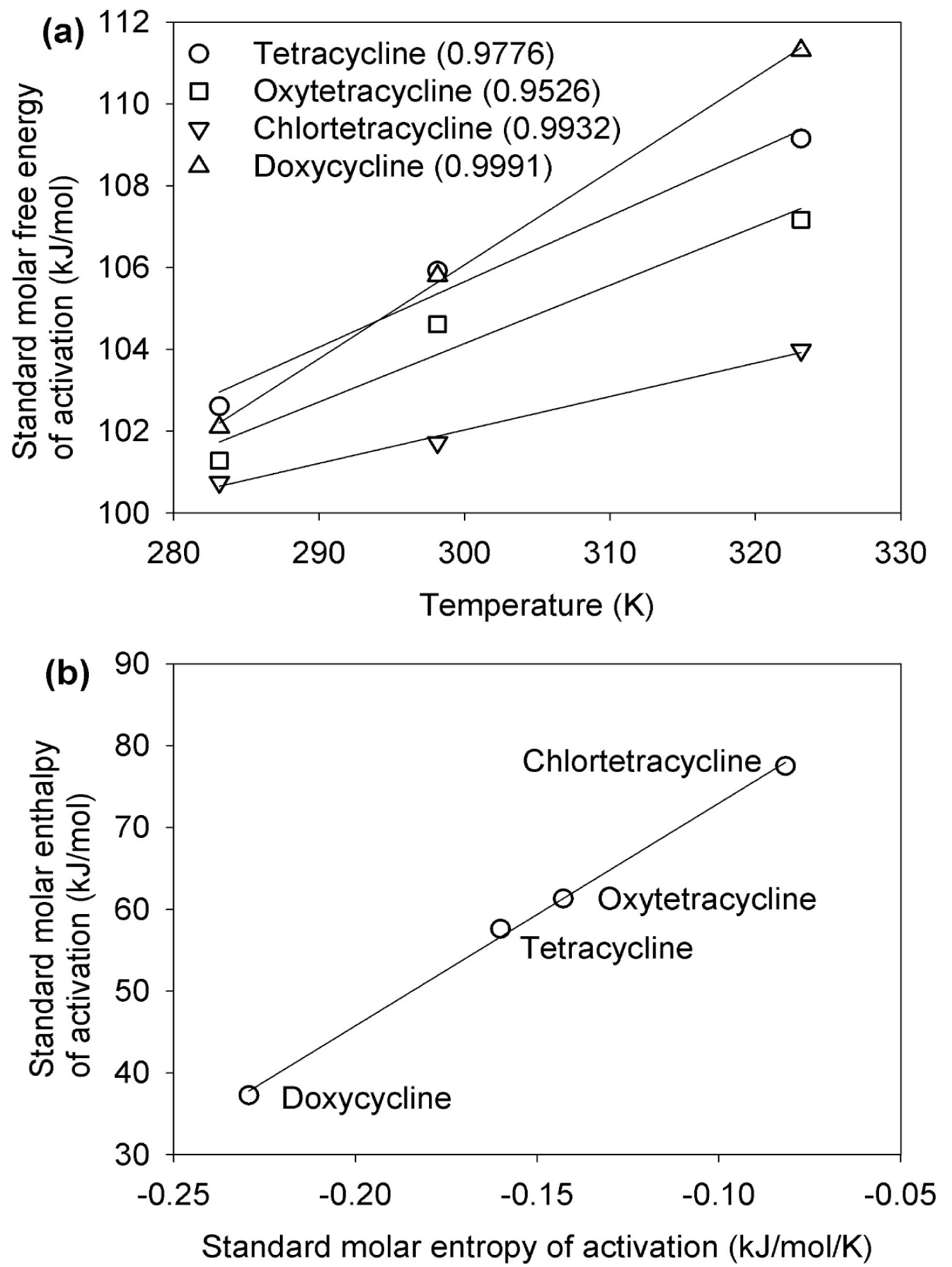
Where R is the ideal gas constant, k is the rate constant, h is Planck's constant, and kB is the Boltzmann constant. Prior to the calculations, a conversion of the units of rate constant from per hour as in the paper [1] to the standard units of per second was made to properly utilize the Eyring equation (Eq. 1). The derived values of ΔG°‡ for the hydrolytic degradation of tetracycline are shown as a function of temperature in Figure 1a. A direct correlation is exhibited between ΔG°‡ and temperature through all four tetracycline antibiotics. As seen in Figure 1a, the differences of ΔG°‡ in the hydrolysis converge together at low temperatures and diverge at high temperatures. The hydrolysis rate of chlortetracycline was the fastest amongst the four, followed by oxytetracycline, tetracycline, and lastly doxycycline at 323.15 K (50 °C). This was also stated in the original paper [1]; however, it lacked the thermodynamics basis of the phenomenon.
To elucidate this phenomenon using thermodynamics, a linear regression of ΔG°‡ with the function of temperature was generated to obtain the molar entropy ΔS°‡ and enthalpy ΔH°‡ of activation in tetracycline hydrolysis using Eq. 2 [2]:
In the regression, the slope and y-intercept correspond to -ΔS°‡ and ΔH°‡, respectively. The resulting values of ΔH°‡ and ΔS°‡ are displayed in Figure 1b. For all four tetracycline antibiotics, ΔS°‡ are negative values, whereas the ΔH°‡ values are all positive. A linear regression line in Figure 1b demonstrates a clear correlation between the two thermodynamics parameters and advocates that the stability of these antibiotics is not directed solely upon the molar enthalpy or entropy magnitudes, but by a combination of both that varies significantly in proportion yet remains near constant in total value represented by ΔG°‡ (Eq. 2).
The thermodynamic features can be concluded from the acquired results. First, the hydrolytic degradation is kinetically unfavorable with regards to both enthalpy and entropy throughout all tetracycline compounds. Second, each compound exhibited a different thermodynamic basis despite having high hydrolytic stability. For instance, the stability of chlortetracycline is credited to its high value of ΔH°‡, whereas the stability for doxycycline is attributed to its low value of ΔS°‡. Through the visual representation, we observed a relatively strong correlation of 0.9982 between ΔH°‡ and ΔS°‡. This phenomenon is known as the enthalpy-entropy compensation, which is widely invoked as an illustrative principle for the limited variation of Gibbs free energy change despite significant variations in the enthalpy and entropy values. From the data, the ΔH°‡ values for the tetracycline ranged from 37.27 to 77.51 kJ/mol and the ΔS°‡ values ranged from -0.082 to -0.229 kJ/mol/K. Contrastingly, the ΔG°‡ values displayed slight variations at all temperatures. It is evident that enthalpy-entropy compensation is accountable for the relatively small variation in ΔG°‡ of the hydrolytic degradation of tetracyclines (Figure 1a).
In conclusion, our thermodynamic analysis specifies that the combination of ΔH°‡ and ΔS°‡ indicates the hydrolytic degradation of tetracycline. The compensation between ΔH°‡ and ΔS°‡ limits the variation of ΔG°‡. Further investigation can be conducted to observe the behavior other tetracyclines and see if they follow a similar trend.
References
- Zhong SF, Yang B, Xiong Q, et al. (2022) Hydrolytic transformation mechanism of tetracycline antibiotics: Reaction kinetics, products identification and determination in WWTPs. Ecotoxicol Environ Saf 229: 113063.
- Chang R (2000) Physical chemistry for the chemical and biological sciences. University Science Books, Sausalito, CA, USA.
- Fox JM, Zhao M, Fink MJ, et al. (2018) The molecular origin of enthalpy/entropy compensation in biomolecular recognition. Annu Rev Biophys 47: 223-250.
Corresponding Author
Jonghoon Kang, Department of Biology, Valdosta State University, 1500 N. Patterson St., Valdosta, Georgia 31698, USA, Tel: 1-229-333-7140.
Copyright
© 2022 Patel YJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.