The Immune Response in Post-Acute COVID-19 Syndrome
Abstract
Hyper-inflammation caused by COVID-19 is related to worsening of symptoms and, probably, to development of Post-acute COVID-19 Syndrome, whose probable mechanisms contributing to the pathophysiology of post-acute COVID-19 include: Virus-specific pathophysiologic changes; immunologic aberrations and inflammatory damage in response to the acute infection; and sequelae of post-critical illness. A mini-review was carried out to elucidate what are the potential immunological determinants related to the clinical presentation of the post-acute COVID syndrome (PACS). This literature review sought to answer the question: what are the potential immunological determinants related to the post-acute COVID syndrome? Studies that evaluated immunological markers in PACS, published in English, Portuguese, or Spanish were included; and literature reviews, case reports and case series, animal studies, or in vitro studies, excluded. Performed searches on PubMed, Web of Science, Scopus databases; used the descriptors "immune markers" and "post-acute COVID-19 syndrome"; identified a total of 343 studies, of which 135 were duplicate records; of these, only 9 studies met the adopted eligibility criteria. Potential mechanisms contributing to the pathophysiology of PACS include virus-specific damage and inflammatory damage in response to the acute infection; and sequelae of post-critical illness. The Practice Community continues waiting for research to propose innate and specific immunity biomarkers that point out patients' profiles PASC susceptible.
Keywords
COVID-19, Post-acute Syndrome, Immune Response, Inflammatory damage
Background of Review
COVID-19 is a multiorgan disease caused by the SARS-CoV-2 virus, which infects cells through the interaction between the viral protein SPIKE and the angiotensin-2 converting enzyme (ACE2).When virus replication is no longer observed, some patients have new, recurring, or ongoing symptoms, called Post-acute COVID-19 syndrome (PACS) or long-COVID, defined as a multisystem disease, characterized by the development of sequelae or persistence of symptoms 4 weeks from the onset of acute COVID-19 [1]. It can be classified into two types: subacute, from 4 to 12 weeks after infection, and chronic, beyond this period. PACS can occur in patients who have had varying degrees of illness during acute infection, including those who had mild or asymptomatic infections [2].
At this moment, longitudinal surveillance data on PACS are lacking and the prevalence is challenging to estimate, ranging from 5% to 80%, because there is no unanimity in case definition for post-COVID conditions, or temporal criteria used, neither population included, and how conditions are researched [3].
PACS have been more commonly reported in female sex [4], as well as in patients who need admission to intensive care units (ICUs), with previous severe clinical status, with a high number of comorbidities or body mass index (BMI), older groups of age [5,6], health professionals [7] and black, Asian or ethnic minority populations, in Europe [8]. Evidence suggests that PACS occurs in children and adolescents as well as adults in good health, and despite the good prognosis, they present visits to the doctor, consumption of symptoms and absences from school and work, respectively [9].
Large cohorts show that, like the acute COVID-19, clinical manifestations can affect several systems, being the most prevalent: Fatigue and post-exertional malaise and/or poor endurance, anosmia, ageusia and fever; alopecia and rash; arthralgia, myalgia and impaired daily function and mobility, followed by: Headache, depression, cognitive and sleep disorders, encephalitis and myelitis; dyspnea, cough, prolonged dependence on oxygen therapy; venous thromboembolic events, chest pain and palpitations; thyroiditis and diabetic ketoacidosis, acute kidney injury; abdominal pain and diarrhea [10,11].
The pathophysiology of PACS, although complex and not fully elucidated, potential determinants seems to be related to direct viral damage and immune system dysfunction, which triggers a state of hyper inflammation, with increased production of cytokines (such as interleukins 1, 2 and 6, TNF-α, TGF-β, IFN-γ), and consequently, hypercoagulation, tissue damage, emergence of new pathologies and worsening of previous diseases [1,12].
Therefore, given the important influence of immune changes in the generation of complications and persistent symptoms post-acute COVID syndrome, it was decided to review studies on the topic, which can enable the development of prophylaxis and therapeutics capable of improving the prognosis of affected patients.
Methods
This literature review sought to answer the question: what are the potential immunological determinants related to the post-COVID syndrome? Based on this question, the following inclusion criteria were adopted: Clinical trials (n = 0), cohort (n = 6) and transversal studies (n = 3) that evaluated immunological markers in post-COVID syndrome, published in English, Portuguese or Spanish. Were excluded: case series (n = 2), case reports (n = 7), in vitro studies (n = 5), another topic (n = 34) and literature review (n = 151).
We performed searches on November 1, 2021, in the following databases: PubMed, Web of Science, Scopus. We use the descriptors "Immune Markers" and "post-acute COVID-19 syndrome", and similar terms to construct the search strategy. More details about search strategies can be found in the Supplementary Table S1.
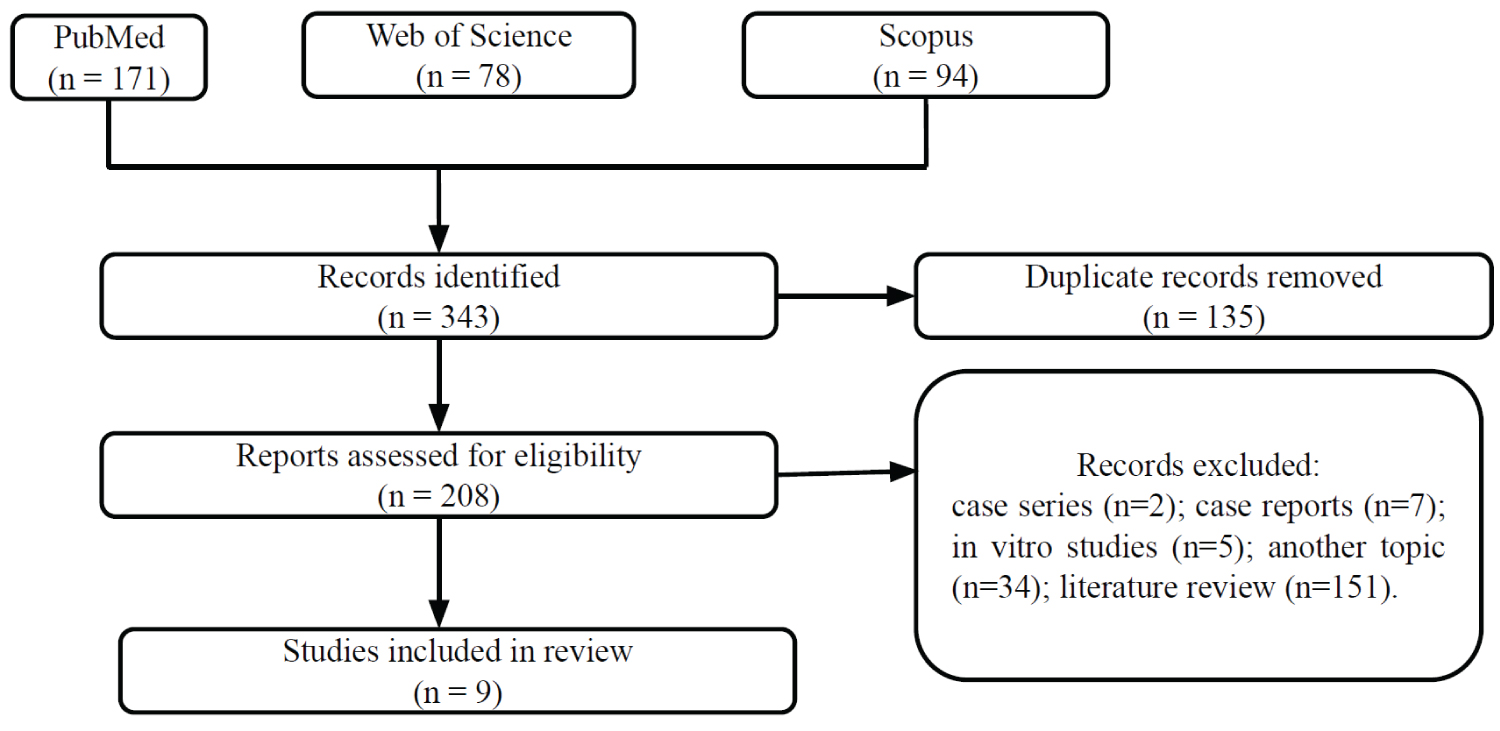
Regarding the study selection process, the searches identified a total of 343 studies, of which 135 were duplicate records. Therefore, 208 studies were evaluated in the selection process. Of these, only 9 studies met the adopted eligibility criteria (Figure 1).
Findings
The articles selected in this review are summarized in Table 1, as well as their main findings. Of the nine selected articles, six were cohort trials and three were cross-sectional studies.
Theoretical reference
The link between innate immune activation and Post-acute COVID-19 Syndrome
Hyper-inflammation caused by COVID-19 is related to worsening of symptoms and, probably, to the development of PASC. In accordance with Nalbadian, et al., 2021, the potential mechanisms contributing to the pathophysiology of post-acute COVID-19 include virus-specific damage and inflammatory damage in response to the acute infection; and sequelae of post-critical illness [12]. SARS-CoV-2 appears to trigger a prolonged production of pro-inflammatory mediators like IL-6, IL-1β, TNF-α, and CXCL8 (IL-8) by macrophages and other innate immune cells, a fact that causes lung damage and thrombosis observed in later stages of acute COVID-19 [13]. In the pathogenesis of PACS, we found articles evaluating the role of neutrophils and mast cells.
Mast cells: Mast cells are innate immune cells that play pathogenic roles in COVID-19 [14] and evidence points to its relationship with the PACS [15]. Pulmonary fibrosis occurs in patients with PACS and the activity of fibroblasts is stimulated by cytokine storm mediated by immune cells, including mast cells [16]. According to Afrin, et al., the prevalence of severe COVID-19 is similar to that of mast cell activation syndrome (MCAS) and the mast cell alterations of MCAS may underlie chronic Covid-19 illness [17]. In a study conducted by Weinstock, et al. [15], of 136 patients with long-COVID-19 symptoms, 80 subjects have had MCAS. Both groups before treatment had identical MCA symptom and severity analyses. Moreover, MCA symptoms were increased in post-COVID-19 patients. Potential limitations of this study are gender recruitment imbalance since sex could influence the immune response of both groups. Together, these facts suggest the involvement of mast cells in the pathogenesis of PACS. No study has directly assessed the activity and production of cytokines by mast cells in PACS, which suggests that further studies are needed.
Neutrophils: Neutrophils are involved in the immunology of COVID-19 and disease severity. Besides the antiviral roles, an unbalanced neutrophil immune response may contribute to lung tissue damage and thrombosis observed in COVID-19 patients [18]. According to Sieminska, et al. [19], post COVID-19 convalescents patients, even 3 months after infection, have an elevated number of granulocytic myeloid-derived suppressor cells, including neutrophils, in the blood, which correlates negatively with the number of CD8+ cells. Moreover, low-density neutrophils and normal density neutrophils may interfere directly with the production of anti-SARS-CoV-2 neutralizing antibodies. These neutrophils also showed a suppressive anti-CD3-induced proliferation of autologous T cells associated with a high expression of immunosuppressive PD-L1. In conclusion, these results suggest important alterations in the neutrophil activity that can help the study of the pathophysiology of PACS. However, the small number of patients and controls included in the study [13] makes it necessary that further research be carried out.
Alterations in cytokine levels and Post-acute COVID-19 Syndrome
The cytokine storm is an important proven mechanism related to the severity of COVID-19. The sequelae attributed to this disease and observed in the PACS can be partly caused by the intense production of proinflammatory cytokines [20]. Patterson, et al. [21] evaluating 29 control subjects, 26 patients with moderate COVID-19, 48 patients with severe COVID-19, and 121 subjects with PACS, and related that CCL5/RANTES, IL-2, IL-4, CCL3, IL-6, IL-10, IFN-γ, and VEGF were elevated when compared to controls, while the levels of CCL4 and GM-CSF were reduced. The authors established a binary model for separating cases of PASC and non-PASC according to the evaluated markers. "PASC Score" was defined as (IFN-g + IL-2)/CCL4-MIP-1b and the threshold of "PASC score" was 0.5. This cut-off has 97.5% of sensibility and specificity of 100% for healthy control and mild-moderate cases and 85% for severe cases. The authors hypothesize that, in PASC, the IFN-g and IL-2 cytokines would create a favorable microenvironment to Th1 polarization, however, the low levels of CCL4 can affect the recruitment of these cells impairing the antiviral response and leading to inflammatory myeloid cell activation, as evidenced by the augmented frequency of inflammatory CD14+, CD16+, CCR5+ monocytes in the PASC group compared to healthy donors [21].
Peluso, et al. [22] evaluated the levels of inflammation soluble in a SARS-CoV-2 cohort at early (< 90 days) and late (> 90 days) time points. The patients who developed PACS had higher levels of TNF-alpha (1.28 -fold higher) and IP-10 (IFN-gamma-induced protein 10, 1.28 -fold higher). Among those with post COVID symptoms, there were higher IL-6 levels in late recovery. However, in another study of the same group, the authors did not observe alterations in IL-6, IL-10, IP-10 and IFN-α levels in post-COVID patients [23].
Adaptative Immune response and Post-acute COVID-19 Syndrome
T cells: T cells play an important role in the response against the SARS-CoV-2, and evidence shows that it may also be part of the dynamics of PACS. According to Patterson, et al. [21], there is a significant decrease in regulatory T-cells frequency on PACS in comparison to healthy patients, which may exacerbate the hyper immunity on PACS. On top of that, there is evidence that even convalescent patients may present immune abnormalities. The reduction of the frequency of exhausted T cells (CD4+PD1+/CD8+PD1+) was observed in PACS patients when compared with health and acute COVID-19 individuals. A possible limitation of this study was the use of refrigerated samples for immunophenotyping analysis, which can influence the expression of cell markers depending on the cryopreservation protocol used, and the imbalance between the number of participants in each sample group. In a study by Townsend, et al. [24], the convalescent patient may even present T cell abnormalities up to 3 months after the SARS-CoV-2 infection, as lymphocyte count reduction. In a similar case, another source suggests that T-cell response against SARS-CoV-2 may be stable during the 8 months following the infection onset [23]. In addition, Shuwa, et al. showed that the Infection with SARS-CoV-2 presented alteration in the functional potential of the T CD8+ cells up to 6 months after the hospital discharge, outlining a continuous expression of the cytotoxic activity. It was observed a great response of T CD8+ cells, with high expression of perforin and granzyme. This high cytotoxic activity was also present in convalescent patients, although the CD8+ cells in these patients weren’t actively degranulating or proliferating. Additionally, it was also observed a reduced expression of CXCR3 and CXCR5 on patients with acute COVID-19, which may reflect the reduction of the direction of the lymphocytes to the lymphatic nodules and follicles, which is described as contributing to the immune dysfunction in other diseases such as advanced HIV [25]. Although this does not make the study unfeasible, the control group consisted of front-line workers at COVID-19. The differences could have been more dramatic if healthy people who were not on the front lines had been included.
B cells and antibodies: B lymphocytes and antibodies appear to play a notable role in PACS. One study verified the expansion of B lymphocytes negative for IgD and CD27 expression in critically ill and convalescent patients and worse clinical outcomes in convalescent patients with lower production of interleukin-10 by B lymphocytes [25]. Regarding the influence of antibodies during the post-acute period, a study with 70 individuals previously infected by the coronavirus showed that the level of neutralization exerted by IgG against the virus molecules N, S and RBD (receptor binding domain) decreased four months after the onset of symptoms [23]. Furthermore, in a study that enrolled 34 patients with persistent symptoms of COVID-19, all 15 people who tested positive for the interferon-gamma ELISPOT test had at least one positive serologic test: 14 (93.3%) for anti-RBD, 11 (73.3%) total for IgG/IgM anti-RBD, 12 (80%) for IgG anti-S and 11 for (73.3%) IgG anti-N [26]. It is also worth mentioning that one study revealed the development, greater than in other acute diseases, of autoantibodies against a limited repertoire of antigens (such as skeletal, epidermal and muscle) during and after COVID-19. In the post-acute period, this repertoire can increase. In addition, the study revealed that severe cases of COVID-19, when compared with cases that did not require hospitalization, generate a greater increase in the development of autoantibodies [27].
Conclusion
The spectrum of patients discharged from the hospital ranges from sequelae to new and persistent manifestations linked to COVID-19. On the other hand, any patient can develop numerous conditions related to Post-acute COVID syndrome. Despite the research groups' efforts to point out clinical-epidemiological and etiopathological determinants, the most appropriate guidance yet is to register patients and provide clinical-immunological follow-up, approached by a multidisciplinary primary care team, for those patients who need it. Meanwhile, the Practice Community continues waiting for research to propose innate and specific immunity biomarkers that point out patients' profiles PACS susceptible.
Author Contribution Statement
The authors confirm contribution to the paper as follows: Study conception and design: GFA; CF; SMAF; SGF. Data collection: CF; SMAF; SGF. Analysis and interpretation of results: GFA; ACMT; CF; GRFG; NRCR; PVTF; SMAF; SGF; TPRB. Draft manuscript preparation: GFA; ACMT; CF; GRFG; NRCR; PVTF; SMAF; SGF, TPRB. All authors reviewed the results and approved the final version of the manuscript.
References
- Nalbandian A, Sehgal K, Gupta A, et al. (2021) Post-acute COVID-19 syndrome. Nat Med 4: 601-615.
- Havervall S, Rosell A, Phillipson M, et al. (2021) Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA 18: 2015-2016.
- Cabrera Martimbianco AL, Pacheco RL, Bagattini ÂM, et al. (2021) Frequency, signs and symptoms, and criteria adopted for long COVID: A systematic review. Int J Clin Pract 11: e14357.
- Huang C, Huang L, Wang Y, et al. (2021) 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 397: 220-232.
- Carvalho-Schneider C, Laurent E, Lemaignen A, et al. (2021) Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect 2: 258-263.
- Moreno-Perez O, Merino E, Leon-Ramirez JM, et al. (2021) Post-acute COVID-19 syndrome. Incidence and risk factors: A Mediterranean cohort study. J Infect 5: 378-383.
- Pavli A, Theodoridou M, Maltezou HC (2021) Post-COVID syndrome: Incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res 52: 575-581.
- Halpin SJ, McIvor C, Whyatt G, et al. (2021) Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol 2: 1013-1022.
- Say D, Crawford N, McNab S, et al. (2021) Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health 6: e22-e23.
- Chevinsky JR, Tao G, Lavery AM, et al. (2021) Late conditions diagnosed 1-4 months following an initial COVID-19 encounter: A matched cohort study using inpatient and outpatient administrative data - United States, March 1-June 30, 2020. Clin Infect Dis 73: S5-S16.
- Lund LC, Hallas J, Nielsen H, et al. (2021) Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: A Danish population-based cohort study. Lancet Infect Dis 21: 1373-1382.
- Carod-Artal FJ (2021) Síndrome post-COVID-19: Epidemiología, criterios diagnósticos y mecanismos patogénicos implicados. Revista de Neurología 11: 384-396.
- Girija ASS, Shankar EM, Larsson M (2020) Could SARS-CoV-2-induced hyperinflammation magnify the severity of coronavirus disease (CoViD-19) leading to acute respiratory distress syndrome? Front Immunol 11: 1206.
- Gebremeskel S, Schanin J, Coyle KM, et al. (2021) Mast cell and eosinophil activation are associated with COVID-19 and TLR-Mediated viral inflammation: Implications for an anti-siglec-8 antibody. Front Immunol 12: 650331.
- Weinstock LB, Brook JB, Walters AS, et al. (2021) Mast cell activation symptoms are prevalent in Long-COVID. Int J Infect Dis 112: 217-226.
- Conti P, Al Caraffa, G Tetè, et al. (2020) Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19. J Biol Regul Homeost Agents 34: 1629-1632.
- Afrin LB, Weinstock LB, Moldering GJ (2021) Covid-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. Int J Infect Dis 100: 1373-1382.
- Cui S-N, Tan H-Y, Fan G-C (2021) Immunopathological roles of neutrophils in virus infection and COVID-19. Shock 56: 345-351.
- Sieminska I, Weglarczyk K, Surmiak M, et al. (2021) Mild and asymptomatic COVID-19 convalescents present long-term endotype of immunosuppression associated with neutrophil subsets possessing regulatory functions. Front Immunol 12: 748097.
- Rokni M, Hamblin MR, Rezaei N (2020) Cytokines and COVID-19: Friends or foes? Hum Vaccin Immunother 16: 2363-2365.
- Patterson BK, Guevara-Coto J, Yogendra R, et al. (2021) Immune-based prediction of COVID-19 severity and chronicity decoded using machine learning. Front Immunol 12: 700782.
- Peluso MJ, Lu S, Tang AF, et al. (2021) Markers of immune activation and inflammation in individuals with post-acute sequelae of SARS-CoV-2 infection. J Infect Dis 224: 1839-1848.
- Peluso MJ, Deitchman AN, Torres L, et al. (2021) Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep 36: 109518.
- Townsend L, Dyer AH, Naughtone A, et al. (2021) Longitudinal analysis of COVID-19 patients shows age-associated T Cell changes independent of ongoing Ill-Health. Front Immunol 12: 676932.
- Shuwa HA, Shaw TN, Knight SB, et al. (2021) Alterations in T and B cell function persist in convalescent COVID-19 patients. Med 2: 720.e4-735.e4.
- Scherlinger M, Felten R, Gallais F, et al. (2021) Refining "Long-COVID" by a prospective multimodal evaluation of patients with long-term symptoms attributed to SARS-CoV-2 Infection. Infect Dis Ther 10: 1747-1763.
- Richter AG, Shields AM, Karim A, et al. (2021) Establishing the prevalence of common tissue-specific autoantibodies following severe acute respiratory syndrome coronavirus 2 infection. Clin Exp Immunol 205: 99-105.
Corresponding Author
Prof. Dr. Gislei Frota Aragão, Estadual University of Ceará - UECE. Dr. Silas Munguba Avenue, 1700. Campus do Itaperi, Fortaleza, Ceará, 60.714.903, Brazil, Tel: +55-85-3101-9601
Copyright
© 2022 Aragão GF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.