Prevalence, Antimicrobial Susceptibility and Macrolide Molecular Study in Vaginal Group B Streptococcus: A Five-Year Study among Pregnant Women Attending Antenatal Clinics in a Tertiary Care Hospital in Tunisia
Abstract
Background
Group B Streptococcus (GBS) is an important perinatal pathogen. It is considered as a leading cause of stillbirth and early onset neonatal infections. The epidemiology of maternal GBS colonization is poorly understood in Tunisia. We investigated genital GBS carriage prevalence and its associated risk factors in pregnant women. Antibiotic susceptibility was evaluated for isolated strains; macrolide gene resistance and capsular serotyping were also performed.
Methods
A retrospective study conducted during 4 years at Aziza Othmana hospital of Tunis, Tunisia in pregnant women. Sampling was performed by vaginal swab. GBS was isolated by culture on selective medium; capsular serotyping was performed by rapid agglutination on Latex (PastorexStrepto B, Biorad®).Antibiotic susceptibility was performed according to the recommendations of the Antibiogram Committee of the French Society of Microbiology. Macrolide resistance was studied by a multiplex PCR assay.
Results
A total of 3839 pregnant women was enrolled in the study; mean age was 31 ± 5 years A carriage GBS prevalence was estimated to 12.7% (486/3869). It was significantly associated to primiparity, third gestational trimester, altered vaginal flora and Candida co-infection. No significant association wasobserved with gestational diabetes, membranes premature rupture and premature delivery threat. The most frequent serotypes were serotype III (33.3%), II (12.8%) and I (5.1%). All GBS strains were sensitive to beta-lactams and glycopeptides; only one strain was resistant to pristinamycin and another had high level resistance to gentamycin. Tetracycline resistance was observed in 96.1% of cases. Resistance to macrolides and lincosamides was detected in 37% and 32.4% respectively. A constitutive macrolide-lincosamide-streptogramin B (MLS(B)) phenotype was observed in 73.5% of cases, the inducible type MLS(B) in 14.8% of cases and the M phenotype in 11.7% of cases. Erm(B), erm(TR) and mef (A) genes were found in 79.4%, 15.3% and 12.9% of erythromycin resistant strains.
Conclusion
Vaginal GBS screening and intrapartum antibiotic prophylaxis are the most effective way to reduce early neonatal bacterial infections. Beta-lactams remain the antibiotics of choice. When allergy is diagnosed, antibiotic susceptibility is compulsory to choose an alternative such as clindamycine.
Keywords
Strptocccus agalactiae, Vaginal carriage, Pregnancy, Antibiotic succeptibility
Introduction
Group B Streptococcus (GBS), is a commensal bacterium of gastrointestinal tract and vagina [1]. Despite of its opportunistic nature, it is involved in several serious infections mainly in elderly, immunocompromised patient and neonates [2]. Its virulence is related to different bacterial factors such as polysaccharide capsule, hyaluronidase, adhesion, hemolytic pigment and pili [3].
GBS is considered as the major cause of neonatal sepsis, meningitis and prematurity [4]. Mother to infants' transmission occurs mostly during labor from contaminated vaginal fluids or during pregnancy through ascending infection [2]. GBS vaginal colonization is considered as the primary risk factor for neonatal GBS early-onset disease, that's why CDC guidelines recommend screening by vaginal culture in late pregnancy, with intrapartum antibiotic prophylaxis for carriers [4]. This approach reduced neonatal morbidity and mortality in US with incidence of neonatal GBS sepsis, estimated to 0.41/1000 live births [5]. In other countries, to avoid side effects of systematic antibiotic therapy and particularly antibiotic resistance, antibiotic prophylaxis is given only when increasing risk of SGB transmission was suspected such as premature rupture of the membranes or past history of infants with invasive GBS disease [6]. The World Health Organization (WHO) recommends adapting guidelines to local policy and incidence of GBS disease in the concerning country [7].
Treatment of GBS infection is based, in first line, on β-lactam antibiotics; macrolide and clindamycin are used as alternative when allergy to β-lactam is diagnosed or for patients with less severe infections. If GBS continues to be sensitive to Penicillin G, its resistance to second-line antibiotics, however, has been identified since the early 1990s with a steady increase. Macrolide resistance is caused by a specific ribosomal methylation encoded by erm genes with as results a cross-resistance to lincosamide and streptogramin B (MLSB). The phenotypic expression of this resistance can be inducible (iMLSB) or constitutive (cMLSB). The second resistance mechanism identified is related to an active antibiotic efflux; it is caused by a membrane-bound protein encoded by mef gene resulting to the M phenotype of resistance.
In Tunisia, few data were published about GBS carriage in pregnancy and neonatal GBS early-onset disease. Incidence of early-onset bacterial infections was evaluated to 12.85‰ and GBS was associated to 50% of them [8]. Reported incidence of neonatal GBS sepsis was about 2.3/1000 live births [3]. GBS colonization in Tunisian pregnant women was estimated to 13% in 2006 [9]. However, this rate differs largely between studies. During a period of three years from 2001 to 2003, no case of GBS colonization in vagina of mothers for infected neonates was reported [8]. Moreover, no national strategy was adopted to prevent neonatal GBS infection. In another hand, poor informations are available about the profile of antibiotic-resistance strains.
The aim of this study was to estimate prevalence of GBS carriage in pregnancy and to identify risk factors implicated in this colonization. Antibiotic susceptibility, capsular serotyping and molecular mechanisms of macrolide resistance were also assessed.
Material and Methods
This retrospective study was conducted over five years; from January the 1st, 2014 to December the 31th, 2018. It included all pregnant women, referred to the laboratory of Microbiology-Biochemistry of Aziza Othmana Hospital (Tunis, Tunisia), at 34 to 37 gestation weeks for GBS screening. Pregnant women with preterm membranes rupture or delivery threat and those referred for microbiological exploration of a genital infection were also investigated.
Demographic and clinical data were collected through a questionnaire about maternal age, comorbidities, gestational age and parity. All patients who received antibiotics or antifungal treatment one week before examination were excluded.
Two vaginal swabs were taken from the third lower part of the vagina. Gram stain smear was performed to assess vaginal flora with reference to Nugent score [10]. Cultures were immediately achieved on a Columbia Agar supplemented by 5% fresh blood +/-nalidixic acid then incubated at 37 ℃ for 24 hours under 5% CO2 atmosphere as described previously by Chhuy, et al. [11].
Samples were also inoculated on Chloramphenicol-Sabouraud and Chapman media to detect Candida and Staphylococcus respectively for symptomatic patients when candidiasis or bacterial vaginitis was suspected. When isolated, GBS was identified by characteristics colonies with narrow β hemolysis, Gram-positive cocci, and absence of catalase. Detection of capsular polysaccharide antigen by Lancefield serogrouping was performed by Pastorex™ Strep(Biorad®) USA.
Antibiotic susceptibility pattern of isolated GBS was performed according to recommendations of the Antibiogram Committee of the French Society of Microbiology [12].
Minimal inhibitory concentration (MIC) for macrolides, azithromycin, clarithromycin, clindamycin, quinupristin-dalfopristin and linezolid was performed, by E-Test (Biomérieux) Franceaccording to the manufacturer's instructions when resistance to erythromycin was detected. The erm(B), erm(A) and mef(A) erythromycin-resistance genes were performed by multiplex PCR as described by Lonardo, et al. [13]. Briefly, bacterial DNA was extracted using Instagene Matrix (Biorad®) USA; 10 ml DNA was then added to 40 ml PCR mixture containing 2.5 U GoTaq DNA polymerase (Promega®) USA, 1.5 mM MgCl2, 0.2 mM of each deoxynucleoside triphosphate and primers of 3 genes ermB, erm TR and mefA. Primers were 5'-GAAAAGGTACTCAACCAAATA-3', 5'-AGTAACGGTACTTAAATTGTTTAC-3 for ermB gene. For ermTR, primers were 5'- TTGGGTCAGGAAAAGGA-3' and 5'-GGGTGAAAATATGCTCG and finally for mefA gene primers were 5'-CGTAGCATTGGAACAGC-3' and 5'-TGCCGTAGTACAGCCAT-3'. Amplification was carried out in a Perkin-Elmer 2400 GeneAmp thermal cycler (UK) under following conditions: Initial denaturation at 95 ℃ for 3 min, 35 cycles of denaturation at 95 ℃ for 1 min, primer annealing at 57 ℃ for 1 min and extension at 72 ℃ for 1 min and a final elongation reaction at 72 ℃ for 5 min. Capsular serotyping was performed by a commercial latex agglutination technique to detect serotypes I, II and III (PastorexStrepto B, Biorad®) USA.
Data were analyzed by SPSS version 22 software. Categorical variables were compared by chi-square or Fisher's exact tests. Odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated to define the relative risk associated with maternal GBS colonization. A p-value less than 0.05, was considered as statistically significant.
Results
During studied period, 3839 patients were enrolled with a mean age of 31 ± 5 years. No history of previous pregnancy was reported for 56% of patients. Gestational diabetes, premature delivery threat and premature membrane rupture were found in 11.1%, 0.8% and 0.5% of cases and respectively. An altered vaginal flora was observed in 30% of cases; it was classified as vaginosis in 6.6% of them.
The profile of altered vaginal flora was highly correlated with candidiasis; it was observed in 37% of cases (p = 0.001). However, no significant association was observed between candidiasis and gestational diabetes (p = 0.912) or term of pregnancy (p = 0.134).
Prevalence and risk factors of GBS carriage
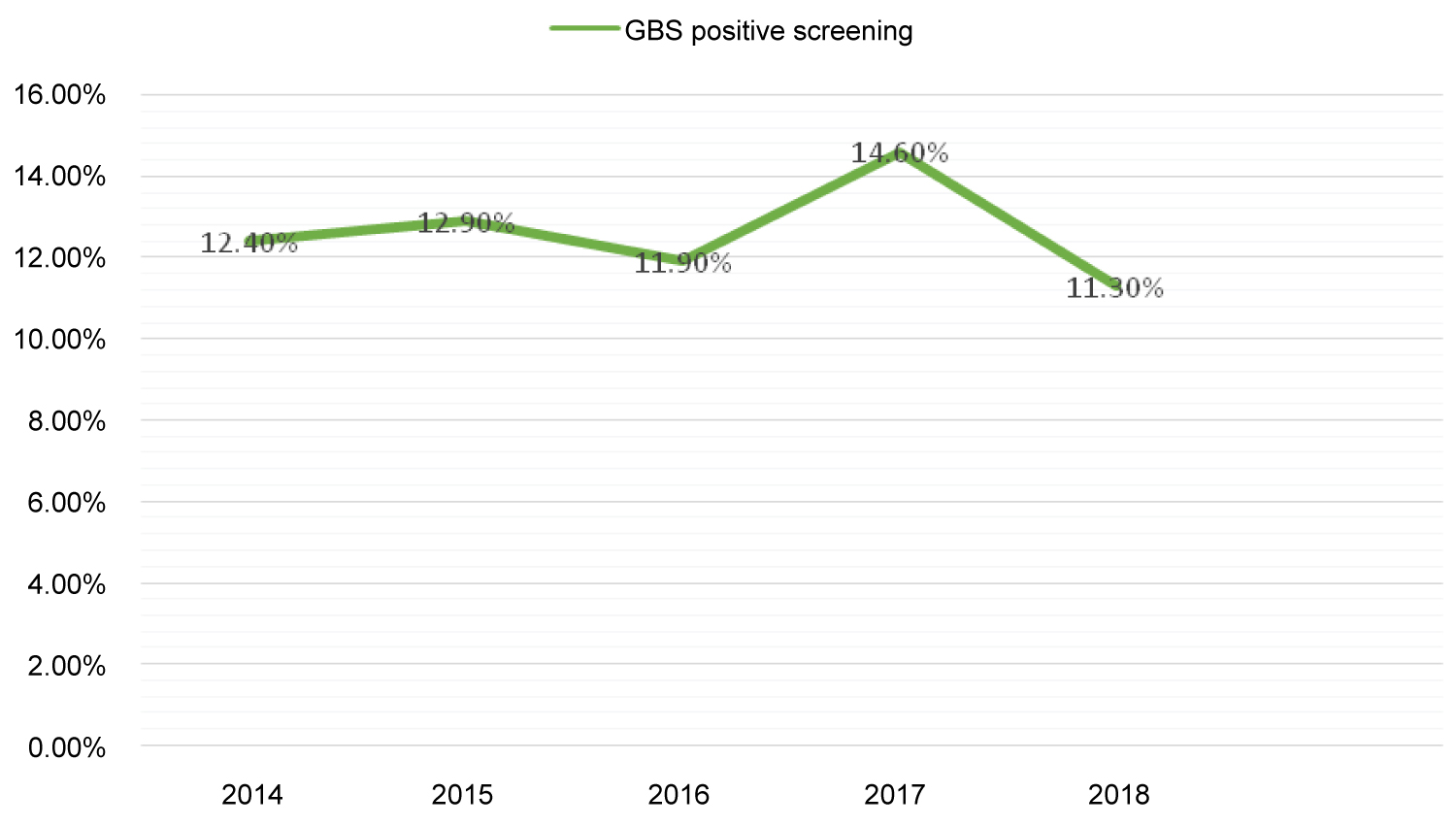
Overall, GBS colonization rate was 12.7% (n = 486/3839); it varied slightly over the studied period, from 11.30% to 14.60% as shown in Figure 1 (Spearman's r = 0.005). Higher rates were associated with primiparity (p = 0.001), third trimester of gestation (p = 0.001), altered vaginal flora (p < 0.001) and vaginal candidiasis (p < 0.001). No significant association was observed for GBS isolation with age of the patients, history of premature rupture of membranes or threat of preterm birth as reported in Table 1.
Antimicrobial susceptibility testing
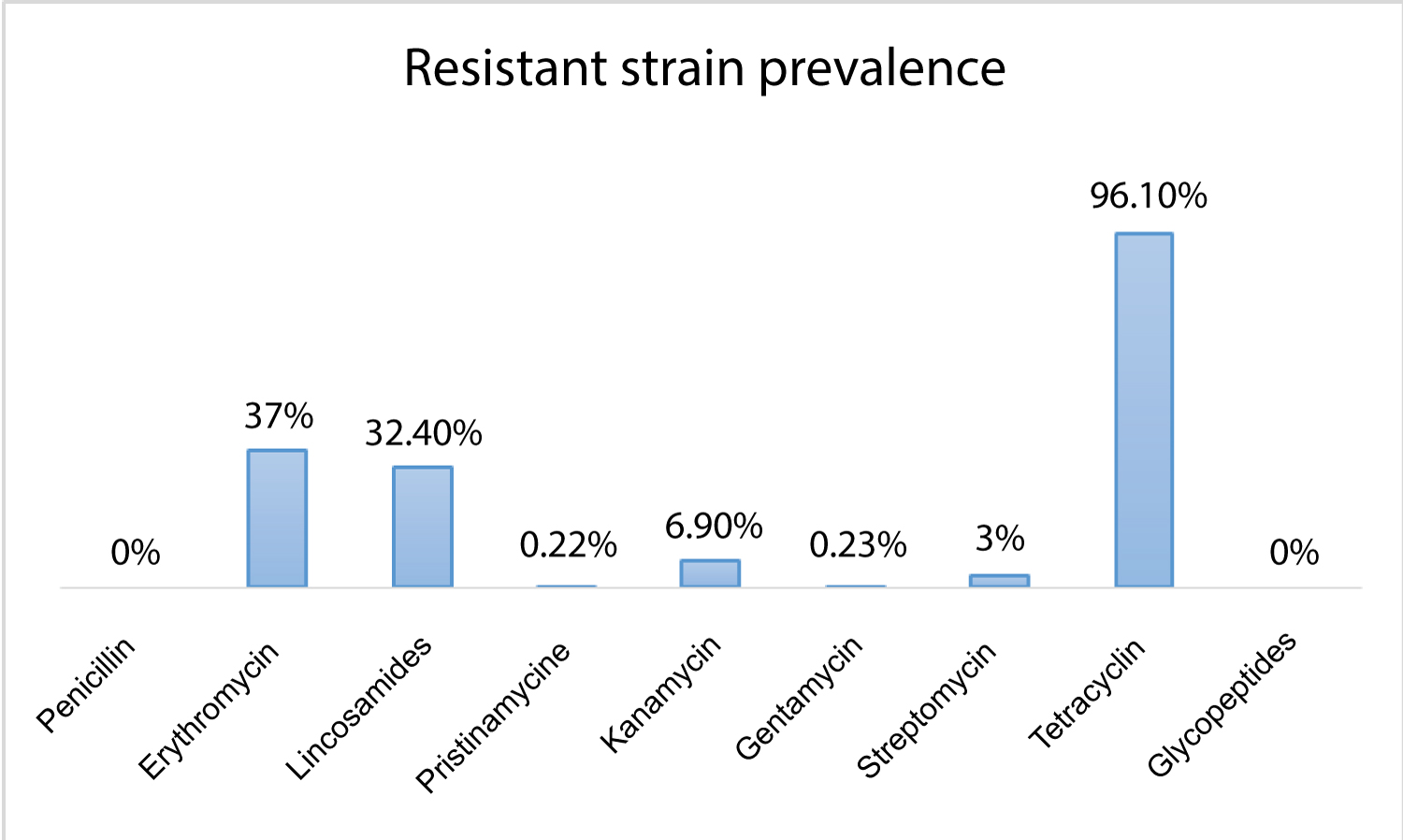
All GBS isolated were sensitive to beta-lactam and glycopeptides. The highest rate of resistance was observed for tetracyclins (96.3%), macrolides (37%) and lincosamides (32.4%). Only, 6.9% of strains had high level of resistance to kanamycin; this rate was estimated to 3% and 0.2% for streptomycin and gentamycin respectively. Resistance to pristinamycin was observed in 0.2% (Figure 2). A constitutive macrolide-lincosamide-streptogramin B (MLS(B)) phenotype was observed in 73.5% of cases. The inducible MLS(B) phenotype was identified in 14.8% of cases and the M phenotype in 11.7% of cases.
As reported in Table 2, MLS groups were characterized by high MIC of macrolides and clindamycin compared to the M phenotype. For all strains resistant to macrolides, MIC 50 and 90 of quinupristinedalfopristine (QDF) and linezolid were less than 0.5 mg/ml.
A molecular study of the 32 isolates resistant to macrolide identified erm (B), erm (TR) gene and mef (A) genes in 79.4%, 15.3% and 12.9% of cases respectively. Erm (B) and erm(TR) genes were associated to the cMLS(B) phenotype in 93.5% and 6.5% of cases andtothe iMLS(B) in 40% and 60%. Mef (A) gene was detected in only 6.5% of cMLS(B) strains but in all isolates with M phenotype.
GBS serotype III was found in 33.3% of cases with macrolides resistant isolates; 69.2% of them were associated to cMLS(B) phenotype, 23.1% to iMLS(B) and 7.7% to M phenotype. Serotypes II was detected in 12.8% of cases and serotype I in 5.1% of cases; all stains of serotypes I and II showed a MLS(B) phenotype. Other serotypes were found in 48.7%.A distribution of different serotypes and their profile of resistance are shown in Table 3.
Discussion
To our knowledge, this is the largest study conducted in Tunisia to assess prevalence and risk factors for vaginal GBS carriage in Tunisian pregnant women during the latest thirty years [14]. GBS colonization was detected in 12.7% of pregnant women. This result is consistent with what was published previously in the region of the center of Tunisia; 13% of pregnant women were positive for GBS during the third trimester of pregnancy [9].
Throughout the world, a large variation in prevalence rate of GBS colonization was reported; a meta-analysis reviewing 78 studies conducted in 37 countries and looking for 73791 pregnant women estimated it to 17.9% worldwide [15,16]. It was 22.4% in Africa, 19% in American and European countries and 11% in Asia [3]. This large disparity observed for rates can be explained by race, foods and sex habits, climate and maternal hygiene [17]. Growing conditions used for bacterial isolation, such as, culture methods, type of medium used or type of sample used, can also interfere and conduct to GBS inhibition with as results lower prevalence in some cases. As well, using an enrichment broth before agar culture improves significantly GBS detection. Adding rectal swab increases also a sensitivity of GBS screening in pregnant women [18].
GBS is the most common pathogen causing neonatal infections with high mortality. It remains a frequent cause of preterm birth; about 3.5 million babies among the estimated 15 million born prematurely each year, are contaminated by GBS [19]. These significant rates and the seriousness of neonatal GBS infection support the importance of GBS screening in pregnant women allowing to the establishment of different recommendations. The French National College of Gynecologists and Obstetricians and the National Agency for Accreditation and Health Assessment recommend a screening to all pregnant women between 34 and 38 week of amenorrhea by isolation of GBS from vagina; an intrapartum prophylaxis should be given systematically when culture is positive and for all pregnant women having a GBS bacteriuria or reporting an history of GBS maternal-fetal infection [20].
The American College of Obstetrics and Gynecology, the Center for Disease Control and Prevention and the Committee of Obstetricians and Gynecologists of Canada recommend similarly intrapartum prophylaxis to women with positive culture at 35-37 weeks gestation, GBS bacteriuria and past history of an infant with invasive GBS disease. However, to improve GBS isolation, they recommend combining sampling from rectum and vagina [4,21].
Regarding to our results, GBS screening should be performed systematically for all pregnant women at 35 weeks of gestation with starting antibioprophylaxis when culture is positive. It also should be done for cases with altered vaginal flora and vaginal candidiasis; these two latest seem to be risk factors to GBS colonization since they increased its positivity rate at least twice times. Oral lactobacillus, reported to be efficient to reduce microbial proliferation such as Candida, Gardnerella vaginalis. Its efficiency was also observed for GBS; it seems able to eradicate GBS colonization in 42% women [22]. That's why; it can be administered in this way. However, we did not identify, as described elsewhere, maternal age, gestational diabetes, premature rupture of membranes and premature delivery threat as risk factors for GBS colonization [9,23]. Other risk factors were also associated to GBS colonization such as leukorrhea and prolonged labor [6,24]. Knowledge about risk factors associated to maternal colonization will be useful for better care of pregnant women and to reduce morbidity and mortality related to GBS diseases. That's why, further studies are needed.
All GBS strains isolated in this study were sensitive to betalactamin and glycopeptides. This result was shared by different studies in Tanzania [25], South Africa [26], China [27], Brazil [28] and UK [29]. Surprisingly, a recent American study reported high resistance rate to penicillin (about 15%). This rate reached 33% for GBS from serotype II; the same study estimated resistance to vancomycin to 30% [30].
When resistance or allergy to penicillin was detected, lincosamides can be prescribed for prevention of GBS infection. However, susceptibility assessing should be done before; resistance to macrolides and lincosamides were frequently described. It was reported in 38% of cases for erythromycin and 21% for clindamycin by an American study [31]. Rates were estimated to 37% and 32.4% respectively in our study. Resistance to macrolides and lincosamides varied largely through regions; it was lower in South Africa (21.1% and 17.2% respectively) but higher in China where estimated to more than 70.8% for erythromycin [26,32]. A Korean study reported 54% of resistance to Clindamycin for GBS isolates [33].Very low rates were reported in Netherlands (5.3%) and Scotland (4.3%) [34,35].
A cMLS (B) phenotype characterized a majority of our erythromycin-resistant GBS isolates; few strains (14.8%) had an iMLS(B) phenotype. The same profile was reported in Egyptwhere these phenotypes were observed in 82.4% for cMLS(B) and in 5.9% for iMLS(B) [36]. In Ethiopia, however, these two phenotypes were distributed equally between strains, 26.1% and 15.2% respectively [37]. M phenotype was observed in 11% for our isolates; this rate was higher than reported previously in Tunisia (2.2% and 7.5% for the two studies) [38,39]. This increase should be the consequence of widespread of use of these molecules leading to high resistance. It, also, reflects a probable change in the resistance mechanism against these molecules. Phenotype M is frequently associated to a resistance by active molecule efflux; this mechanism is mediated by emf gene. In our study, this gene was associated to all strains with M phenotype; these results support this hypothesis. However, it is not the only resistance mechanism for our isolates since, as described recently, macrolides and lincosamides resistance was predominantly associated to erm(B) gene [40]. However, mef (A) gene was reported in 12.1% of erythromycin resistant GBS which was lower than that reported in an Italian study (46.5%) [41].These findings are in favor to the involvement of the methylation of the target gene in the resistance to macrolides. All these finding showed that, in a short period of time, macrolides and lincosamides will no longer be a reliable alternative therapy for penicillin-allergic women.
GBS resistance to pristinamycine was described at first in 2015 in Tunisia; it remained low as reported previously (0.7% for Tunisian strains in 2017) [42].
As reported elsewhere, the highest rates of resistance were observed for tetracyclineshowing a limited action of this antibiotic on streptococcal bacteria. It was estimated to 90%-98% in Egypt, Tunisia and Brasil [36,38,43]. In Japan, however, only 46% of GBS were resistant to tetracycline [44]. Finally, gentamycin high level resistance was estimated to be 0.2%; it was much lower than reported previously in non invasive strains by a Tunisian multicenter study (13.3%) [27].
The serotype III, identified in 25.7% of cases is the current study, was reported to be the predominant serotype in invasive strains mostly in late onset neonatal infection; its prevalence can reach 88% [45]. This serotype was isolated in 33.3% and 26.3% of maternal carriage GBS [43,44]. Data collection about serotype distribution among pregnant women may be helpful for the development of vaccine proposals against invasive bacteria.
Conclusion
The present study showed high prevalence of maternal Group B Streptococcus colonization which remains the primary risk factor for neonatal early-onset disease [46]. Neonatal infections prevention strategy is important to decrease morbidity and mortality. Systematic Group B Streptococcus screening during last weeks of pregnancy as well as intrapartum antibiotic prophylaxis are essential to limit the risk. The gold standard method to identify this bacterium is still the microbiological approach with culture from vaginal swabs. Aminopenicillin remains the first antibiotic to use in women carrying Group B Streptococcus in order to prevent neonatal sepsis. However, identification of antibiotic susceptibility profile is very important for choosing a molecule than can be used as alternative when allergy to penicillin is diagnosed.
All authors had contributed in this manuscript. Dr Hannachi and Miss Lahmar: Collecting data and writing manuscript. Dr Bouyahia and Dr Hamdoun designed the study. Pr Zhioua, Pr Ferchiou and Pr Bahri reviewed the manuscript. All bacteriological data were collected anonymously.
Government and industry support
None.
Conflicts of interest
None.
References
- Onile BA (1985) Review of group B streptococci and their infections. Afr J Med Med Sci 14: 131-143.
- Fry RM (1938) Fatal infections by Haemolytie Streptococcus Group B. Lancet 199-201.
- Vornhagen J, Adams Waldorf KM, Rajagopal L (2017) Perinatal Group B streptococcal infections: Virulence factors, immunity, and prevention strategies. Trends Microbiol 25: 919-931.
- Jennifer R Verani, Lesley McGee, Stephanie J Schrag (2010) Prevention of Perinatal Group B Streptococcal Disease. Revised Guidelines from CDC, 2010. MMWR 59: 1-32.
- Simonsen KA, Anderson-Berry AL, Delair SF, et al. (2014) Early-onset neonatal sepsis. Clin Microbiol Rev 27: 21-47.
- Le Doare K, O'Driscoll M, Turner K, et al. (2017) Intrapartum antibiotic chemoprophylaxis policies for the prevention of Group B streptococcal disease worldwide: Systematic review. Clin Infect Dis 65: S143-S151.
- (2015) WHO recommendations for prevention and treatment of maternal peripartum infections. In: Green ink. WHO, Geneva, Switzerland.
- https://www.latunisiemedicale.com/article-medicale-tunisie_1012_fr.
- Ferjani A, Abdallah HB, Saida NB, et al. (2006) Portage vaginal de Streptococcus agalactiae chez la femme enceinte en Tunisie?: Facteurs de risque et sensibilité aux antibiotiques des isolats. Bull Soc Pathol Exot.
- Nugent RP, Krohn MA, Hillier SL (1991) Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 29: 297-301.
- Chhuy T, Mansour G, Zejli A, et al. (2005) Dépistage du streptocoque de groupe B pendant la grossesse: A propos de 1 674 prélèvements. J Gynecol Obstet Biol Reprod (Paris) 34: 328-333.
- https://resapath.anses.fr/resapath_uploadfiles/files/Documents/2013_CASFM.pdf.
- Lopardo HA, Vidal P, Jeric P, et al. (2003) Six-month multicenter study on invasive infections due to group B streptococci in Argentina. J Clin Microbiol 41: 4688-4694.
- Gizachew M, Tiruneh M, Moges F, et al. (2020) Streptococcus agalactiae maternal colonization, antibiotic resistance and serotype profiles in Africa: A meta-analysis. Ann Clin Microbiol Antimicrob 18: 14.
- Blencowe H, Cousens S, Chou D, et al. (2013) Born too soon: The global epidemiology of 15 million preterm births. Reprod Health 10: S2.
- Kwatra G, Cunnington MC, Merrall E, et al. (2016) Prevalence of maternal colonisation with group B streptococcus: A systematic review and meta-analysis. Lancet Infect Dis 16: 1076-1084.
- Schuchat A, Wenger JD (1994) Epidemiology of group B streptococcal disease. Risk factors, prevention strategies, and vaccine development. Epidemiol Rev 16: 374-402.
- Meyn LA, Krohn MA, Hillier SL (2009) Rectal colonization by group B Streptococcus as a predictor of vaginal colonization. Am J Obstet Gynecol 201: 76.e1-76.e7.
- Seale AC, Bianchi-Jassir F, Russell NJ, et al. (2017) Estimates of the burden of Group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin Infect Dis 65: S200-S219.
- https://www.hassante.fr/upload/docs/application/pdf/prevention_antenatale_du_risque_infectieux_bacterien_-_rec.pdf
- Money D, Allen VM (2016) Prévention de l'infection néonatale à streptocoques du groupe B d'apparition précoce. J Obstet Gynaecol Can 38: S336-S347.
- Martín V, Cárdenas N, Ocaña S, et al. (2016) Rectal and vaginal eradication of streptococcus agalactiae (gbs) in pregnant women by using lactobacillus salivarius cect 9145, a target-specific probiotic strain. Nutrients 11: 810.
- Cools P, Jespers V, Hardy L, et al. (2012) A multi-country cross-sectional study of vaginal carriage of Group B Streptococci (GBS) and Escherichia coli in resource-poor settings: Prevalences and risk factors. PLoS ONE 11: e0148052.
- Kruk CR, Feuerschuette OHM, da Silveira SK, et al. (2013) Epidemiologic profile of Streptococcus agalactiae colonization in pregnant women attending prenatal care in a city of southern of Brazil. The Braz J Infect Dis 17: 722-723.
- Joachim A, Matee MI, Massawe FA, et al. (2009) Maternal and neonatal colonisation of group B streptococcus at Muhimbili National Hospital in Dar es Salaam, Tanzania: Prevalence, risk factors and antimicrobial resistance. BMC Public Health 9: 437.
- Bolukaoto JY, Monyama CM, Chukwu MO, et al. (2015) Antibiotic resistance of Streptococcus agalactiae isolated from pregnant women in Garankuwa, South Africa. BMC Res Notes 8: 364.
- Lu B, Li D, Cui Y, et al. (2014) Epidemiology of Group B streptococcus isolated from pregnant women in Beijing, China. Clin Microbiol Infect 20: O370-373.
- Melo SCCS de, Santos NC de S, Oliveira M de, et al. (2016) Antimicrobial susceptibility of streptococcus agalactiae isolated from pregnant women. Rev Inst Med Trop Sao Paulo 58: 83.
- Gopal Rao G, Nartey G, McAree T, et al. (2017) Outcome of a screening programme for the prevention of neonatal invasive early-onset group B Streptococcus infection in a UK maternity unit: An observational study. BMJ Open 7: e014634.
- Burcham LR, Spencer BL, Keeler LR, et al. (2019) Determinants of Group B streptococcal virulence potential amongst vaginal clinical isolates from pregnant women. PLoS One 14: e0226699.
- Gygax SE, Schuyler JA, Kimmel LE, et al. (2006) Erythromycin and clindamycin resistance in group B streptococcal clinical isolates. Antimicrob Agents Chemother 50: 1875-1877.
- Guo D, Cao X, Li S, et al. (2018) Neonatal colonization of group B Streptococcus in China: Prevalence, antimicrobial resistance, serotypes, and molecular characterization. Am J Infect Control 46: e19-e24.
- Lee BK, Song YR, Kim MY, et al. (2010) Epidemiology of group B streptococcus in Korean pregnant women. Epidemiol Infect 138: 292-298.
- Buter CC, Mouton JW, Klaassen CH, et al. (2010) Prevalence and molecular mechanism of macrolide resistance in beta-haemolytic streptococci in The Netherlands. Int J Antimicrob Agents 35: 590-592.
- Amezaga MR, McKenzie H (2006) Molecular epidemiology of macrolide resistance in beta-haemolytic streptococci of Lancefield groups A, B, C and G and evidence for a new mef element in group G streptococci that carries allelic variants of mef and msr(D). J Antimicrob Chemother 57: 443-449.
- Shabayek S, Abdalla S (2014) Macrolide- and tetracycline-resistance determinants of colonizing group B streptococcus in women in Egypt. J Med Microbiol 63: 1324-1327.
- Gizachew M, Tiruneh M, Moges F, et al. (2019) Streptococcus agalactiae from Ethiopian pregnant women; prevalence, associated factors and antimicrobial resistance: Alarming for prophylaxis. Ann Clin Microbiol Antimicrob 18: 3.
- Hraoui M, Boutiba-Ben Boubaker I, Rachdi M, et al. (2012) Macrolide and tetracycline resistance in clinical strains of Streptococcus agalactiae isolated in Tunisia. J Med Microbiol 61: 1109-1113.
- Rachdi M, Boutiba-ben Boubaker I, Hraoui M, et al. (2010) High rates of macrolide resistance among clinical isolates of Streptococcus agalactiae in Tunisia. Arch Inst Pasteur Tunis 87: 35-42.
- Bergal A, Loucif L, Benouareth DE, et al. (2015) Molecular epidemiology and distribution of serotypes, genotypes, and antibiotic resistance genes of Streptococcus agalactiae clinical isolates from Guelma, Algeria and Marseille, France. Eur J Clin Microbiol Infect Dis 34: 2339-2348.
- De Francesco MA, Caracciolo S, Gargiulo F, et al. (2012) Phenotypes, genotypes, serotypes and molecular epidemiology of erythromycin-resistant Streptococcus agalactiae in Italy. Eur J Clin Microbiol Infect Dis 31: 1741-1747.
- https://www.infectiologie.org.tn/pdf_ppt_docs/resistance/1544218503.pdf.
- Dutra VG, Alves VM, Olendzki AN, et al. (2014) Streptococcus agalactiae in Brazil: Serotype distribution, virulence determinants and antimicrobial susceptibility. BMC Infect Dis 14: 323.
- Ueno H, Yamamoto Y, Yamamichi A, et al. (2012) Characterization of group B streptococcus isolated from women in Saitama city, Japan. Jpn J Infect Dis 65: 516-521.
- Wu B, Su J, Li L, et al. (2019) Phenotypic and genetic differences among group B Streptococcus recovered from neonates and pregnant women in Shenzhen, China: 8-year study. BMC Microbiol 19: 185.
- (2019) Prevention of Group B streptococcal early-onset disease in newborns: ACOG Committee Opinion. Obstet Gynecol 134: e19-e40.
Corresponding Author
Hela Hannachi, Academic Hospital Assistant, Faculty of Medicine, Microbiology and Biochemistry Laboratory, Aziza Othmana Hospital, ElManar University, Tunisia, Tel: 0021625551028, Fax: 0021671260327.
Copyright
© 2020 Hannachi H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.