Mycobacterium Kansasii: A Case of Blood Stream Infection with Multiple Metastatic Abscesses
Abstract
We describe a patient, who presented to our hospital with a prolonged febrile illness and was diagnosed with Acquired Immunodeficiency Syndrome (AIDS) with CD4 count of 12 cells/mm³ and viral load of 738000 copies/mL. The CT scan of the chest did not show any abnormalities. Routine cultures were negative. The CT scan of the abdomen showed a single liver abscess, the routine cultures of liver abscess were negative. The MRI scan of the head revealed focal enhancing lesion within the right parietal bon which was not pursued further at this point. About eight weeks later, the AFB blood culture was reported to have grown Mycobacterium Kansasii. Because of the high index of suspicion, aspiration of the liver abscess was repeated, and mycobacteria consistent with M. kansasii were grown. The biopsy from the right parietal dura and right parietal bone showed acid fast bacilli on the stain which did not grow on the cultures, perhaps representing post-treatment non-viable mycobacteria.
Abbreviations
M. kansasii: Mycobacterium Kansasii; MAC: Mycobacterium Avium Complex; NTM: Non-Tuberculous Mycobacteria; AFB: Acid Fast Bacilli; HIV: Human Immunodeficiency Virus; AIDS: Acquired Immunodeficiency Syndrome; HAART: Highly Active Antiretroviral Therapy; MRI: Magnetic Resonance Imaging; CT: Computed Tomography; CXR: Chest X Ray; FNA: Fine Needle Aspiration; CDI: Clostridium Difficile Infection
Introduction
Mycobacterium kansasii is the second most common cause of Non-Tuberculous Mycobacteria (NTM) disease in the United States including patients with HIV infections. M. kansasii causes pulmonary disease in over half of infected patients late in the course of AIDS [1-3]. However, this presentation is more common in patients with underlying lung diseases. The clinical presentations of M. kansasii pulmonary disease are similar to those associated with pulmonary M. tuberculosis [4,5].
Disseminated M. kansasii disease is less likely in HIV negative individuals and often associated with advanced HIV. The average CD4 count of patient with M. kansasii and AIDS co-infection was less than 50 cells/mm³ in most studies. Like other opportunistic infections, the incidence of disseminated M. kansasii infection has declined after the introduction of Highly Active Antiretroviral Therapy (HARRT) [1,4,5].
Blood stream, aorta, mediastinal lymph nodes, pericardium, gingivomandibular, sinuses, bone, tendon and joints, liver, appendix, brain infections have been reported in advanced HIV and immunocompromised individuals [2-5].
Case Report
35-year-old female, without a significant past medical history, presented to our hospital with complaints of fever, malaise, weight loss and chronic diarrhea.
On physical examination she was thin and chronically ill appearing, she had a maximum temperature of 103.9 °F, blood pressure of 107/63 mmHg, heart rate of 120 beats/min, respiratory rate of 18 breaths/min, and oxygen saturation of 97% on room air. Thrush and poor dentition were found on oral exam. Abdomen, chest, lung and skin examination were unremarkable.
She was found to be HIV infected with a very low CD4 count of 12 cells/mm³ (3.1%); HIV viral load of 738,000 copies/mL, and the HIV genotyping did not show any resistance to antiretroviral agents.
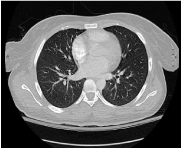
During her prolonged hospitalization, she had unexplained persistent fever, and the extensive work up included multiple negative blood cultures, negative CXR. The CT scan of the chest (Figure 1) showed no evidence of pulmonary infiltrate to suggest pneumonia, The CT scan of the abdomen and pelvis (Figure 2) demonstrated hepatic abscess. The patient underwent CT guided liver abscess drainage with 3 cc of pus removed and routine cultures did not grow any organisms. Initially the impression was that the liver abscess was secondary to dental abscesses which had been found on Panorex and was treated with appropriate antibiotics. Thereafter, the patient developed leukocytosis and diarrhea and the clostridium difficile toxin in the stool was positive. She was treated for C. difficile infection which resulted in resolution of leukocytosis and diarrhea.
However, the patient continued to have persistent fever, so we ordered blood cultures for Acid fast bacilli to rule out disseminated MAC infection. Approximately 4 weeks later, the blood cultures were reported to grow acid fast bacilli other than MTB. We started her on treatment for presumptive disseminated MAC infection with Azithromycin and Ethambutol while awaiting speciation. Patient did not attain any clinical improvement so Rifabutin was added. At 8 weeks the AFB in blood cultures was identified as Mycobacterium kansasii.
Azithromycin was changed to Isoniazid plus pyridoxine. Rifabutin and Ethambutol were continued. A repeat CT scan of abdomen (Figure 3 and Figure 4) showed improvement of liver abscess and demonstration of a 1.8 × 1.0 cm area of hypoattenuation in the left hepatic lobe, which could represent hypoenhancement due to infarct from prior abscess versus residual or recurrent abscess. A second aspiration of the liver abscess was done. Pathology from liver lesion on second Fine Needle Aspiration (FNA) was negative for malignant cells, but there was acute inflammation which consistent with abscess formation. The specimen was sent to a reference laboratory where the AFB stain was reported to be positive for numerous organisms consistent with M. kansasii.
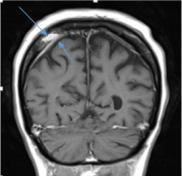
Patient was also found to have a focal enhancing lesion within the right parietal bone on MRI scan (Figure 5). The biopsy from the lesion was taken from right parietal dura and right parietal bone. Histopathology showed mixed inflammatory reaction with plasmacytosis involving bone and underlying dura mater which was felt to be consistent with reactive process. Ziehl-Neelsen staining was performed at the reference laboratory showed rare acid fast bacilli that did not grow on cultures, this can easily be explained by the presence of post-treatment non-viable mycobacteria.
After 6 weeks of treatment for M. kansasii infection, with the aim of avoiding Immune Reconstitution Inflammatory Syndrome (IRIS), patient was started on antiretroviral therapy. She is being followed in our clinic and is doing well. Her most recent CD4 count is 87 cells/mm³ and HIV viral load is 710 copies/mL. And she is into 9 months of therapy for M. kansasii infection. Patient was also placed on prophylaxis for Pneumocystis jirovecii pneumonia, Toxoplasmosis and Mycobacterium avium complex.
Discussion
Mycobacterium kansasii was first described by Buhler and Pollack in 1953. It is an acid-fast, photo chromogenic (produces a yellow pigment when exposed to light) mycobacterium, stains poorly with gram staining, grows slowly over more than 7 days to reach mature growth and appears longer and broader than Mycobacterium tuberculosis often with a beaded or cross-barred appearance on Ziehl-Neelsen staining. Unlike other NTM, M. kansasii is not readily isolated from environmental sources. Tap water is likely the major reservoir causing human disease. Human to human transmission likely does not occur [1-4].
The reported incidence of M. kansasii in HIV patients in the United States is 0.14%-0.44% [5]. Almost 20% of patients with HIV infection, who develop M. kansasii infection, eventually develop disseminated disease. The average CD4 count of patient with M. kansasii and HIV co-infection was less than 50 cells/mm³ in most studies [1,4,6-8].
M. kansasii causes pulmonary disease in patients with underlying lung diseases, such as chronic obstructive pulmonary disease, bronchiectasis, prior tuberculosis, pneumoconiosis and malignancy [1,4,5]. The symptoms of M. kansasii pulmonary disease are commonly indistinguishable to those associated with pulmonary M. tuberculosis [4,5]. The most common symptoms of pulmonary M. kansasii infection include cough, weight loss, shortness of breath, chest pain, hemoptysis, and fever [9].
Disseminated M. kansasii disease is uncommon in HIV negative patients and usually associated with advanced immune compromised status. Nonetheless, the incidence of disseminated M. kansasii infection has declined after the debut of Highly Active Antiretroviral Therapy (HARRT). The risk factors for disseminated M. kansasii infection include acquired immunologic defects such as AIDS and iatrogenic immunosuppression, or genetic disorders like as INF-γ and IL-12 pathway defects [10,11].
Isolated bloodstream infection cause by M. kansasii is less common than with MAC in HIV infected patients and is seen in less than 25% of infected individuals [1,4,5]. The organs involved in disseminated M. kansasii disease include lung, bone, intestines, central nervous system, spleen, bone marrow, lymph nodes, skin, and genitourinary system [5]. Other cases reported include pericarditis in a kidney transplant patient [10], aortic pseudoaneurysm in myelodysplastic syndrome [12], mediastinal lymphadenopathy or abscesses [11], hepatic abscesses in a renal transplant recipient [2]. A case of M. kansasii and MAC co-infection in an AIDS patient was reported, but the liver abscess revealed MAC only [13]. Smith, et al. reported that M. kansasii was isolated from granuloma in the portal area of the liver [5].
Common causes of liver abscess in HIV infected patients include pyogenic abscesses due to the Streptococcus milleri group, Staphylococcus aureus, Streptococcus pyogenes, and other Gram positive cocci, other pathogens like Klebsiella pneumoniae, Candida species, Mycobacterium tuberculous, MAC, Burkholderia pseudomallei, Amoebiasis, Histoplasmosis, Leishmaniasis, Pneumocystis jirovecii have been reported as well [14,15].
In our extensive literature review, we came across a report of liver abscess due to M. kansasii in a patient with HIV with low CD4 count and without pulmonary involvement [16]. However, case of M. kansasii infection involving blood stream infection with liver abscess and bone next to the dura mater was not reported. Our case extends this previous reported case to include cranial bone and dura mater involvement in addition to blood stream infection and liver abscess in disseminated infection caused by M. kansasii.
Conclusion
M. kansasii is an important pathogen in immune compromised patients, especially those with advanced AIDS. The prevalence of M. kansasii increased with the HIV pandemic. Disseminated M. kansasii infection is rare in HIV negative individuals. HIV and M. kansasii co-infection is often associated with advanced immune compromised status, usually with CD4 count less than 50 cells/mm³ in most studies. Pulmonary disease is seen in over 50% of patients and isolated bloodstream infection is far less common than with MAC and occurs in less than 25% of infected individuals. M. kansasii should be considered in immune suppressed individuals with pulmonary, extrapulmonary and systemic infection. The case reported here adds liver abscess and cranial (parietal bone) lesions to the spectrum of organs involved in disseminated diseases due to M. kansasii infection. The case also highlights the significant contribution histopathologic and microbiologic evaluation made to the diagnosis and management of this patient. For a number of reasons, the initial specimen may not be diagnostic but a second biopsy was done in this case, can be revealing and very useful.
References
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. (2007) An official ATS/IDSA statement: diagnosis, treatment and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175: 367-416.
- Kaur P, Fishman JA, Misdraji J, et al. (2011) Disseminated Mycobacterium kansasii infection with hepatic abscesses in a renal transplant recipient. Transpl Infect Dis 13: 531-535.
- Horsburgh CR, Selik RM (1989) The epidemiology of disseminated nontuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am Rev Respir Dis 139: 4-7.
- Johnston JC, Chiang L, Elwood K (2017) Mycobacterium kansasii. Microbiol Spectr 5.
- Smith MB, Molina CP, Schnadig VJ, et al. (2003) Pathologic features of Mycobacterium kansasii Infection in patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med 127: 554-560.
- Campo RE, Campo CE (1997) Mycobacterium Kansasii disease in patients infected with human immunodeficiency virus. Clin Infect Dis 24: 1233-1238.
- Block KC, Zwerling L, Pletcher MJ, et al. (1998) Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann Intern Med 129: 698-704.
- Levine B, Chaisson RE (1991) Mycobacterium kansasii: a cause of treatable pulmonary disease associated with advanced human immunodeficiency virus (HIV) infection. Ann Intern Med 114: 861-868.
- Evans SA, Colville A, Evans AJ, et al. (1996) Pulmonary Mycobacterium kansasii infection: comparison of the clinical features, treatment and outcome with pulmonary tuberculosis. Thorax 51: 1248-1252.
- Cho JH, Yu CH, Jin MK, et al. (2012) Mycobacterium kansasii pericarditis in a kidney transplant recipient: a case report and comprehensive review of the literature. Transpl Infect Dis 14: E50-E55.
- Lovell JP, Zerbe CS, Olivier KN, et al. (2016) Mediastinal and Disseminated Mycobacterium kansasii Disease in GATA2 Deficiency. Ann Am Thorac Soc 13: 2169-2173.
- Ehsani L, Sujan CR, Mosunjac M, et al. (2015) Fatal aortic pseudoaneurysm from disseminated Mycobacterium kansasii infection: case report. Hum Pathol 46: 467-470.
- Massenkeil G, Opravil M, Salfinger M, et al. (1992) Disseminated coinfection with Mycobacterium avium complex and Mycobacterium kansasii in a patient with AIDS and liver abscess. Clin Infect Dis 14: 618-619.
- Poblete RB, Rodriguez K, Foust RT, et al. (1989) Pneumocystis carinii hepatitis in the acquired immunodeficiency syndrome (AIDS). Ann Intern Med 110: 737-738.
- Poles MA, Dieterich DT, Schwarz ED, et al. (1996) Liver biopsy findings in 501 patients infected with human immunodeficiency virus (HIV). J Acquir Immune Defic Syndr Hum Retrovirol 11: 170-177.
- Elsia Y, Sammanodi M, Zhao A, et al. (2017) Mycobacterium kansasii liver abscess in a patient with HIV. Open J Clin Med Case Rep 3: 1231.
Corresponding Author
Nancy Khardori, MD, PhD, Division of Infectious Disease, Department of Internal Medicine, Eastern Virginia Medical School, USA.
Copyright
© 2018 Kulla B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.