Comparison between Letrozole with and without Gonadotropins Injection on Pregnancy Rates in Infertile PCOS Patients: A Multicenter Randomized Observational Trial
Abstract
Background: We developed a multicenter randomized observational trial to assess the effectiveness of letrozole monotherapy and the co-therapy of letrozole and gonadotropins on pregnancy outcomes including the ovulation induction, pregnancy rate, estradiol (E2) levels and endometrial thickness in infertile women with polycystic ovarian syndrome (PCOS).
Methods: At three in vitro fertilization (IVF) centers in Egypt, we conducted our study since May 2019 until we have recruited the planned number of patients. Infertile PCOS patients were recruited after they met our criteria. We had two intervention groups based on the treatment regimen. Moreover, baseline parameters, outcomes, and hormonal assessment was conducted throughout the study period.
Results: We included 90 patients in this study and were equally randomized into two groups; the letrozole monotherapy (Group A) and the letrozole with gonadotropins (Group B) groups. The mean age was significantly higher in group A [26 (± 3.5)] than group B [24.2 (± 3.1)] (P = 0.015). Both groups showed resulted in similar pregnancy rates (31.1%) (P = 0.079). Moreover, group B [2.4 (± 0.8)] resulted in a significantly higher (P = 0.023) mean number of follicles than group A [2.0 (± 0.7)]. On the other hand, no statistical significance was estimated in terms of estradiol levels and endometrial thickness (P = 0.209 and 0.485, respectively).
Conclusion: We concluded that letrozole monotherapy and in combination with gonadotropins obtain better pregnancy rates in PCOS patients. Moreover, the combination may be useful in reducing gonadotropin adverse events with the same acceptable rates.
Keywords
Gonadotropin, Letrozole, Ovulation induction, Polycystic ovarian syndrome
Introduction
During women's reproductive age, the prevalence of polycystic ovarian syndrome (PCOS) ranges between 9 and 18%. PCOS is one of the commonest endocrine disorders and the most prevalent cause of anovulatory infertility at this age [1-3]. The diagnosis is dependent on the presence of ovulatory dysfunction, hyperandrogenism, or ultrasound-guided detection of PCO morphology according to the Rotterdam criteria [4]. Many therapeutic approaches have been effectively reported to enhance the outcomes of the syndrome. The most common drug which has been widely used historically as an estrogen-receptors (ER) modulator is called clomiphene citrate (CC) and is mainly used to induce ovulation. However, arousing concerns have now been rising about the quality of this drug based on its adverse effects and its effectiveness on the overall outcomes [5]. Moreover, a rate of 20% of women receiving CC is CC-resistant [6]. Although many approaches have been made to overcome this, like combining the drug with metformin, investigations have been directed into navigating through the efficacy of other therapies which may be safer and more effective [7].
Letrozole, an aromatase inhibitor, has been reportedly effective in ovulation induction and increasing birth rate especially in CC-resistant patients [8]. It acts by indirect stimulation of the hypothalamic-pituitary axis resulting in the excessive release of gonadotropin-releasing hormone (GnRH) and follicle-stimulating hormone (FSH) that lead to ovulation induction [9]. Furthermore, letrozole has positive effects on the endometrial thickness, flocculation genesis and frequency (mono-follicular), and cervical mucus secretions which may enhance the rate of pregnancy and decrease the morbidities in PCOS patients [10].
In addition, gonadotropins have been reported to effective in this field and are usually used in combinations with other first-line drugs. As for the CC-resistant case, two gonadotropins preparations have been found to induce ovulation in PCOS patients. These are the urinary and recombinant FSH (rFSH) [11]. However, gonadotropin administration must be done under wise supervision and continuous sonographic checking and hormonal evaluation. Many adverse events have been associated with gonadotropins application. These include ovarian hyperstimulation, multiple pregnancies, cycle cancellation, and are not cost-effective [12-15]. Hyperstimulation can result from high doses, while low doses may cause no responses and are inefficacious [13]. However, when used in combination with other drugs, as CC, better outcomes were obtained than when used alone [16]. Therefore, bearing in mind the efficacy of letrozole and its fewer side effects, and the usefulness of gonadotropins when used in combinations, we aim to develop a randomized trial to compare between letrozole when used alone and when combined with gonadotropins on enhancing ovulation induction and pregnancy rates in PCOS infertile patients after normal intercourse.
Methods
Study design and population
In this randomized clinical trial, data collection occurred at three different in vitro fertilization (IVF) centers including Beni-Suef University Hospital, Beni-Suef, Egypt, Omuma IVF center Fayoum, Egypt, and El-Nada IVF center, Beni-Suef, Egypt on May 2019 until our target sample size was obtained. Patients at the centers were screened against our inclusion and exclusion criteria to find the most suitable ones to the aim of our study. Our inclusion criteria were: 1) Infertile with PCOS according to the Rotterdam criteria (4); (2) < 35-years-old; 3) With normal thyroid-stimulating hormone and prolactin levels; 4) Menstrual third day FSH levels < 12 IU/ml, (5) normal hysterosalpingography (HSG) and (5) Abnormal semen analysis while patients were excluded if they: 1) Were infertile but did not meet any of the Rotterdam criteria; 2) > 35-years-old; (3) Abnormal thyroid-stimulating hormone and prolactin levels; 4) Abnormal semen analysis; 5) Menstrual third day FSH levels > 12 IU/ml, and 5) Abnormal hysterosalpingography (HSG) ornormal hysterosalpingography (HSG).
Sample size calculation was done using PS Power and Sample Size Calculations software, version 3.0.11 for MS Windows (William D. Dupont and Walton D. Vanderbilt, USA). It is mainly based on comparing the occurrence of pregnancy rates after normal intercourse in infertile PCOS patients that received the study intervention (and based on other ratios from previously published studies) which primarily aimed to induce ovulation in these patients. We had two interventions including administration of letrozole alone and administration of letrozole together with gonadotropins. Accordingly, patients were randomized into two groups with a 1:1 ratio using the Chi test, the α-error level, which was fixed at 0.05, and the power which was set at 80%.
After that patients were randomly allocated into two groups based on the interventions that were used in this study by using a random sequence generation method. These groups include the letrozole only group which will be referred to as (Group A) and the letrozole and gonadotropins group, referred to as (Group B). The dose of letrozole was 2.5 mg twice daily from the 2nd to the 5th days of the menstrual cycle for both groups while gonadotropins administration was inaugurated on the 7th day of the menstrual cycle and the applied doses were based on many factors including age, BMI, basal FSH level, medical history. Moreover, doses were adjusted based on the follicular response and estradiol (E2) levels of the patients to prevent any major adverse events.
Data collection and outcome measures
Data collection was done mainly through a pre-designed questionnaire. Examinations were done through the 2nd to the 5th days of menstruation and included patient's demographics as age, main body weight and length, body mass indexes (BMI), and previous periods of infertility. Besides, hormonal levels were also assessed as FSH, luteinizing hormone (LH), E2, progesterone, prolactin, and TSH levels. These were measured by an immune-enzymatic approach (ELISA) on the 2nd to 3rd of patients' menstrual cycles. After that, transvaginal ultrasonography was used to follow-up patients from the 9th day of menstruation and continued after the greatest follicle was measured and recorded 14 mm or more. We have also estimated the follicular size means from two different dimensions together with the thickness of the endometrium thickness which was done between two echogenic surfaces in a longitudinal axis of the uterus. Moreover, after the greatest follicle has reached the size of 17-18 mm, a maximum shot of 10,000 IU HCG was given to the patient intramuscularly with a boosting dose within 36h after the injection. Patients continued to be assessed after normal intercourse, after the injection until the outcomes of our study have been achieved.
The number of follicles, endometrial thickness, and serum estradiol levels will be measured in both groups with no difference between the two doses of letrozole. Pregnancy rates, endometrial thickness, estradiol levels, and frequency of flocculation were our primary outcomes, according to which, we studied the statistical significance between the two study groups.
Statistical analysis
We used SPSS 25 program (SPSS Inc, Chicago, IL, USA) to perform the analysis. For continuous variables, we have estimated the descriptive statistics and represented them by mean ± standard deviation (SD). On the other hand, nominal variables were examined by the Chi-square test and presented as counts and percentages. Normal distributions for continuous data were assessed by the Kolmogorov-Smirnov test. Based on this, further assessment was conducted by the t-test or the Mann-Whitney-U test. Statistical significance has been decided if the P-value < 0.05 of any of the assessed variables.
Results
Characteristics of the study population
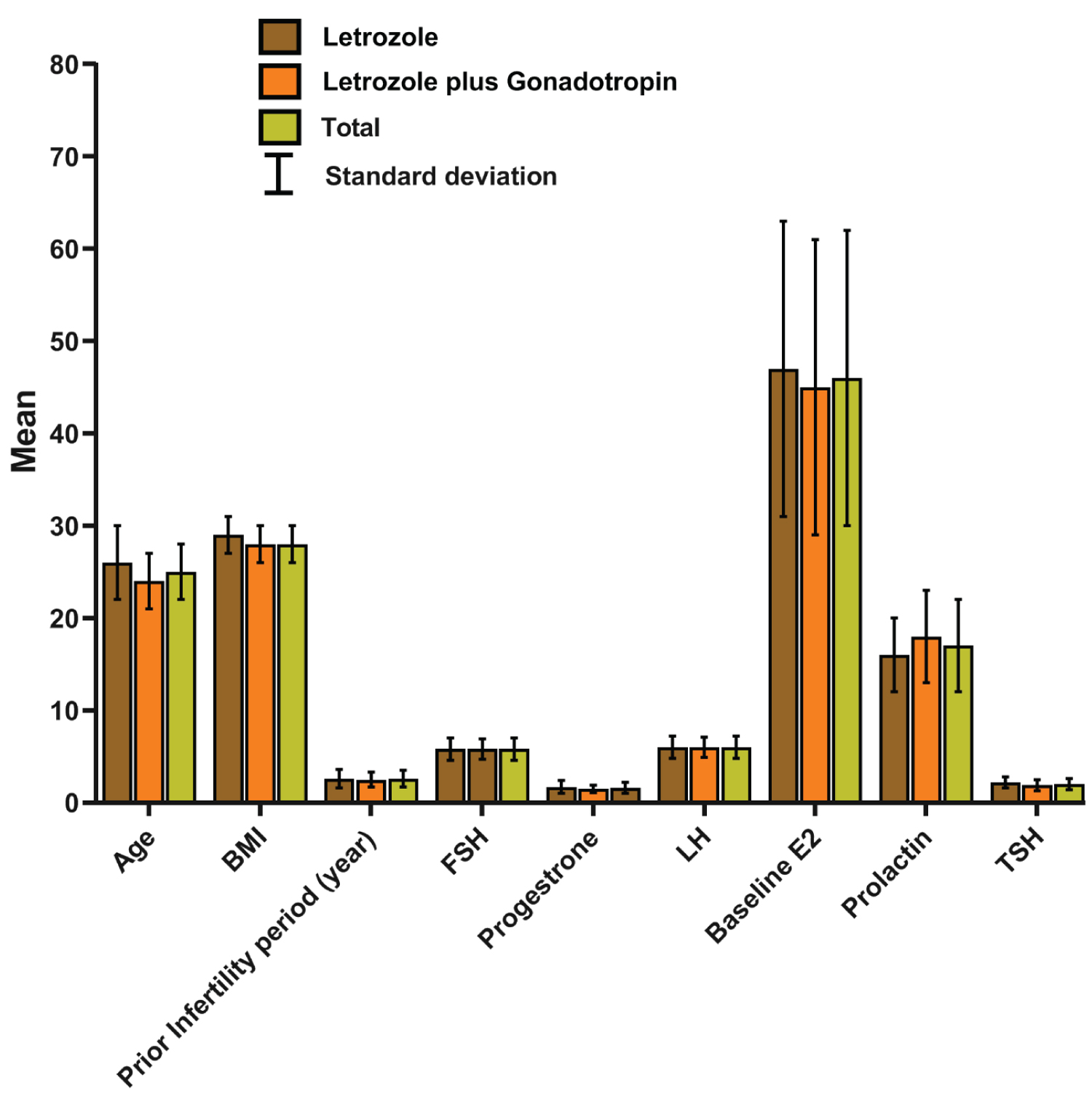
The study population of the present study involved 90 patients with infertility due to PCOS. Each of the study groups; groups A and B, included 45 patients with a mean age of 26 (± 3.5), and 24.2 (± 3.1), respectively. Detailed patient demographics and other variables are presented in Table 1. The mean BMI was 28.6 (± 2.3) and 28.0 (± 2.1) kg/m2 for groups A and B, respectively. The mean infertility period before enrollment in the study was 2.6 (± 1.0) and 2.5 (± 0.8) for groups A and B, respectively. Even though group A showed higher values of these variables than the other group, on statistical comparison, only the age variable was significant (P= 0.015) while BMI and prior infertility period were not (P = 0.156 and 0.902, respectively). Moreover, Figure 1 shows that the baseline of the assessed hormones was similar between the two groups. As none of these variables were normally distributed, we used the Mann-Whitney-U test to compare their values between the two groups. None of the studied baseline variables showed significance (Table 1) except for FSH level which was significantly higher in Group A than group B (P = 0.018).
Assessed outcomes
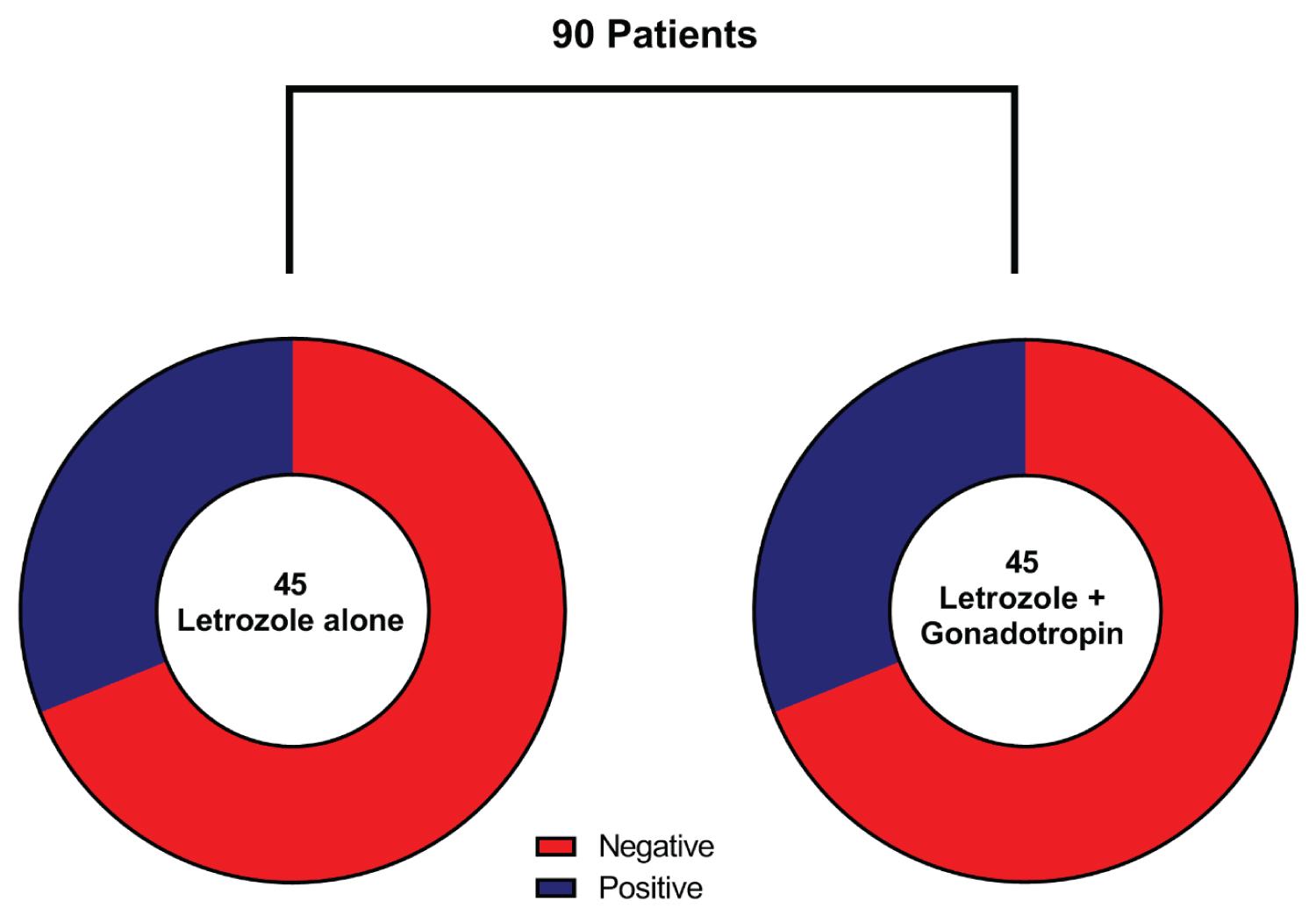
The pregnancy rate was 31.1% while 62 (68.9%) patients were not pregnant after letrozole administration with and without gonadotropins. Based on the subgroup analysis, no difference (P = 0.079) was found between groups A and B in the pregnancy rate (31.1% for each group) (Table 2 and Figure 2). The mean number of follicles was significantly higher (P = 0.023) in group B [2.4 (± 0.8)] (where gonadotropins were administered with letrozole) than group A [2.0 (± 0.7)] (where letrozole was used alone). Other assessed outcomes include endometrial thickness and E2 levels. The overall mean endometrial thickness was 7.7 (± 1.9) mm with no statistical significance between the two groups (P = 0.485). Additionally, group B [981.1 (± 126.7)] was associated with more elevated E2 levels than group A [946.7 (± 152.8)], however, the difference was not significant (P = 0.209) (Table 3).
Discussion
The management of PCOS-infertility depends on the prevention of follicular atresia which turns against the follicular formation. This requires re-establishing a state of balance in the synthesis of intra-ovarian hormones that take part in the maturation and ovulation process. In the PCOS case, patients will first undergo a regimen of lifestyle modification followed by the administration of certain drugs to induce ovulation [17]. Aromatase inhibitors are used as a second-line treatment in this condition after CC. This group has a minimal effect on androgens preventing their conversion into estrogen which inhibits the feedback on the hypo-thalamic-pituitary axis leading to the release of normal GnRH and FSH levels [18]. Moreover, together with aromatase inhibitors, low doses of gonadotropins have been used to induce ovulation. However, many efforts are needed to administrate the optimum required doses only, otherwise, many side effects will develop. In addition, after gonadotropins application, the following cycles will be interrupted and further administration of gonadotropins will be contradicted due to their hyperstimulation effect and multi-follicles formation [19,20].
To reduce the need for gonadotropins, CC has been used in combination with it, and the results showed no negative impact on pregnancy rate as the used gonadotropin dose was reduced to 80% [21]. On the other hand, other studies have shown that maintaining the number of ovulatory follicles in women receiving combined therapy as double as those receiving gonadotropins alone is the key element to equal pregnancy rates. Without this condition, patients on the combined group were prone to more side effects and less positive outcomes than when using gonadotropins alone [22,23]. Apart from CC, letrozole has been reported to be an efficacious therapy with fewer adverse effects. No toxic events have been reported on the endometrium due to the short plasma half-life of the drug. In addition, it does not cause ovarian hyperstimulation as it maintains FSH at lower levels [24-26]. Although many investigations have reported the use of many treatment regimens and used comparative approaches to formulate evidence on the overall superiority of these regimes, scarce reports have compared between using letrozole as a monotherapy and with gonadotropins, therefore, among a very minimal number of reports in this field, we have conducted a randomized clinical trial to compare between the two regimens in terms of ovulation induction and related factors.
The results of our study showed a total of 31.1% successful pregnancy rates for both groups with no difference between them. Although Alizzi, et al. [27] reported higher rates in the letrozole group than the other group which used letrozole combined with gonadotropins, no statistical significance was found between the two rates. On the other hand, Chen, et al. [28] results showed that the difference in pregnancy rates was significant in favor of the combined treatment regimen over the letrozole alone. In general, the overall rates for both groups were similar to these studies [29]. Furthermore, our study demonstrated that total endometrial thickness was generally acceptable and both groups, the two administered regimens did not affect it. However, we did not find any significance between the two groups. Similarly, Alizzi, et al. [27] reported no significance. On the other hand, Chen, et al. [28] found that letrozole alone does not significantly reduce the thickness of the endometrium. However, Yu, et al. [29] showed that patients that received the combined regimen significantly had thicker endometria than others that received letrozole monotherapy. Another study by Healey, et al. [30] reported that letrozole negatively affected the endometrium thickness in a significant relation. However, the authors argued that this may be due to its negative on E2 production [31]. However, in our study, no significance was reported between the two groups on E2 production. On the other hand, Chen, et al. [28] supported Healy, et al. as their results indicated that E2 levels were significantly decreased with letrozole monotherapy. In other terms, hyperstimulation in the combined group could still be noticed as the number of follicles was significantly higher than in the group where letrozole was used alone (P = 0.023). Moreover, the baseline values of FSH and LH were identical in both groups, while TSH was significantly lower (which has a major role on lowering gonadotrophin levels through positive feedback on thyroid-stimulating hormones and prolactin which inhibits more gonadotropins release) in group 2 which indicates the results that hyperstimulation was due to the exogenous administration of gonadotropins.
Limitations to our study include the small sample size of the included patients. Moreover, although patients were randomly allocated into their groups, we found significance in age between the two of them as group one had higher ages. The differences in other baseline features were not significant, though. When comparing these results to other studies, we found that Alizzi, et al. [27] and Chen, et al. [28] reported significance in terms of age, BMI, and mean duration of infertility before recruitment. Therefore, we can argue that these characteristics may have an impact on the study outcomes, and therefore, more defined and specific criteria regarding them should be considered in future investigations.
Conclusion
In this study, we found that both letrozole monotherapy and letrozole combined with gonadotrophins results in acceptable rates of pregnancy. Moreover, both groups showed similar rates with no significance together with the non-significance in terms of endometrial thickness and E2 levels. On the other hand, the only disadvantage that was noticed for the combined therapy is that it leads to the production of significantly higher numbers of follicles. Therefore, gonadotrophins injection with letrozole can enhance the outcomes, however, it should be carefully used with precise dosages and continuous monitoring.
Ethical Statement
None.
Conflict of Interest
None.
Funding
None.
References
- Broekmans F, Knauff E, Valkenburg O, et al. (2006) PCOS according to the Rotterdam consensus criteria: Change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG 113: 1210-1217.
- Sirmans SM, Pate KA (2014) Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol 6: 1.
- ESHRE Capri Workshop Group (2012) Health and fertility in World Health Organization group 2 anovulatory women. Hum Reprod Update 18: 586-599.
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod 19: 41-7.
- Legro RS, Barnhart HX, Schlaff WD, et al. (2007) Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 356: 551-566.
- Kamphuis EI, Bhattacharya S, van der Veen F, et al. (2014) Are we overusing IVF? BMJ Clinical Research 348: g252.
- Lord JM, Flight IHK, Norman RJ (2003) Metformin in polycystic ovary syndrome: Systematic review and meta-analysis. BMJ (Clinical research ed) 7421: 951-327.
- Biljan MM, Hemmings R, Brassard N (2005) The Outcome of 150 Babies Following the Treatment With Letrozole or Letrozole and Gonadotropins. Fertil Steril 84: S95.
- Casper RF, Mitwally MFM (2006) Review: Aromatase inhibitors for ovulation induction. J Clin Endocrinol Metab 91: 760-771.
- Legro RS, Kunselman AR, Brzyski RG, et al. (2012) The Pregnancy in Polycystic Ovary Syndrome II (PPCOS II) trial: Rationale and design of a double-blind randomized trial of clomiphene citrate and letrozole for the treatment of infertility in women with polycystic ovary syndrome. Contemporary Clinical Trials 33: 470-481.
- (2008) Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod 23: 462-477.
- Herman A, Ron-El R, Golan A, et al. (1993) Overstimulated cycles under low-dose gonadotrophins in patients with polycystic ovary syndrome: Characterization and management. Human Reproduction 8: 30-34.
- Fritz MA, Speroff L (2012) Clinical Gynecologic Endocrinology and Infertility: Wolters Kluwer Health.
- McNeilly AS (1988) The control of FSH secretion. Acta Endocrinol Suppl (Copenh) 288: 31-40.
- Mitwally MF, Casper RF (2001) Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril 75: 305-309.
- Amer SA, Li TC, Ledger WL (2004) Ovulation induction using laparoscopic ovarian drilling in women with polycystic ovarian syndrome: Predictors of success. Hum Reprod 19: 1719-1724.
- Ghanem ME, Elboghdady LA, Hassan M, et al. (2013) Clomiphene citrate co-treatment with low dose urinary FSH versus urinary FSH for clomiphene resistant PCOS: Randomized controlled trial. J Assist Reprod Genet 30: 1477-1485.
- Tanbo T, Mellembakken J, Bjercke S, et al. (2018) Ovulation induction in polycystic ovary syndrome. Acta Obstet Gynecol Scand 97: 1162-1167.
- Dale O, Tanbo T, Lunde O, et al. (1993) Ovulation induction with low-dose follicle-stimulating hormone in women with the polycystic ovary syndrome. Acta Obstet Gynecol Scand 72: 43-46.
- Orvieto R, Homburg R (2009) Chronic ultra-low dose follicle-stimulating hormone regimen for patients with polycystic ovary syndrome: One click, one follicle, one pregnancy. Fertil Steril 91: 1533-1535.
- Lu PY, Chen ALJ, Atkinson EJ, et al. (1996) Minimal stimulation achieves pregnancy rates comparable to human menopausal gonadotropins in the treatment of infertility**Presented at the 50th Annual Meeting of The American Fertility Society, San Antonio, Texas, November 7, 1994. Fertil Steril 65: 583-587.
- Dickey RP, Olar TT, Taylor SN, et al. (1993) Sequential clomiphene citrate and human menopausal gonadotrophin for ovulation induction: Comparison to clomiphene citrate alone and human menopausal gonadotrophin alone. Human Reproduction 8: 56-59.
- Ransom MX, Doughman NC, Garcia AJ (1996) Menotropins alone are superior to a clomiphene citrate and menotropin combination for superovulation induction among clomiphene citrate failures. Fertil Steril 65: 1169-1174.
- Akhtar M, Njar VCO, Neville Wright J (1993) Mechanistic studies on aromatase and related C-C bond cleaving P-450 enzymes. The Journal of Steroid Biochemistry and Molecular Biology 44: 375-387.
- Weil SJ, Vendola K, Zhou J, et al. (1998) Androgen receptor gene expression in the primate ovary: Cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab 83: 2479-2485.
- Buzdar A, Howell A (2001) Advances in aromatase inhibition: Clinical efficacy and tolerability in the treatment of breast cancer. Clinical cancer research: An Official Journal of the American Association for Cancer Research 7: 2620-2635.
- Alizzi F (2018) Letrozole with or without gonadotropin as a first-line ovulation induction in anovulatory infertile women due to polycystic ovary syndrome. Asian Journal of Pharmaceutical and Clinical Research 11: 129.
- Chen Z, Zhang M, Qiao Y, et al. (2016) Effects of letrozole in combination with low-dose intramuscular injection of human menopausal gonadotropin on ovulation and pregnancy of 156 patients with polycystic ovary syndrome. Pak J Med Sci 32: 1434-1438.
- Yu X, Cao Z, Hou W, et al. (2019) Effects of letrozole combined with human menopausal gonadotrophin in ovarian stimulation for intrauterine insemination cycles. Annals of Translational Medicine 7: 771.
- Healey S, Tan SL, Tulandi T, et al. (2003) Effects of letrozole on superovulation with gonadotropins in women undergoing intrauterine insemination. Fertil Steril 80: 1325-1329.
- Geisler J, Haynes B, Anker G, et al. (2002) Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. Journal of clinical oncology: Official journal of the American Society of Clinical Oncology 20: 751-757.
Corresponding Author
Ahmed Ragab Ali, Fayoum General Hospital, Fayoum, Egypt.
Copyright
© 2023 Ali HAA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.