The Efficacy of Letrozole with or without Gonadotropins on Pregnancy Rates after Intrauterine Insemination in Women with Polycystic Ovarian Syndrome: A Randomized Clinical Trial
Abstract
Background: In this study, we aim to assess the efficacy of letrozole with and without gonadotropins on pregnancy outcomes especially the pregnancy rate, ovulation induction, and endometrial thickness in women suffering from polycystic ovarian syndrome (PCOS).
Methods: We have developed a randomized clinical trial where we included 90 infertile women due to PCOS that met our criteria. Data collection took place at three in vitro fertilization (IVF) centers in Egypt since May 2019 until we reached the target population number.
Results: Patients were randomized into two groups; the letrozole alone (A) and the letrozole with gonadotropin (B) groups. The mean age for both groups was 27.3 (± 3.6) and 25 (± 3), respectively. The total pregnancy rate was 31.1% with no statistical significance (P = 0.788) between group A (17.8%) and B (20%). Additionally, the mean number of follicles was significantly higher (P < 0.001) in the B [2.82 (± 0.89)] than A [1.89 (± 0.71)] groups. We also found no significance regarding the endometrial thickness and estradiol levels between the two groups (P = 0.303 and 0.496, respectively).
Conclusion: Administration of letrozole alone or in combination with gonadotropins enhances the rates of pregnancy in PCOS patients together with the minimal adverse events regarding endometrial thickening and frequency of ovulation.
Keywords
Gonadotropin, Letrozole, Ovulation induction, Polycystic ovarian syndrome
Introduction
Although polycystic ovary syndrome (PCOS) poses a high prevalence rate among pregnant women [1], the etiology is still unclear, and many factors have been involved. Patients with PCOS are at risk of developing many complications. These include all members of metabolic syndrome especially type 2 diabetes mellitus (DM), dyslipidemia, and hypertension [2-5]. Moreover, previous research demonstrated that PCOS can complicate pregnancy with an increased incidence of preterm labor, preeclampsia, and gestational diabetes [6-9]. Moreover, around 75% of PCOS patients develop metabolic abnormalities and ovulation disturbances leading to infertility or sub fertility [10-12]. Therefore, proper diagnosis and treatment are essential in reducing the prevalence rate and adverse events which can be achieved by understanding the pathophysiology of the disease. Hyperinsulinemia and hyperandrogenism (HR) have been found to take part in the development of the syndrome, the mechanism of which, however, is still unknown [13].
Many approaches have been presented for the management of PCOS. These include lifestyle modifications as weight reduction and dietary interventions. Intrauterine insemination (IUI) has been performed to induce pregnancy in many infertility events including ovulatory disorders, unexplained infertility and male-related infertility [14]. However, many pharmacological regimens have been introduced for further ovarian stimulation and pregnancy induction. Various treatment regimens have been reported as clomiphene citrate (CC), letrozole, and gonadotrophins. Other measures include laparoscopic ovarian surgery (LOS), and in-vitro fertilization (IVF). Despite being used as a first-line drug in treating PCOS, CC-resistance, which is a condition when pregnant women fail to ovulate or conceive, develops in 20% of pregnant women [15].
The administration of human Menopausal Gonadotropin (HMG) or follicle-stimulating hormone (FSH) has been used with or without anti estrogens or aromatase inhibitors to inhibit ovarian hyperstimulation and multiple pregnancies [16]. Letrozole, a potent third-generation aromatase inhibitor, has been effectively reported to enhance pregnancy outcomes with low multiple gestation rates [17]. Several investigations have reported potentially accepted pregnancy rates after letrozole application. Some of these showed that letrozole was superior to other drugs in terms of enhancing pregnancy rates [18-23]. The main advantages of letrozole include mono-ovulation and the short plasma half-life of the drug (45 hours) so that it would be rapidly eliminated from the body. Besides, the anti-estrogen effect of the drug will not persist due to the minimal effect on estrogen receptors (ER). Therefore, minimal/no adverse events may occur on the endometrium and cervical mucosa [24]. However, not enough evidence can be concluded for routine letrozole use.
In this study, we aim to compare the efficacy of letrozole alone and letrozole together with gonadotrophins in terms of mild ovarian stimulation for obtaining favorable pregnancy rates in pregnant women undergoing IUI.
Methods
Study design and participants
This multicenter study was conducted at the following IVF centers in Egypt: Beni-Suef University, Beni-Suef, the Omuma IVF center, Fayoum, and El-Nada IVF center, Beni-Suef. Patients were included in this study if they were 1) Infertile with PCOS according to the Rotterdam criteria [25]; 2) < 35-years-old; 3) With normal thyroid-stimulating hormone and prolactin levels; 4) Menstrual third day FSH levels < 12 IU/ml; 5) Normal hysterosalpingography (HSG), and 6) Abnormal semen analysis. Patients were excluded if any of the mentioned criteria were not achieved.
Sample size calculation was done using the comparison of occurrence of ongoing pregnancy through intrauterine insemination between infertile PCO women treated with letrozole and those treated with letrozole plus gonadotropins for induction of ovulation. The calculation was done based on comparing two proportions from independent samples in a prospective study using the Chi test, with a fixed α-error level at 0.05. The power was set at 80% and the intervention groups (case: Control) ratio was set at 1. Sample size calculation was done using PS Power and Sample Size Calculations software, version 3.0.11 for MS Windows (William D. Dupont and Walton D. Vanderbilt, USA).
The allocation of patients was done randomly using a random sequence generation method and patients were equally randomized into the letrozole alone (Group A) and the letrozole plus gonadotropins (Group B) groups. Group A received 2.5 mg letrozole twice daily on day 2-5 of the menstrual cycle while group Breceived gonadotropins starting from day 7 plus letrozole 2.5 mg twice daily on the day 2-5 of the menstrual cycle. Gonadotropin doses were determined according to the age, basal FSH level, BMI, former treatment regimens of the patient, and adjusted according to the follicular response and estradiol (E2) levels of the patient. Then intrauterine insemination has been conducted for all patients.
Data collection
The study has started in May 2019 and continued data collection until reaching the target number of cases. Data have been collected through a pre-designed questionnaire. Each patient was examined on the 2-5 days of menstruation for their weight and length. Body mass indexes (BMI) were recorded. FSH, luteinizing hormone (LH), E2, progesterone, prolactin, and TSH levels were measured by immune-enzymatic method (ELISA) on the 2nd-3rd day of menstruation.
Follow-up and outcome measures
Patients were evaluated by transvaginal ultrasonography on the 9th day of menstruation and lasted daily after the greatest follicle reached 14 mm. For computing, the follicular size mean of two dimensions was calculated. Endometrial thickness was measured in the longitudinal axis of the uterus between echogenic surfaces. Moreover, a 10,000 IUHCG was administered intramuscularly when the greatest follicle reached a 17-18 mm size and 36 h after HCG administration.
The main outcomes of our study include the pregnancy rates in both groups and the significance between both. In addition, the significance in endometrial thickening and the number of follicles will be also estimated. By this, we hope to determine whether letrozole alone is better than the combination of gonadotropin and letrozole administration with the pre-specified doses in terms of the aforementioned outcomes.
Statistical analysis
Data will be analyzed by SPSS 25 (SPSS Inc, Chicago, IL, USA). Descriptive statistics for continuous variables were done by mean ± standard deviation (SD) and nominal variables were done by variable counts and percentages. Moreover, the Chi-square test will be used to examine the nominal variables. For continuous variables, the normal distribution was tested using the Kolmogorov-Smirnov test and according to the results, either the independent samples t-test or the Mann-Whitney-U test were used. A P value < 0.05 will be considered statistically significant.
Results
Patients characteristics
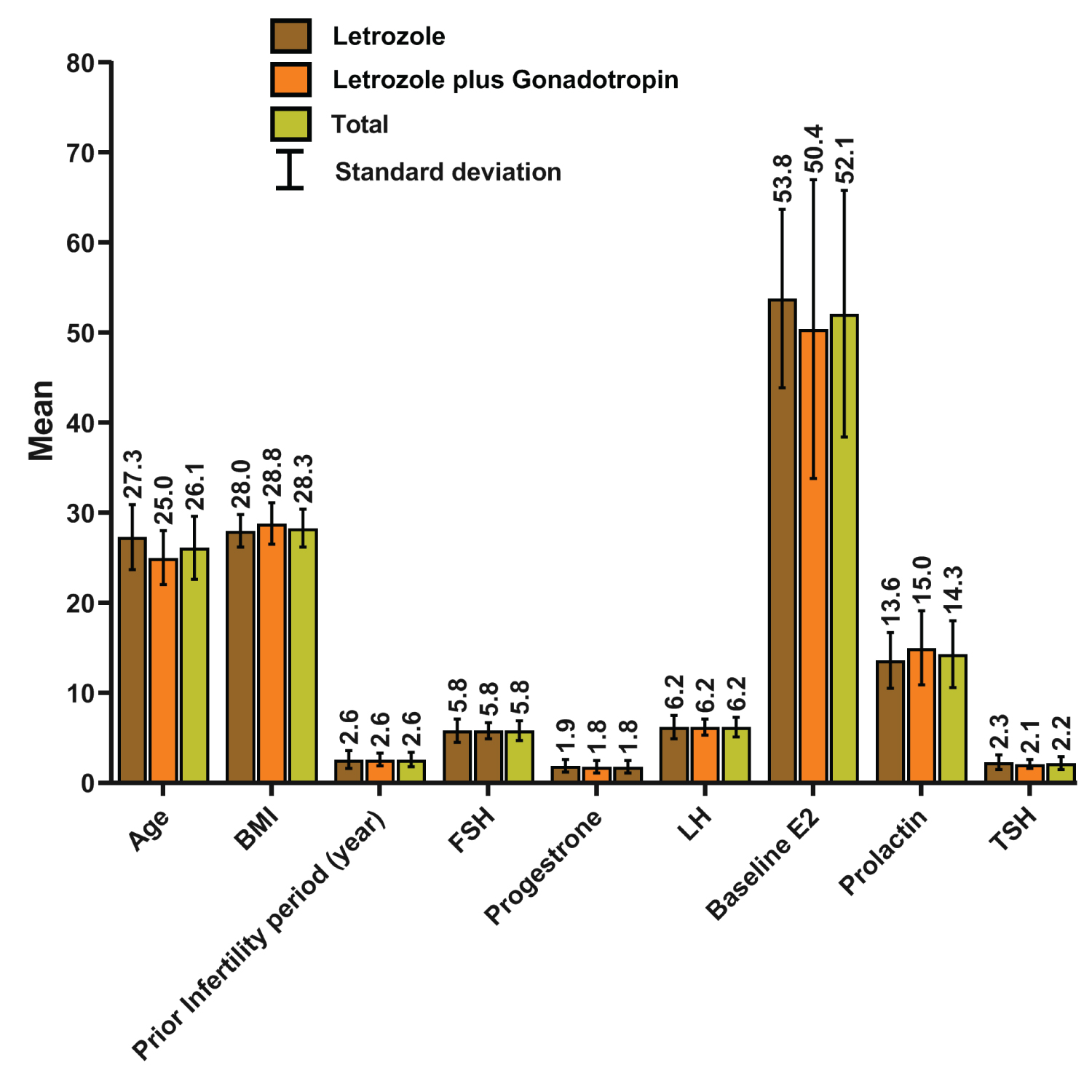
We have included 90 patients with a total mean infertility period of 2.6 (± 0.8) years. Patients were equally randomized into 45 patients in each group. The mean age was 27.3 (± 3.6) and 25 (± 3) years for groups A and B, respectively. The mean BMI was 28 (± 1.8) and 28.8 (± 2.3) kg/m2 for groups A and B, respectively, with no statistical significance (P = 0.091). Other variables including the FSH, LH, TSH, progesterone, prolactin, and baseline E2 have been compared between the two groups with the Mann-Whitney-U test and no statistical significance was found. Detailed values and outcomes of these variables are presented in Table 1 and Figure 1.
Study outcomes
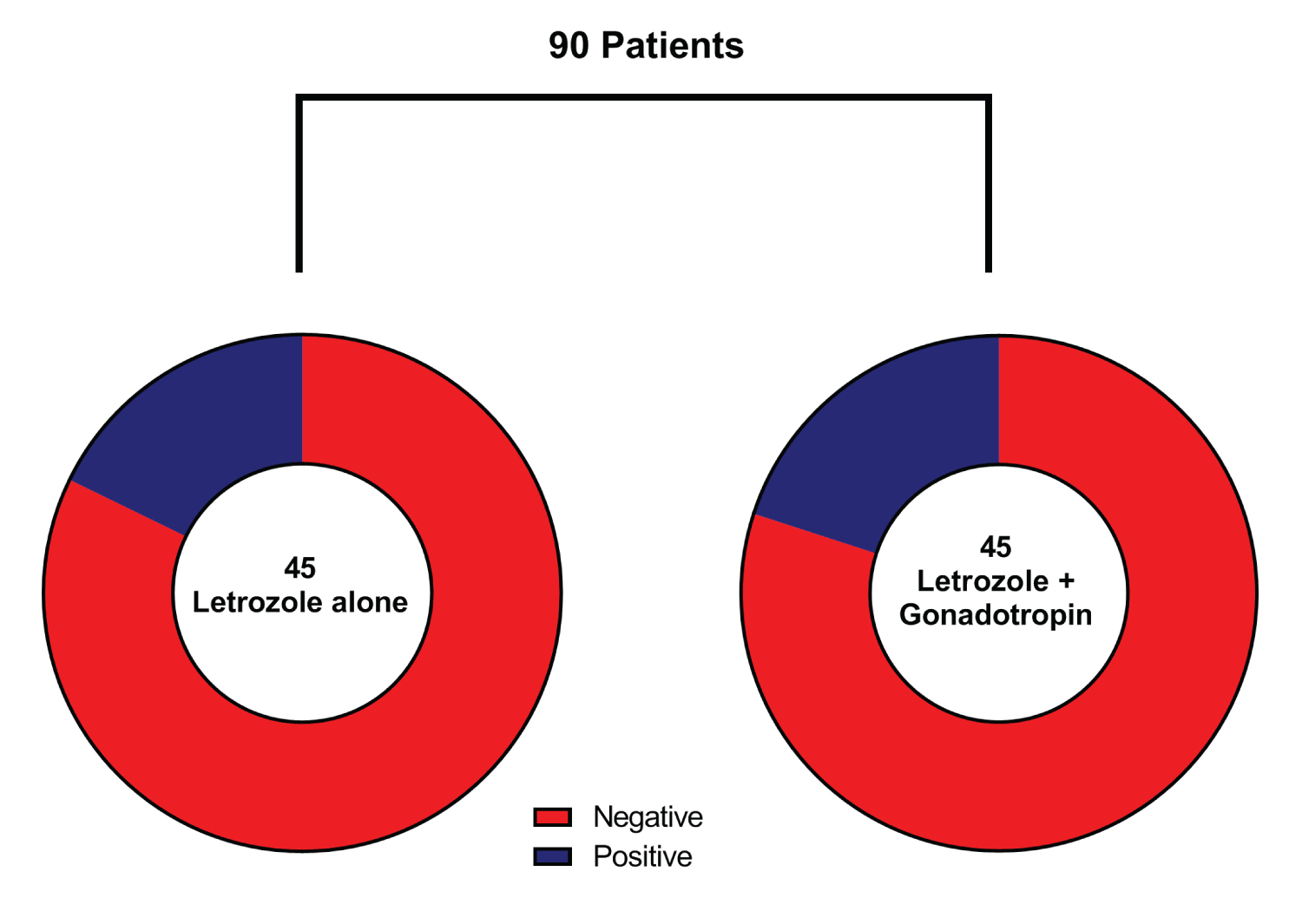
A total of 73 patients (68.9%) have shown negative pregnancy tests, while 17 (31.1%) patients were pregnant (Table 2). On comparing the pregnancy rate in both treatment groups, no statistical significance was estimated (P = 0.788) with 17.8% pregnancies in the letrozole group versus 20% in the combined treatment group (Figure 2). The mean number of follicles was 1.89 (± 0.71), and 2.82 (± 0.89) in the letrozole only and the combined letrozole and gonadotropin groups, respectively. The mean endometrial thickness was 7.6 1 (± 1.35) and 7.31 (± 1.56) mm in group A and B, respectively. Furthermore, the mean E2 levels were 928.89 (± 125.45) and 908.89 (± 124.9) pg/mL in group A and B, respectively. None of these variables showed normal distribution on post-treatment assessment. Accordingly, the Mann-Whitney-U test was used to compare the two groups, and by which, the number of follicles was the only significant variable (P < 0.001) between the two groups (Table 3).
Discussion
Letrozole has been recently introduced as the most effective therapy in treating infertility and increasing the pregnancy rates in PCOS patients [26,27]. Although CC has been widely used as the first-line treatment for this condition, letrozole has been proposed as an alternative of it due to its harmful side effects [28]. Letrozole has now been in practice to induce ovulation in both non ovulatory and ovulatory women with infertilities [29-31]. It can lead to ovulation in 62% of CC-resistant patients and can boost the pregnancy rate by up to 14.7% [31]. Besides, minimal side effects have been reported with the drug, therefore, it can be the first line of therapy in patients who tolerate CC, but with thin endometrial [32]. Moreover, gonadotropins are usually used as the second line of treatment behind CC. However, as a result of the minimal adverse effects and the economic advantages of using letrozole, it has recently been widely used instead of gonadotrophins in CC-resistant patients. Gonadotrophins can lead to serious adverse events as ovarian hyperstimulation and even death, and therefore, they should not be used alone [32]. In this study, we aimed to compare the efficacy of letrozole as an ovulation-inducing agent with a combination of letrozole and gonadotropins in terms of enhancing the pregnancy rate, the effect on endometrial thickening, and the number of released follicles per each group.
The difference in demographics among the two study groups was significant in terms of participants age only (P = 0.002) while other demographics as BMI and previous periods of infertility were not. On the other hand, Alizzi, et al. reported significant findings regarding these variables. Regarding the administered amounts of letrozole and gonadotrophins, no consensus has been reported concerning the optimal daily and cumulative doses. However, as reported by Badawy, et al. [33] and Polyzos, et al. [34] we used a fixed daily dose of 5 mg of letrozole which is said to be more efficient than other doses. On the other hand, the used doses of gonadotrophins in our study were dependent on many factors as the level of endogenous gonadotropins, and the demographics of the patients.
The results of our study indicate the fact that letrozole can be used as a second line-treatment after CC and before surgical intervention without embryo cryopreservation. On the other hand, our results also showed that letrozole can minimize the adverse events of gonadotrophins and obtain better outcomes. In this study, the total pregnancy rate was 31.1%. Moreover, we found no significance between using letrozole alone (17.8%) or in combination with gonadotropins (20%) (P = 0.788). Our results are consistent with the results of Yu, et al. which reported similar pregnancy rates [35]. Unlike our study, Alizzi, et al. [36] reported a total higher pregnancy rate among their population. Additionally, the authors reported that higher pregnancy rates were associated with using letrozole alone, however, this was not statistically significant when comparing it to the combined use of both letrozole and gonadotrophins. Moreover, Chen, et al. [37] reported significantly higher pregnancy rates with the combination of letrozole with gonadotrophins over letrozole alone.
Among other outcomes that were assessed by our study, we found that the mean number of follicles was significantly higher in the combined letrozole and gonadotropins group than the letrozole group. Moreover, the effect of both regimens on endometrial thickness and E2 levels was not statistically significant, however, higher estimations were recorded when using letrozole alone. On the other hand, Chen, et al. [37] found that letrozole alone was associated with significantly lower levels of E2 and increased thickness of endometrium while Yu, et al. [35] reported that letrozole was significantly associated with a thinner endometrium and Alizzi, et al. [36] found no significance.
Limitations to our study include the small sample size of patients that met our inclusion criteria which may have influenced the results of our outcomes.
Conclusion
Our results showed that both regimens of letrozole and letrozole with gonadotropins can enhance the rate of pregnancy after performing IUI. However, favoring one of them over the other required statistical significance which we did not find in this study for many variables. In addition, our study favors the use of the letrozole which showed significant results in terms of increasing the number of follicles unlike combining it with gonadotrophins which have shown a persistent over-stimulatory effect.
Ethical Statement
None.
Conflict of Interest
None.
Funding
None.
References
- March WA, Moore VM, Willson KJ, et al. (2010) The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 25: 544-551.
- Legro RS, Kunselman AR, Dodson WC, et al. (1999) Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84: 165-169.
- Chen M-J, Yang W-S, Yang J-H, et al. (2007) Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension 49: 1442-1447.
- Legro RS, Kunselman AR, Dunaif A (2001) Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med 111: 607-613.
- Glueck CJ, Papanna R, Wang P, et al. (2003) Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism 52: 908-915.
- Boomsma CM, Eijkemans MJ, Hughes EG, et al. (2006) A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 12: 673-683.
- Falbo A, Rocca M, Russo T, et al. (2010) Changes in androgens and insulin sensitivity indexes throughout pregnancy in women with polycystic ovary syndrome (PCOS): relationships with adverse outcomes. J Ovarian Res 3: 23.
- Qin JZ, Pang LH, Li MJ, et al. (2013) Obstetric complications in women with polycystic ovary syndrome: A systematic review and meta-analysis. Reprod Biol Endocrinol 11: 56.
- Roos N, Kieler H, Sahlin L, et al. (2011) Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study. BMJ 343: 6309.
- Diejomaoh M, Jirous J, Al-Azemi M, et al. (2003) The relationship of recurrent spontaneous miscarriage with reproductive failure. Med Princ Pract 12: 107-111.
- Chakraborty P, Goswami SK, Rajani S, et al. (2013) Recurrent pregnancy loss in polycystic ovary syndrome: role of hyperhomocysteinemia and insulin resistance. PLoS One 8: e64446.
- Pasquali R (2006) Obesity and androgens: Facts and perspectives. Fertil Steril 85: 1319-1340.
- Schuring AN, Schulte N, Sonntag B, et al. (2008) Androgens and insulin-two key players in polycystic ovary syndrome. Recent concepts in the pathophysiology and genetics of polycystic ovary syndrome. Gynakol Geburtshilfliche Rundsch 48: 9-15.
- Tomlinson MJ, Amissah-Arthur JB, Thompson KA, et al. (1996) Prognostic indicators for intrauterine insemination (IUI): Statistical model for IUI success. Human reproduction (Oxford, England) 11: 1892-1896.
- Imani B, Eijkemans MJ, te Velde ER, et al. (1998) Predictors of patients remaining anovulatory during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J Clin Endocrinol Metab 83: 2361-2365.
- Nargund G, Fauser BC, Macklon NS, et al. (2007) The ISMAAR proposal on terminology for ovarian stimulation for IVF. Hum Reprod 22: 2801-2804.
- Mitwally MF, Biljan MM, Casper RF (2005) Pregnancy outcome after the use of an aromatase inhibitor for ovarian stimulation. Am J Obstet Gynecol 192: 381-386.
- Banerjee Ray P, Ray A, Chakraborti PS (2012) Comparison of efficacy of letrozole and clomiphene citrate in ovulation induction in Indian women with polycystic ovarian syndrome. Arch Gynecol Obstet 285: 873-877.
- Kar S (2012) Clomiphene citrate or letrozole as first-line ovulation induction drug in infertile PCOS women: A prospective randomized trial. J Hum Reprod Sci 5: 262-265.
- (2012) Letrozole and Clomiphene Citrate Effect on Endometrial and Subendometrial Vascularity in Treating Infertility in Women with Polycystic Ovary Syndrome. Journal of Gynecologic Surgery 28: 405-410.
- Elsedeek MS-E-A, Elmaghraby HAH (2011) Predictors and characteristics of letrozole induced ovulation in comparison with clomiphene induced ovulation in anovulatory PCOS women. Middle East Fertility Society Journal 16: 125-130.
- Roy KK, Baruah J, Singla S, et al. (2012) A prospective randomized trial comparing the efficacy of Letrozole and Clomiphene citrate in induction of ovulation in polycystic ovarian syndrome. J Hum Reprod Sci 5: 20-25.
- Kamath MS, Aleyamma TK, Chandy A, et al. (2010) Aromatase inhibitors in women with clomiphene citrate resistance: A randomized, double-blind, placebo-controlled trial. Fertil Steril 94: 2857-2859.
- Sh Tehrani Nejad E, Abediasl Z, Rashidi BH, et al. (2008) Comparison of the efficacy of the aromatase inhibitor letrozole and clomiphen citrate gonadotropins in controlled ovarian hyperstimulation: A prospective, simply randomized, clinical trial. J Assist Reprod Genet 25: 187-190.
- (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19: 41-47.
- Badawy A, Shokeir T, Allam AF, et al. (2009) Pregnancy outcome after ovulation induction with aromatase inhibitors or clomiphene citrate in unexplained infertility. Acta Obstet Gynecol Scand 88: 187-191.
- Elnashar A, Fouad H, Eldosoky M, et al. (2006) Letrozole induction of ovulation in women with clomiphene citrate-resistant polycystic ovary syndrome may not depend on the period of infertility, the body mass index, or the luteinizing hormone/follicle-stimulating hormone ratio. Fertil Steril 85: 511-513.
- Healey S, Tan SL, Tulandi T, et al. (2003) Effects of letrozole on superovulation with gonadotropins in women undergoing intrauterine insemination. Fertil Steril 80: 1325-1329.
- Homburg R (2008) Polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol 22: 261-274.
- Mitwally MF, Casper RF (2001) Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril 75: 305-309.
- Abu Hashim H, Shokeir T, Badawy A (2010) Letrozole versus combined metformin and clomiphene citrate for ovulation induction in clomiphene-resistant women with polycystic ovary syndrome: A randomized controlled trial. Fertil Steril 94: 1405-1409.
- Fritz MA, Speroff L (2012) Clinical gynecologic endocrinology and infertility. Wolters Kluwer Health.
- Badawy A, Abdel Aal I, Abulatta M (2009) Clomiphene citrate or letrozole for ovulation induction in women with polycystic ovarian syndrome: A prospective randomized trial. Fertil Steril 92: 849-852.
- Polyzos NP, Tzioras S, Badawy AM, et al. (2009) Aromatase inhibitors for female infertility: A systematic review of the literature. Reprod Biomed Online 19: 456-471.
- Yu X, Cao Z, Hou W, et al. (2019) Effects of letrozole combined with human menopausal gonadotrophin in ovarian stimulation for intrauterine insemination cycles. Ann Transl Med 7: 771.
- Alizzi F (2018) Letrozole with or without gonadotropin as a first-line ovulation induction in anovulatory infertile women due to polycystic ovary syndrome. Asian Journal of Pharmaceutical and Clinical Research 11: 129.
- Chen Z, Zhang M, Qiao Y, et al. (2016) Effects of letrozole in combination with low-dose intramuscular injection of human menopausal gonadotropin on ovulation and pregnancy of 156 patients with polycystic ovary syndrome. Pak J Med Sci 32: 1434-1438.
Corresponding Author
Ahmed Mahmoud Sadek, Fayoum General Hospital, Fayoum, Egypt.
Copyright
© 2022 Ali HAA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.