Series of 9386 IUI Cycles: The Impact of the Number of Motile Spermatozoa Inseminated Varies According to the Female Age
Abstract
Introduction: Many authors have studied the effects of the NMSI on the success of IUI, but a real consensus has not yet been achieved, and NMSI and female age have rarely been studied together. In addition, the upper limit of NMSI has rarely been studied while some fertility centers perform a dilution of the sperm sample to obtain a NMSI between 1 × 106 and 10 × 106. What is the impact of the number of motile spermatozoa inseminated (NMSI) on clinical pregnancy rates (CPR) in IUI with partner sperm according to female age?
Methods: This is a retrospective cohort study, in which 3535 couples completed 9386 IUI cycles with partner sperm. The NMSI was calculated by multiplying the final sperm concentration by the percentage of progressive motile sperm after washing and preparation volume. The main outcome measures were pregnancy rate per cycle (PR), defined as a positive pregnancy test 14 days after the IUI and CPR per cycle, defined as a fetal heartbeat on ultrasound at 7 weeks.
Results: The 9386 cycles were categorized into four groups based on the NMSI as follows: < 1 × 106; 1 to 4 × 106; 5 to 9 × 106; and ≥ 10 × 106. Regarding the NMSI independent of patient age: PR and CPR were significantly lower if the NMSI was < 1 × 106. A NMSI ≥ 10 × 106 was associated with a higher PR than a NMSI between 1 × 106 and 4 × 106 (respectively 13.8% vs. 10.8%, p < 0.001). For patients between 30 and 34, with NMSI ≥ 10 × 106, PR was 14.85%, which is significantly higher than that the PR with NMSI between 1 and 4 × 106 (9.97%, p = 0.01). For women between 35 and 39, PR with NMSI ≥ 10 × 106 (13.64%) was significantly higher than the one with NMSI between 5 and 9 × 106 (8.80%, p = 0.03).
Conclusion: This study demonstrates that a high NMSI does not decrease pregnancy rates. On the contrary, the higher the female age is, the higher the NMSI needs to be.
Keywords
Intrauterine insemination, Number of motile spermatozoa inseminated, Female age, Clinical pregnancy rate
Introduction
Intrauterine insemination (IUI) is a commonly used assisted reproductive technique because it is inexpensive, minimally invasive and well accepted by couples [1]. IUI treatment is mainly offered to infertile couples with moderate male factor, ovulatory dysfunction and unexplained infertility. IUI is often the step that precedes in vitro fertilization (IVF), a more expensive and invasive treatment option, but also more effective. Indeed, the main disadvantage of IUI remains its low success rate. The latest data from the European Society of Human Reproduction and Embryology (ESHRE) indicated delivery rates at 8.9% per cycle after IUI using husband/partner's semen [2].
Access to infertility treatment is not homogenous in developing countries. The coverage of infertility treatment by government medical insurances varies among countries. Some governments support the infertility services but the majority of them apply some restrictions. For this reason, the financial aspect plays an important role in the choice of treatment. Many patients will prefer to attempt treatment using IUI even if their results suggest better success rates in IVF. That is why it is extremely important to offer good quality counseling to these patients. However, there is a lack of literature on prognostic factors for success in IUI. In addition, it is necessary to personalize the advice given to the patient taking into account the different variables that can influence the results in IUI.
Since time-to-pregnancy is an important element to be taken into account in fertility treatment, it seems important to optimize IUI indications as much as possible in order to select the population with the highest chances of success. For this, a few prognostic factors have been identified: maternal age and number of motile spermatozoa inseminated (NMSI) are among the most important [3,4].
Many authors have studied the effects of the NMSI on the success of IUI, but a real consensus has not yet been achieved. Current literature is too limited to determine sperm parameter thresholds for which IUI should not be recommended [1]. A minimum of 1 million motile spermatozoa inseminated is often quoted as required to optimize IUI but several authors question this threshold. Some studies have shown better results if the NMSI exceeds 2 × 106, or even 5 × 106 [5,6]. However, NMSI and female age have rarely been studied together. In addition, the upper limit of NMSI has rarely been studied. In Europe, some fertility centers perform a dilution of the sperm sample to obtain a NMSI less than 10 × 106 [7]. However, no study has shown that an elevated NMSI could decrease the chances of pregnancy.
This study aimed to evaluate the impact of the NMSI on the clinical pregnancy rates (CPR) in IUI with partner sperm according to female age.
Materials and Methods
Population and study design
This was a retrospective cohort study, in which 3535 patients completed 9386 IUI cycles with partner sperm, between January 2011 and December 2015, at a University-affiliated private Assisted Reproductive Technology clinic in Montreal, Canada. The study period was a period in which IUI and IVF were funded by the government in Quebec. All patients who started their first IUI during the study period, for primary or secondary infertility, were included. Cycles in which the woman was over 43-years of age were excluded. Donor sperm was excluded.
All patients tried to conceive without success for at least 1-year. In order to assess the cause of infertility, patients underwent a full fertility workup including; for men: a semen analysis, and for women: Ovarian reserve testing including antral follicular count and hormone measurements (estradiol, FSH and AMH) between the second and fifth day of the menstrual cycle. Evaluation of the uterine cavity and tubal patency was done by hysterosalpingography or hysterosonography. Indications for IUI included moderate male factor, ovulatory dysfunction and unexplained infertility. The moderate male factor was defined by a moderate oligospermia (sperm concentration between 5 and 14 millions/ml), on a semen analysis performed as described in Björndahl, et al. [8].
Ovulation induction
The majority of IUI was performed after mild ovarian stimulation. Ovarian stimulation was performed according to three different protocols, decided at the discretion of the physician depending on the ovarian reserve: 1) either Clomiphene Citrate (Serophene®, EMD Serono Canada or Clomid®, Sanofi-Aventis Canada, 50 to 200 mg/day), Letrozole (2.5 to 5 mg/day) or Tamoxifen Citrate (20 to 60 mg/day) for 5 days from the third day of the menstrual cycle; 2) recombinant FSH (Gonal F®, EMD Serono Canada, or Puregon®, Merck Canada) or purified urinary FSH (Bravelle®, Ferring Canada) or hMG Human menopausal gonadotropins (Menopur®, Ferring Canada, or Repronex®, Ferring Canada) at a dose of 37.5 to 300 UI per day or every 2 days from the second or third day of the menstrual cycle; 3) a combination of either Clomiphene Citrate or Letrozole with gonadotropins.
Ovulation was monitored by vaginal ultrasound to evaluate the number and size of ovarian follicles and the endometrial thickness. When at least one mature follicle reached 18 mm, a subcutaneous injection of recombinant hCG (Ovidrel®, EMD Serono Canada, 250 µg) was administrated for the ovulation triggering.
Semen assessment and preparation
A full semen analysis was carried out during patient evaluation according to Björndahl, et al. [8]; however, a simplified assessment was carried out on the day of sperm preparation for insemination as described in the following paragraph.
Sperm samples were collected at the laboratory after 2-3 days of sexual abstinence. They were analyzed after liquefaction at 37 ℃ for 30 minutes. The initial sperm variables were determined from 10 µL of semen, on a warmed Makler chamber, with compound microscope at × 100, to estimate the concentration, the mobility and the progression of spermatozoa. Sperm preparation for IUI was performed using density gradient centrifugation, composed of 1mL of 80% colloidal gradient and 1 mL of 40% colloidal gradient (Gynotec Sperm filter, Fertitech Canada). A maximum of 4 mL of the sperm sample was gently deposited on the 40% layer. After a first centrifugation at 400 G for 20 minutes, the concentrated phase containing the most mobile spermatozoa was collected with a sterile Pasteur pipette and resuspended in 5 mL of a wash medium (Gynotec Sperm wash, Fertitech Canada). A second centrifugation was performed at 100G for 10 minutes and the resulting pellet was diluted in 0.5 mL of Sperm Wash (Gynotec Sperm wash, Fertitech Canada).
After sperm washing, a further assessment was performed on the sperm as described earlier and the NMSI was calculated by multiplying the final concentration by the percentage of progressive motile sperm after washing and the volume of the preparation (0.5 mL). In this study, sperm morphology was not evaluated at the time of IUI.
Insemination and pregnancy rate
Insemination was performed with an insemination catheter (Minispace®, CCD) that was inserted into the uterine cavity and the sperm preparation was injected slowly. According to our clinical protocols, the luteal phase was supplemented with micronized progesterone (Prometrium®, Merck Canada, 200 mg/day for 15 days after the IUI) in all cases involving gonadotropins, until 8 weeks of pregnancy. The patients performed a urine pregnancy test 2 weeks after the IUI, then an ultrasound was done at 7 weeks to confirm a clinical pregnancy.
Statistical methods
The main outcome measures were pregnancy rate per cycle (PR), defined as a positive pregnancy test 14 days after the IUI and clinical pregnancy rate per cycle (CPR), defined as a fetal heartbeat on ultrasound at 7 weeks.
Quantitative variables were descriptive in the form of mean ± standard deviation (SD). Qualitative variables were expressed as absolute numbers and proportions. Factors that may influence CPR were compared according to chi2 test, or Fisher's exact test when indicated. A p-value < 0.05 was considered statistically significant.
Results
Descriptive characteristics of the 9386 IUI cycles
The baseline characteristics of the cycles are described in Table 1. The mean age of women was 33.6 years (± 4.4) and that of men was 36.2-years (± 5.7). Only 10.9% of women were between 40 and 43-years. The majority of cycles analyzed fell within the first three IUI attempts (84.4%). The majority of patients had received oral ovarian stimulation alone (79.7%) or coupled with gonadotropins (16.23%). The average NMSI was 38.9 × 106 (± 34.5) and 75.1% of sperm samples had a NMSI ≥ 10 × 106.
Of the 9386 IUI cycles, 1200 resulted in a pregnancy (PR = 12.8%) and 921 in a clinical pregnancy (CPR = 9.8%).
PR and CPR according to female age and NMSI independently
Table 2 compares pregnancy rate (PR) and clinical pregnancy rate (CPR) by woman's age and NMSI, independently.
PR was significantly lower if the female patient was 40-years or older (9.6%, p=0.016), the results being similar between age groups < 30, 30-34 and 35-39-years (respectively 13.5%, 13.7% and 12.5%). However, CPR was significantly higher if the female patient was less than 35 (p < 0.001).
Regarding the NMSI: PR and CPR were lower if the NMSI was under 1 × 106 (respectively 4.2% and 3.1%, p < 0.001). A NMSI ≥ 10 × 106 was associated with a higher PR than a NMSI between 1 × 106 and 4 × 106 (respectively 13.8% vs. 10.8%, p < 0.001). There was no statistical difference in terms of CPR between groups 1- 4 × 106, 5-9 × 106 and ≥ 10 × 106.
PR and CPR according to both the female age and the NMSI
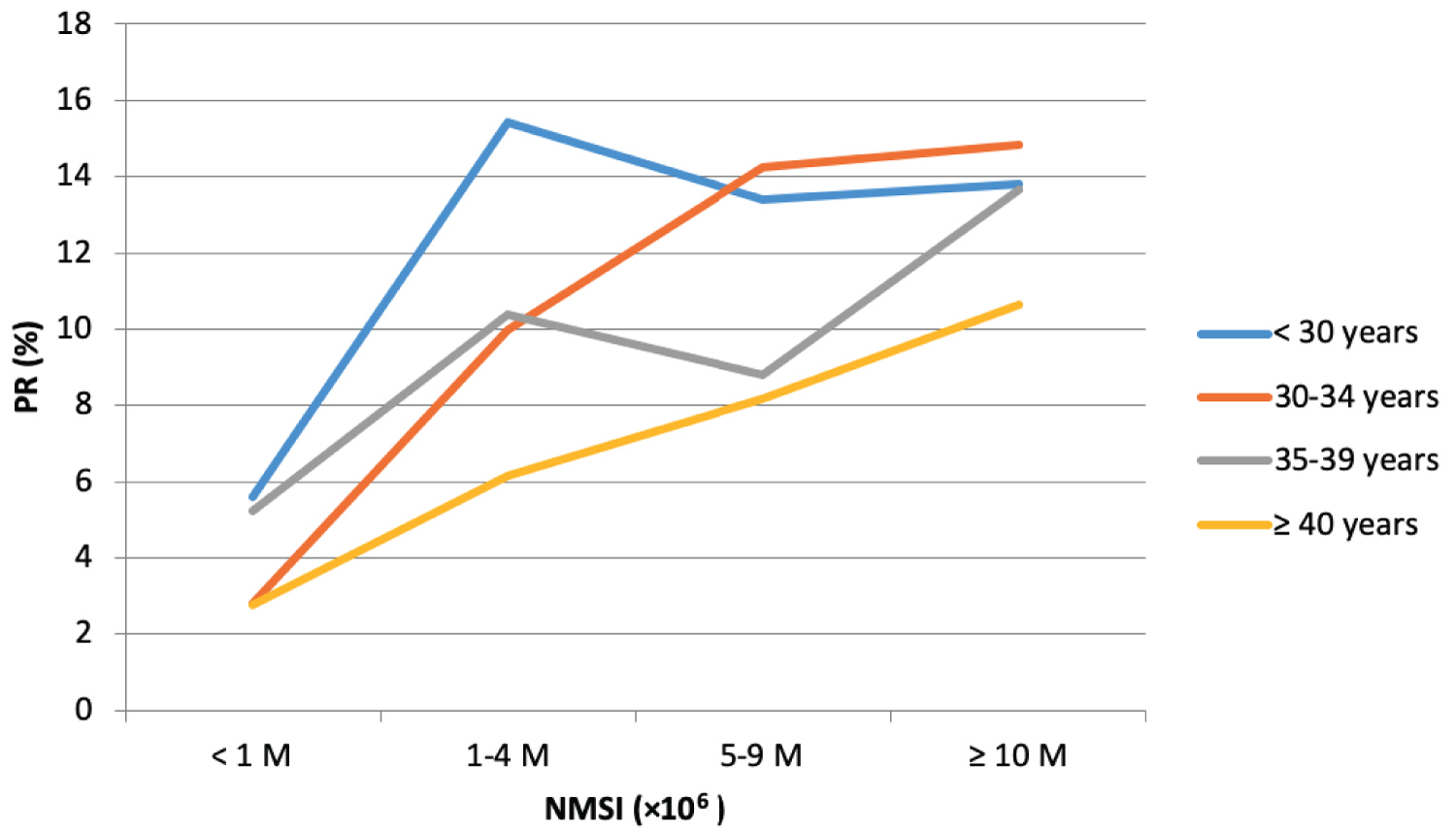
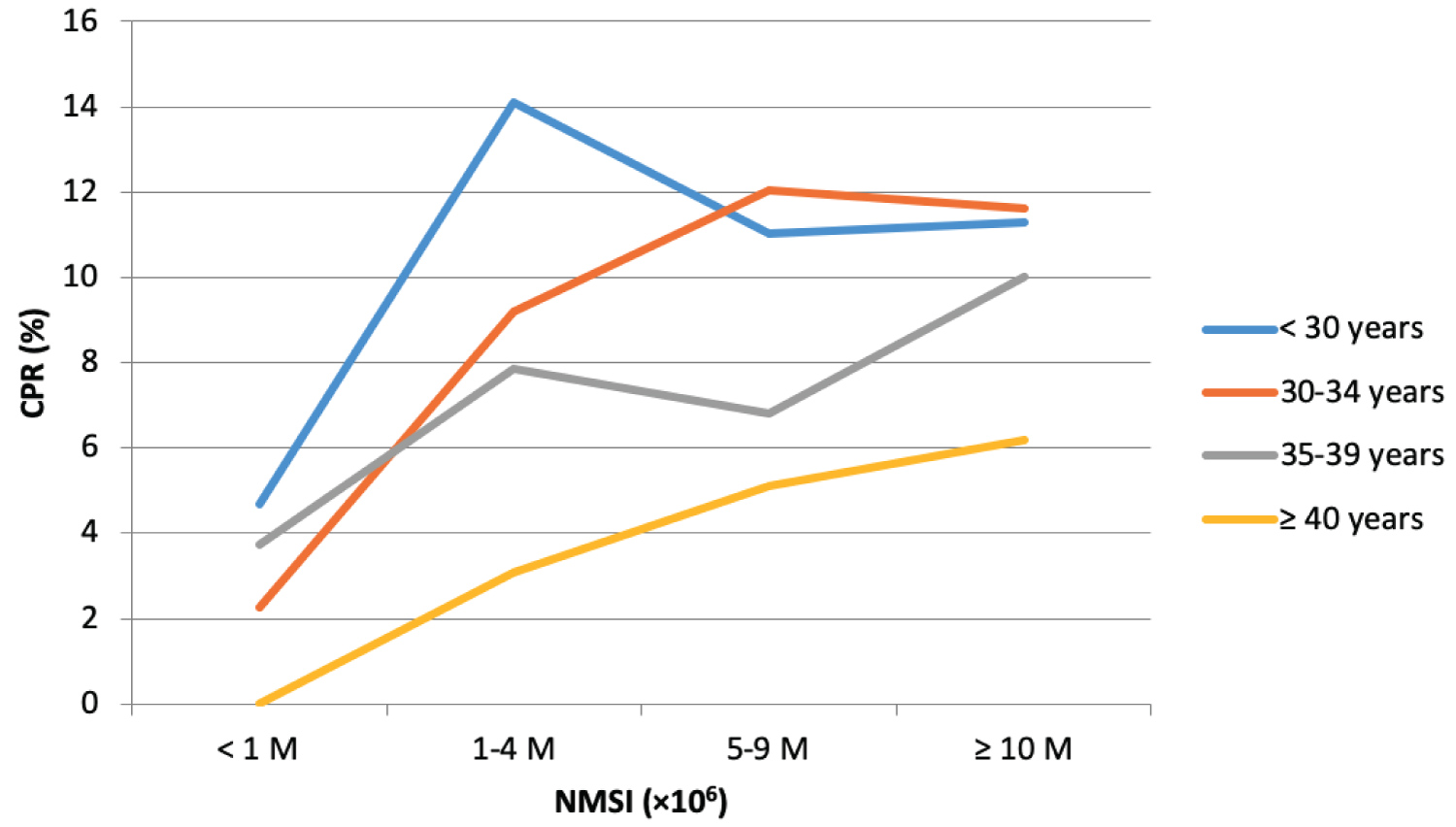
The PR and CPR according to both the female age and the NMSI are described in Table 3, Table 4, Figure 1 and Figure 2.
For patients < 30-years old, PR and CPR tended to be lower if NMSI < 1 × 106 compared to other NMSI groups, however the difference was not statistically significant (p = 0.063 for PR and p = 0.072 for CPR).
For patients between 30 and 34, a NMSI < 1 × 106 was associated with a significantly lower PR and CPR compared to other NMSI groups. Moreover, in this age category, with NMSI ≥ 10 × 106, the PR per cycle was 14.85%, which is significantly higher than that the PR with NMSI between 1 and 4 × 106 (9.97%, p = 0.01). In this same age category, the PR was greatest with NMSI between 5 and 9 × 106 (14.23%) than the one with NMSI between 1 and 4 × 106 (9.97%) although this difference was not statistically significant (p = 0.11).
For women between 35 and 39, PR with NMSI ≥ 10 × 106 (13.64%) was significantly higher than the one with NMSI between 5 and 9 × 106 (8.80%, p = 0.03). Regarding CPR in this age group, it tended to be the highest if NMSI ≥ 10 × 106 compared to other NMSI groups, but the result was statistically different only compared to NMSI < 1 × 106. In this same age category, there was no statistical difference in terms of PR and CPR between NMSI groups < 1 × 106, 1-4 × 106 and 5-9 × 106.
For women aged 40 or over, the PR and CPR were highest in the NMSI group ≥ 10 × 106 (10.64% and 6.18%), followed by the NMSI group 5-9 × 106 (8.16% and 5.10%), then by the NMSI group 1-4 × 106 (6.15% and 3.08%), and the NMSI group < 1 × 106 (2.78% and 0%), although these differences were not statistically significant (p = 0.22 and p = 0.28).
Discussion
This study suggests that a NMSI ≥ 1 × 106 seems to be needed to obtain acceptable pregnancy rates in IUI, without taking into account sperm morphology. In addition, this study demonstrates that a high NMSI does not decrease pregnancy rates. Indeed, we did not observe a decrease in pregnancy rates when the NMSI was greater than 10. Thus, our data suggest that it is not necessary to dilute the sperm sample before IUI. On the contrary, our study tends to show that the higher the female age is, the higher the NMSI needs to be. Our results suggest that in women under 30 years of age, a NMSI ≥ 1 × 106 seems to be sufficient to optimize the chances of pregnancy; however, pregnancy rates could be improved when the NMSI is ≥ 5 × 106 for women aged 30 to 34 and ≥ 10 × 106 for women aged 35 to 39-years. IVF should be considered when the NMSI is under these thresholds.
These results will help us counsel couples in their treatment choice. Indeed, IUI appears to be a relevant first-line treatment for moderate male factor infertility. However, maternal age has to be taken into account. As the woman's age increases, the NMSI must be higher in order to obtain acceptable chances of pregnancy. If both criteria are not met, IVF should be considered as a first option. A more personalized treatment of the couple appears to be judicious regarding the question of IUI indications.
NMSI is relevant in predicting pregnancy rate after IUI. This is in agreement with the literature [9-12]. Many fertility centers consider that IUI can be attempted if the NMSI is greater than 1 million. This threshold is often quoted as required to optimize IUI [13-15]. Dinelli, et al. [3] reported a better PR if the number of progressive motile spermatozoa inseminated was ≥ 1 × 106. However, this study did not attempt to compare other NMSI thresholds.
Several authors questioned this threshold. Some studies have shown that the pregnancy rates were higher if the NMSI exceeds 2 × 106, 5 × 106 or 10 × 106. Cao, et al. [5] proposed a NMSI threshold of 2 × 106. Badawy, et al. [16] evaluated 714 IUI cycles for male factor infertility and they found significantly decreased PR if the NMSI was less than 5 × 106 with a significantly higher PR for NMSI > 5 × 106: 5.55% versus 24.28% (p < 0.001). Miller, et al. [17] found that a NMSI more than 10 × 106 was associated with successful pregnancy.
Nevertheless, few studies have combined NMSI and maternal age in the same analysis. The study of Badawy, et al. [16] evaluated these two parameters concomitantly. They hypothesized that in women younger than 25, a NMSI greater than 5×106 had a better chance of success than a NMSI of less than 5 × 106. There was no statistical difference in women over 25, regardless of the NMSI. However, this study included only men with sperm abnormalities, making this study unrepresentative of all couples performing IUI.
According to Sicchieri, et al. [4], female age is the only variable significantly correlated with IUI success rates. They found no association between sperm parameters and clinical pregnancy rates. In their analysis, they observed the pre- and post-processing concentration and progressive motility, but the NMSI was not calculated due to a lack of data. In addition, their numbers were relatively small with only 237 IUI cycles.
In the study by Lemmens, et al. [18], the group with a NMSI < 1 × 106 had a statistically significant lower probability (OR = 0.42; 95% CI = 0.23-0.76) and the group with a NMSI between 5 × 106 and 10 × 106 a higher probability of becoming pregnant (OR = 1.73; 95% CI = 1.21-2.46) compared with the group with a NMSI > 10 × 106. Furthermore, the probability of becoming pregnant was higher if IUI was done in patients with normal sperm morphology < 4% compared with IUI in patients with normal sperm morphology > 4% (OR = 1.39; 95% CI = 1.06-1.81). It suggests that IUI is an especially relevant treatment for moderate male factor infertility.
However, when other infertility factors add up, such as advanced maternal age (assuming a lower ovarian reserve), then the efficacy of IUI must be questioned and IVF would be a better option. IUI would therefore be a good option in the presence of a moderate male factor in a young woman. But in a woman over 35, sperm parameters need to be higher to optimize this treatment option.
Clinical pregnancy rates per IUI cycle remain low in the literature. It is important to change clinical practice in order to maintain a constant improvement in our treatments. In the literature, Bahadur, et al. [19] suggest several guidelines, including the need to have a sperm sample with a NMSI > 3 × 106 and the use of gonadotrophin stimulation to obtain 2 or 3 follicles. Lemmens, et al. [20] indicate that there is a lack of evidence on most technical aspects of the IUI procedure and some new guidelines are presented, in particular to improve standardization.
It is established that the chances of success in IUI decrease with the female age. There may also be a negative impact of paternal age in IUI [21]. However, literature is poor about the effects of paternal age. In our study, PR was evaluated according to paternal age and there was no statistically significant difference between paternal age groups < 30, 30-34, 35-39 and ≥ 40-years (respectively 13.0%, 13.2%, 13.3% and 11.6%, p = 0.21). Studies have shown a positive correlation between sperm DNA fragmentation and paternal age, which may explain the adverse effects of paternal age on reproductive outcomes [22,23].
Our study population was heterogeneous, with multiple indications for IUI treatment. However, our study was designed to extrapolate the results to all IUI indications, including unexplained infertility, ovulatory dysfunction, and moderate male factor. The main strength of our study comes from the very high number of cycles, which adds strong value to our results and a high statistical power. Furthermore, the NMSI is a standardized data.
Indeed, the NMSI is a simple calculation, based on the sperm concentration and the progressive mobility. It is reliable and reproducible. IUI were performed between 2011 and 2015 because it was a period of time in which IVF and IUI were covered under the provincial government health care system. Therefore, patients with a clear indication for IVF went directly to IVF without attempting IUI. This means that the patients included in the study had a real indication for IUI and the financial aspect of IUI versus IVF did not play a role at that time.
One of the limitations of the study is the inclusion of several IUI cycles for the same patient. The different IUI cycles of a patient are not completely independent of each other and statistical tests are based on the assumption of independent observations. Nevertheless, since the NMSI varies with each IUI cycle within the same patient, it can be assumed that each cycle remains independent.
In conclusion, a NMSI ≥ 1 × 106 is essential to optimize the chances of pregnancy in IUI and a NMSI ≥ 10 × 106 is not detrimental, independent of female age. There may be a tendency for better chances of success if the NMSI is higher among older women. Thus, the NMSI in combination with the female age improves the ability to counsel between IUI and IVF. Artificial intelligence could be an excellent tool to specify prognostic factors in IUI and to establish NMSI limits according to the age of the patient [24,25].
Acknowledgement
The authors acknowledge all the staff of the Departments of Fertility and Research in OVO clinic for their support and cooperation.
Ethical Approval
This retrospective study was conducted as a clinical quality control study. Approval was obtained in March 2018 from the Scientific Review Committee (OVO R&D scientific committee). Patients signed a consent form for the use of their data.
References
- Cohlen B, Bijkerk A, Van der Poel S, et al. (2018) IUI: Review and systematic assessment of the evidence that supports global recommendations. Hum Reprod Update 24: 300-319.
- Wyns C, Bergh C, Calhaz-Jorge C, et al. (2020) European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE), ART in Europe, 2016: Results generated from European registries by ESHRE. Hum Reprod Open.
- Dinelli L, Courbière B, Achard V, et al. (2014) Prognosis factors of pregnancy after intrauterine insemination with the husband's sperm: conclusions of an analysis of 2,019 cycles. Fertil Steril 101: 994-1000.
- Sicchieri F, Silva AB, Silva ACJSRE, et al. (2018) Prognostic factors in intrauterine insemination cycles. JBRA Assist Reprod 22: 2-7.
- Cao S, Zhao C, Zhang J, et al. (2014) A minimum number of motile spermatozoa are required for successful fertilisation through artificial intrauterine insemination with husband's spermatozoa. Andrologia 46: 529-534.
- Wainer R, Albert M, Dorion A, et al. (2004) Influence of the number of motile spermatozoa inseminated and of their morphology on the success of intrauterine insemination. Hum Reprod 19: 2060-2065.
- Fauque P, Lehert P, Lamotte M, et al. (2014) Clinical success of intrauterine insemination cycles is affected by the sperm preparation time. Fertil Steril 101: 1618-1623.
- Björndahl L, Barratt CL, Mortimer D, et al. (2016) How to count sperm properly: Checklist for acceptability of studies based on human semen analysis. Hum Reprod 31: 227-232.
- Nikbakht R, Saharkhiz N (2011) The influence of sperm morphology, total motile sperm count of semen and the number of motile sperm inseminated in sperm samples on the success of intrauterine insemination. Int J Fertil Steril 5: 168-173.
- Ombelet W, Dhont N, Thijssen A, et al. (2014) Semen quality and prediction of IUI success in male subfertility: A systematic review. Reproductive BioMedicine Online 28: 300-309.
- Tomlinson M, Lewis S, Morroll D, et al. (2013) Sperm quality and its relationship to natural and assisted conception: British Fertility Society guidelines for practice. Hum Fertil (Camb) 16: 175-193.
- Tan O, Ha T, Carr BR, et al. (2014) Predictive value of postwashed total progressively motile sperm count using CASA estimates in 6871 non-donor intrauterine insemination cycles. J Assist Reprod Genet 31: 1147-1153.
- Berg U, Brucker C, Berg FD (1997) Effect of motile sperm count after swim-up on outcome of intrauterine insemination. Fertil Steril 67: 747-750.
- Ombelet W, Vandeput H, Van de Putte G, et al. (1997) Intrauterine insemination after ovarian stimulation with clomiphene citrate: Predictive potential of inseminating motile count and sperm morphology. Hum Reprod 12: 1458-1463.
- Wainer R, Merlet F, Bailly M, et al. (1996) Prognostic sperm factors in intra-uterine insemination with partner's sperm. Contracept Fertil Sex 24: 897-903.
- Badawy A, Elnashar A, Eltotongy M (2009) Effect of sperm morphology and number on success of intrauterine insemination. Fertil Steril 91: 777-781.
- Miller DC, Hollenbeck BK, Smith GD, et al. (2002) Processed total motile sperm count correlates with pregnancy outcome after intrauterine insemination. Urology 60: 497-501.
- Lemmens L, Kos S, Beijer C, et al. (2016) Predictive value of sperm morphology and progressively motile sperm count for pregnancy outcomes in intrauterine insemination. Fertil Steril 105: 1462-1468.
- Bahadur G, Homburg R (2019) Prognostic factors in IUI. JBRA Assist Reprod 23: 79-80.
- Lemmens L, Kos S, Beijer C, et al. (2017) Techniques used for IUI: Is it time for a change? Hum Reprod 32: 1835-1845.
- Carballo E, Roque A, Durán-Monterrosas LA, et al. (2013) The value of paternal age on the outcome of intrauterine insemination. Ginecol Obstet Mex 81: 329-333.
- Albani E, Castellano S, Gurrieri B, et al. (2019) Male age: Negative impact on sperm DNA fragmentation. Aging 11: 2749-2761.
- Kaarouch I, Bouamoud N, Madkour A, et al. (2018) Paternal age: Negative impact on sperm genome decays and IVF outcomes after 40 years. Mol Reprod Dev 85: 271-280.
- Curchoe CL, Bormann CL (2019) Artificial intelligence and machine learning for human reproduction and embryology presented at ASRM and ESHRE 2018. J Assist Reprod Genet 36: 591-600.
- Miller DD, Brown EW (2018) Artificial intelligence in medical practice: The question to the answer? Am J Med 131: 129-133.
Corresponding Author
Camille Grysole, Faculty of Medicine, Department of Obstetrics and Gynecology, University of Montreal, Pavillon Roger-Gaudry C.P. 6128, H3C 3J7, Montreal, QC, Canada.
Copyright
© 2021 Grysole C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.