Prostacyclin Activates Survivin Pathway to Enhance Embryo Development
Abstract
Researchers previously demonstrated that inclusion of iloprost, a pharmaceutical grade prostacyclin (PGI2), in culture media improves embryo development and pregnancy rates. Cultured embryos undergo apoptosis and studies have shown protection against apoptosis, rather than cell growth promotion, was the likely cause of iloprost's enhancing effect on embryos. Researchers have also established that embryos produce survivin, an antiapoptotic protein, which appears to be vital to normal development. The objectives of the present study were to determine: 1) The IC-50 of embryos developed in the presence of YM-155, and 2) If iloprost stimulates the survivin pathway as a means of enhancing embryo development. Researchers utilized an animal-based in vitro study of embryo development, with a total of 198 embryos in the IC-50 study and 460 embryos cultured in four separate treatment groups for the iloprost study. Complete embryo hatching was used as an endpoint to determine the extent to which iloprost mitigated the effects of YM-155. Two-cell mouse embryos were harvested and exposed to YM-155 during 32-48 hours of culture; with and without pre-treatment of iloprost, with the endpoint assessed at 120 hours. A significant difference was found when comparing the embryo development of the iloprost treatment group and control (p < 0.05), the YM-155 and control group (p < 0.05), but no difference between control and combination group. These results suggest survivin is one of the pathways activated by iloprost to enhance embryo development. Further, this research may improve future IVF success through addition of iloprost in culture media to prevent embryo apoptosis.
Keywords
Embryo development, Prostacyclin, Iloprost, ym-155, Blastocyst, Apoptosis
Introduction
Embryo culture media has undergone numerous changes since the first successful IVF experiment. Transforming basic culture media to include nutrients and amino acids, embryologist have been working to optimize culture conditions and find the ideal media for over 50 years [1,2]. Previous studies have suggested the inclusion of prostacyclin (PGI2) in culture media during early developmental stages improves embryo quality and pregnancy outcomes [3-6]. PGI2 is present in large quantities in the oviducts during fertilization and early stage embryo development and could potentially be beneficial for fertilization and early embryo growth [3,6,7]. It is suggested that PGI2 stimulates the survivin pathway, which regulates apoptosis in early embryonic stages, and can prevent embryonic apoptosis [4,6-9]. Developing embryos often undergo sudden apoptosis during in vitro culture, which may be reduced by inclusion of PGI2 in the growth media. Improved IVF success would occur through use of a PGI2 additive, such as iloprost, a biosynthetic analogue, and could increase embryo developmental success through reduced apoptosis. As a result, embryologists would have a wider array of embryos to choose from during embryo transfer and could potentially freeze any additional embryos.
The present study focuses on determining the role of PGI2 in activating the anti-apoptotic survivin pathway in a murine model. During cellular stress, or other apoptotic inducing incidents, the survivin pathway can be induced to prevent apoptosis through cellular mechanisms [10,11]. Survivin, which is part of the inhibitor of apoptosis proteins (IAP's), has been expressed in fetal tissues during development and shown to control growth and prevent embryo apoptosis [12]. Survivin is a multifaceted protein that is typically expressed during the G2 and M phases of the cell cycle and helps ensure that cells do not undergo apoptosis during a vulnerable stage of replication [11]. The objective of this study focuses on the relationship between PGI2 and the survivin pathway.
Materials and Methods
Experimental design
All experiments were performed under IACUC approval and guidance from Texas Tech University. Prior to testing the efficacy of iloprost in inducing an anti-apoptotic response in cultured embryos, the IC-50 of YM-155 was determined through logarithmic testing in cultured embryos. YM-155 is a commercially available, apoptosis-inducing drug, that acts by specifically inhibiting survivin's antiapoptotic method of action (Selleck Chem; Houston, TX). Mice were stimulated according to a prior protocol [5]. Cultured embryos were randomized and placed in separate treatment groups with four different concentrations of YM-155: 3.3 nM, 1.0 nM, 0.33 nM, 0.10 nM. After the IC-50 of YM155 was established, experimentation followed with the inclusion of iloprost to determine its role in embryo survival. Mouse embryos were randomly assigned to one of four treatments: 1) Iloprost treatment 2) YM-155 treatment 3) Treatment combining both iloprost and YM-155, and 4) A control - cultured under standard conditions with no additional agents.
Culture media condition preparations
Harvest media preparations: Ham's F-10 media was supplemented with human albumin at the rate of 5 mg/mL and L-glutamine at a rate of 1.0 mM for use during harvest. Four 50 mm petri dishes were prepared with 2 mL of Modified Ham's F-10 media. Similarly, two organ culture dishes (Falcon 3037; Becton Dickinson; St. Louis, Mo) were prepared with 1mL of Modified Ham's F-10 media with an additional 2-3 mL of saline added to the outer well to maintain humidity during culture. Prepared organ culture dishes were placed into the incubator under standard culture conditions (37 ℃, 99% humidity, with 6% CO2) for 1-24 hours to equilibrate.
Embryo development in YM-155: Organ culture dishes were prepared for pre-treatment culture with 600 µl Global Media (Life Global; Guilford, CT) supplemented with 10% synthetic serum substitute (SSS) under an overlay of 600 µl mineral oil and, as with the collection dishes, 2-3 mL of saline was placed into the outer well. The control treatment group was prepared identical to pre-treatment dish, while four separate organ culture dishes were prepared in a similar manner with varying concentrations of YM-155 (Selleck Chem; Houston, TX): 0.1 nM, 0.33 nM, 1.0 nM, or 3.33 nM added to the Global Media supplemented with 10% SSS. For post-treatment culture, four additional organ dishes were prepared as previously described for pre-treatment.
Embryo culture with iloprost: Organ culture dishes were prepared for pre-treatment and control treatment as previously described in preparation for YM-155 treatment. Iloprost (Cayman Chemical; Ann Arbour, MI), and YM-155 underwent standard serial dilutions to achieve a final dilution of 1.0 mM iloprost and 0.145 nM YM-155. The organ culture dish for iloprost treatment was prepared with 1.0 mM iloprost added to Global Media with 10% SSS. The Combination treatment dish was prepared in the same manner as the iloprost treatment dish, with the addition of 0.145 nM YM-155 at 33 hrs post-harvest. The organ culture dish for the YM-155 treatment group was prepared in the same manner as the control dish with the addition of 0.145 nm YM-155. For post-treatment culture, organ dishes were prepared as previously described.
Mice preparations and harvest
Six- to ten-week-old female mice from Charles Rivers (strain CB6F1; Houston, TX) were allowed to acclimate upon arrival, before experimentation. Mice were super ovulated with a subcutaneous injection of pregnant mare serum gonadotropin (PMSG; 5.0 IU IP; Sigma; St. Louis, MO) and injected with human chorionic gonadotropin (hCG; 5.0 IU IP; Sigma; St. Louis, MO) 24 hrs later. After injections were administered, mice were mated, (singularly or in pairs) with a male mouse of proven fertility and were harvested at 42 hrs in an attempt to collect 2-cell embryos.
Embryos were collected at the 2-cell stage from mice using micro-dissection of the oviduct and placed into pre-treatment media. Embryos were then randomly assigned to treatment.
Embryo development and observation
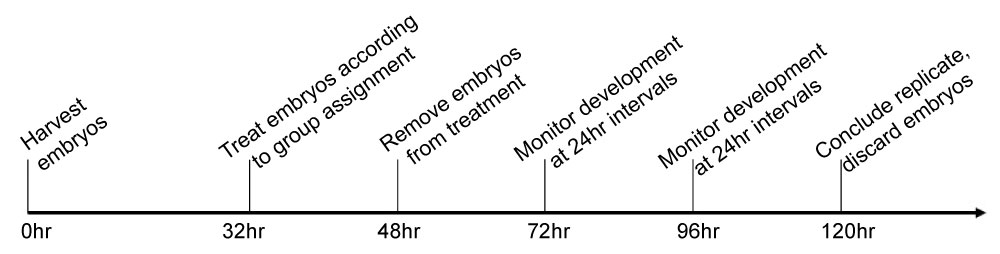
Embryos were allowed to develop in the pre-treatment dishes for 32-33 hrs post-harvest. Embryos undergoing the IC-50 experiment with varying concentrations of YM-155 were transferred into respective treatments at 33 hrs. In the iloprost experiment, embryos were transferred to their respective treatment dishes at 32 hrs. At the 33 hr mark, the dilute 0.145 nM YM-155 was added to the combination treatment group as previously described. Embryos in both experiments developed under treatment conditions for an additional 16 hrs. After 16 hrs in treatment (48 hrs post-harvest), embryos were transferred into their respective post-treatment dishes. Embryos were allowed to develop for 120 hrs. A timeline of the experiment is exhibited in Figure 1.
Embryos in both experiments were evaluated at 24 hr intervals using standard markers for viability used in the ART laboratory. Fragmentation, gross abnormalities in morphological appearance, and excess debris within the zona pellucida were used as markers to determine embryo viability and eliminate embryos from consideration in the study. The study's guidelines to study embryo development would have been compromised by allowing embryos or oocytes that were pre-determined to be unfertilized, stagnant, or dead to be included in the treatment groups.
Hatching was used as an indicator for viability, as a hatched embryo indicates implantation potential and cannot resume in vitro development post hatching. Embryos that ceased development prior to hatching from the zona pellucida were deemed non-viable and to have undergone apoptosis. Embryos that were deemed unhealthy after pre-treatment, and therefore unlikely to progress to the blastocyst stage under normal development, were removed from the study before starting the treatment incubation period.
Statistical analyses
All data were analyzed by Statistical Package for the Social Sciences (SPSS ver. 12, Chicago, IL). Chi-squared analysis was used to determine differences in the experimental model with a one-tail Fisher's exact test performed to determine statistical significance due to the data being a binomial set. A value of p < 0.05 was used to determine statistical significance.
Results
YM-155 IC-50 study
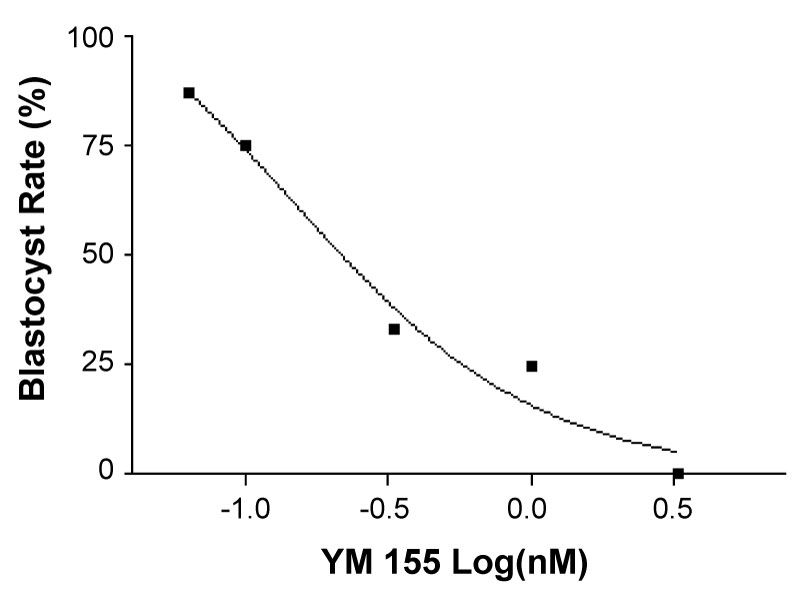
A total of 198 two-cell embryos were recovered and utilized to determine the IC-50 of YM-155 in embryo development. They were cultured as follows: 1) 46 in the control group, 2) 35 in 3.3 nm YM-155, 3) 41 in 1.0 nm YM-155, 4) 36 in 0.33 nm YM-155 and 5) 40 in 0.1 nm YM-155. Development was assessed at 24 hr intervals as described above, and embryos were monitored for the appearance of apoptosis.
The goal of the study was to determine an IC-50, or concentration of YM-155 that causes 50% of the embryos to undergo apoptosis. At the conclusion of 120 hrs, data were collected and the number of embryos that reached the blastocyst stage in a normal span of time are as follows: 1) 69.57% (32/46) in the control, 2) 0% (0/35) in 3.3 nm YM-155, 3) 24.39% (10/41) in 1.0 nm YM-155, 4) 33.33% (12/36) in 0.33 nm YM-155 and 5) 65.00% (26/40) in 0.1 nm YM-155. It was determined that embryos that reached the blastocyst stage, regardless of hatching from the zona pellucida, had failed to undergo apoptosis and were developing normally (Figure 2). The hatching rates of the control group were used as the basis of comparison for the hatching rates of the YM-155 treatment groups.
Iloprost study
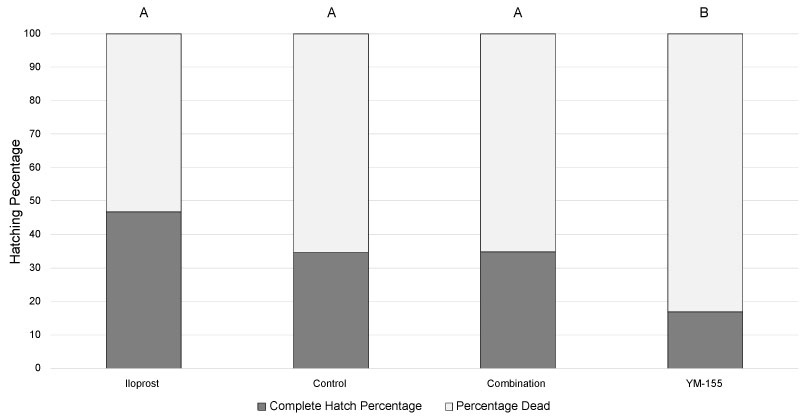
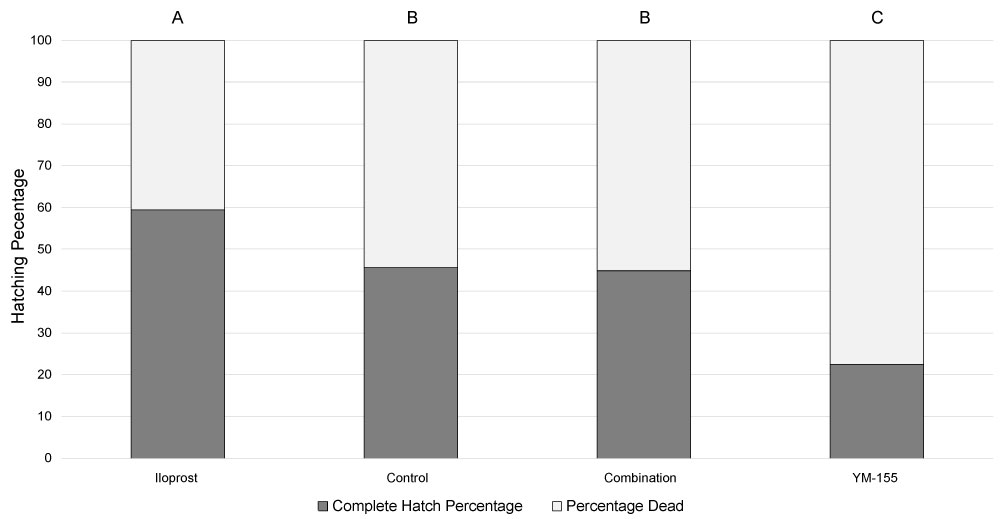
A total of 460 two-cell embryos were recovered and used in the iloprost experiments over the course of thirteen independent replicates. They were cultured as follows: 1) 158 in iloprost alone, 2) 71 in YM-155, 3) 69 in the combined treatment and 4) 162 in the control. Once it was determined that embryos had grown at appropriate rates, the data at each time point was compared to determine the efficacy of iloprost inhibiting the apoptotic effects of YM-155. Hatching from the zona pellucida was monitored within the normal developmental range of 96-120 hrs. As previous preliminary studies have demonstrated significant changes in hatching during this window, data was analyzed at both 96 hr and 120 hr time points. It was determined that embryos which did not hatch completely from the zona pellucida within the 120 hr timeframe would be inadequate and would fail to implant, even if they hatched at a later time-point (Figure 3 and Figure 4).
At 96 hrs, embryos in the control group demonstrated hatching from the zona pellucida at a rate of 34.56% (56/162), as compared to 16.90% (12/71) from the embryos in the YM-155 treatment group (p < 0.05). The embryos treated with iloprost exhibited the highest rate of hatching from the zona pellucida with 46.84% (74/158). As demonstrated in Figure 3 and Figure 4, there is a significant difference in the overall number of embryos that hatched from the zona pellucida at the 96 hr mark, as compared to the 120 hr mark (p < 0.001). This would indicate normal embryo growth and development. However, the number of embryos hatching was treatment dependent at each time point (p < 0.001).
At 120 hrs, embryos in the control group hatched from the zona pellucida at a rate of 45.68% (74/162), as compared with 22.54% (16/71) in the YM-155 treatment group (p < 0.05). Embryos treated with iloprost exhibited the highest rate of embryo hatching of all treatment groups with 59.49% (94/158), similar to results previously reported by Huang, et al. (2007). The embryos cultured in the presence of iloprost and then exposed to YM-155 exhibited hatching rates similar to the control (p = 0.65) and greater than YM-155 alone.
By comparing Figure 3 and Figure 4, it is apparent that all treatments, with the exception of YM-155, had an additional 10% of the embryos hatch over the 24 hr window between 96 and 120 hrs. These results were unable to be corroborated by several studies that terminated data collection at the 96 hr mark [4,5,7,13].
Discussion
The results from this study demonstrate that iloprost influences embryo development through improvement of embryo survival in apoptosis-inducing culture conditions. By demonstrating that iloprost rescued embryos and restored survivability, the research substantiates the original hypothesis that iloprost is a beneficial additive for IVF culture environments, likely by supporting the survivin pathway.
As in previous studies, embryos that developed in media conditions with iloprost developed at a significantly higher rate than the control group under standard conditions [4,7,14]. As expected, YM-155 caused apoptosis in approximately 50% of the embryos when compared to the development of the control group. The addition of iloprost to YM-155 successfully rescued embryos and allowed them to develop at rates similar to the control group without significant difference in hatching rate. This "rescue" effect, while under duress from the apoptotic agent YM-155, signifies an apparent ability of PGI2 to halt apoptosis and induce a biochemical pathway which allows the continued development of the embryos, supporting the hypothesis iloprost supports the survivin pathway.
YM-155 has been shown to specifically inhibit survivin in mammalian tissues, and while it has been used successfully in some cancer research, it is also successful at inducing apoptosis in blastomeres [15]. By utilizing YM-155 to challenge embryo growth, it specifically targeted the survivin protein and allowed embryo apoptosis. The increased embryo survival observed when using iloprost in conjunction with YM-155, appears to support the theory that PGI2 supports expression of the survivin protein, which then prevents apoptosis. However, there is a possibility that YM-155 could act through a different mode of action to induce apoptosis, which has yet to be defined.
The biochemical pathway of apoptosis is well defined and understood; however, finding different mechanisms of action to stop or reverse the effects of apoptosis are not as well characterized [15,16]. By utilizing a chemical that induces apoptosis in a relatively well-defined manner, it is reasonable to accept the theory that PGI2 does, in fact, halt apoptosis in embryos through the expression of survivin. This helps to bring clarity to the proposed mechanisms that are involved in halting apoptosis and potential benefits of iloprost in embryo culture. By analyzing the results of this study, it can be inferred that the inclusion of a PGI2 analogue in the in vitro culture of early-developmental embryos would be beneficial to the overall success rates of IVF and ET protocols. Through the inclusion of iloprost, or a similar PGI2 analogue, embryos will have improved development rates in vitro and higher rates of hatching from the zona pellucida to continue development post-transfer [17,18].
While this study demonstrated correlation between the apoptotic agent YM-155 and iloprost, further testing is required. Even though YM-155 has been proven to stimulate apoptosis by inhibiting the survivin pathway, it is possible a different pathway to induce apoptosis was utilized in these embryos. Further research will be performed to determine the exact interaction between PGI2, survivin, and YM155 in the context of embryo development, while trying to utilize techniques that are minimally invasive and would not halt embryo growth and development.
In conclusion, this research continues to support the inclusion of iloprost in embryo culture media and the concept that iloprost acts by inducing and stabilizing the survivin pathway. It is important to realize that this technique may not be applicable in every IVF protocol but would be useful to improve outcomes of clinical IVF. Data support PGI2 improves embryo in vitro development and might benefit most IVF programs through inclusion in culture media. A previous study by Wun, et al. demonstrated the safety of iloprost to human embryo development when added to culture media [13]. However, future studies will be performed to assess the efficacy of iloprost at activating the survivin pathway in a human model and the potential of including iloprost in IVF and ET protocols. Due to the nature of embryo development and the limitations on which laboratory techniques can be utilized without destroying the embryos, further studies will attempt to elucidate a technique to study the biochemical pathway of apoptosis, while maintaining embryo viability.
Acknowledgements
The authors of this study would like to acknowledge the contributions and assistance of undergraduate research assistants Megan Arroyo and Dalton Tidwell. Their assistance through this study was extremely valuable. The authors would like to thank Leslie Alley for her tremendous support in editing and revising this manuscript. The authors would also like to thank the Laura W Bush Institute for Women's Health for partially funding this experiment.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of Interest
None declared.
References
- JD Biggers (2012) IVF and embryo transfer: Historical origin and development Reprod Biomed Online 25: 118-127
- Y Zhao, P Brezina, CC Hsu, et al. (2011) in vitro fertilization: Four decades of reflections and promises Biochim Biophys Acta 1810: 843-852.
- JC Huang, WS Wun, JS Goldsby, et al. (2003) Prostacyclin enhances embryo hatching but not sperm motility. Hum Reprod 18: 2582-2589.
- JC Huang, JS Goldsby, WS Wun, et al. (2004) Cyclooxygenase-2-derived endogenous prostacyclin enhances mouse embryo hatching. Hum Reprod 19: 2900-2906.
- JC Huang, WS Wun, JS Goldsby, et al. (2007) Prostacyclin receptor signaling and early embryo development in the mouse. Hum Reprod 22: 2851-2856.
- H Lim, R Gupta and W Ma (1999) Cyclooxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev 13: 1561-1574.
- JC Huang, JS Goldsby, F Arbab, et al. (2004) Oviduct prostacyclin functions as a paracrine factor to augment the development of embryos. Hum Reprod 19: 2907-2912.
- F Baratelli, K Krysan, N Heuze-Vourc'h, et al. (2005) PGE2 confers survivin dependent apoptosis resistance in human monocyte-derived dendritic cells. J Leukoc Biol 78: 555-564.
- CM Hao, R Redha, J Morrow, et al. (2002) Peroxisome proliferator-activated receptor delta activation promotes cell survival following hypertonic stress. J Biol Chem 277: 21341-21345.
- J Y Yu, J Silke, P Ekert (2007) Inhibitor of apoptosis proteins and caspases. Apoptosis, Cell Signaling, and Human Diseases 313-334.
- R Colnaghi, CM Connell, RM Barrett, et al. (2006) Separating the anti-apoptotic and mitotic roles of survivin. J Biol Chem 281: 33450-33456.
- E Vassiliou, V Sharma, H Jing, et al. (2004) Prostaglandin E2 promotes the survival of bone marrow-derived dendritic cells. J Immunol 173: 6955-6964.
- WS Wun, R Dunn, R Mangal, et al. (2013) Prostacyclin enhances human IVF success. Fertility and Sterility 100: S1.
- JC Huang, WS Wun, JS Goldsby, et al. (2007) Stimulation of embryo hatching and implantation by prostacyclin and peroxisome proliferator-activated receptor delta activation: Implication in IVF. Hum Reprod 22: 807-814.
- Q Cheng, X Ling, A Haller, et al. (2012) Supression of survivin promoter activity by YM155 involves disruption of Sp1-DNA interaction in the survivin core promoter. Int J Biochem Mol Biol 3: 179-197.
- M Weil, MD Jacobson, HS Coles, et al. (1996) Constitutive expression of the machinery for programmed cell death. J Cell Biol 133: 1053-1059.
- (2016) Complications in a multiples pregnancy. American Pregnancy Association.
- AM van Peperstraten, RP Hermens, WL Nelen, et al. (2010) Deciding how many embryos to transfer after in vitro fertilization: Development and pilot test of a decision aid. Patient Educ Couns 78: 124-129.
Corresponding Author
JC Huang, Department of Obstetrics and Gynecology, Texas Tech University Health Sciences Center, Lubbock, Texas, 79430, USA.
Copyright
© 2019 Schniers BK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.