Expression of PDGF-D in Patients with Valvular Atrial Fibrillation
Abstract
Objective: To investigate the relationship between the over expression of PDGF-D in right atrial tissue and the fibrosis of right atrial in patients with valvular atrial fibrillation (AF).
Methods: A total of 84 patients who needed valve replacement due to rheumatic heart disease were selected. There were 39 patients in the atrial fibrillation group and 45 patients in the sinus rhythm group, respectively. The medical history, examination and examination results of the patients were collected and combed. The right atrial tissue (0.3-0.5 cm 3 ) was obtained during the operation. In this study, the content of collagen in the right atrial tissue of patients in the two groups was determined, and the patients in the atrial fibrillation group and the SR group were measured and compared by real-time PCR and Western-Blot.
Results : By comparing many aspects of all enrolled patients, such as medical history and examination results, there was no statistical significance in the above parameters between the SR group and the AF group(P > 0.05); Masson staining showed that the proportion of collagen in atrial samples obtained from AF group was significantly higher than that from SR group (P < 0.05, t = 271.0); The results of Western Blot and real-time PCR showed that the expression of PDGF-D mRNA and protein in AF group was significantly higher than that in SR group (P < 0.05), Collagen content was positively correlated with PDGF-D mRNA and protein expression (P < 0.05).
Conclusion: The high expression of PDGF-D is closely related to right atrial fibrosis in patients with atrial fibrillation.
Keywords
Atrial fibrillation, Rheumatic heart valvular disease, Collagen content, Atrial remodeling, Platelet-derived growth factor-D
Introduction
Atrial fibrillation a very important public health problem Atrial fibrillation is the most common arrhythmia and a significant contributor to stroke and heart failure, so atrial fibrillation is a very important health problem [1]. In clinical practice, the main cause of atrial fibrillation is valvular heart disease and the most common structural heart disease [2,3]. The most typical pathological change of atrial fibrillation is atrial fibrosis, which refers to abnormal collagen fibers deposited in the normal atrial myocyte interstitium. In other words, the content of collagen fibers increases in the normal atrial myocyte interstitium [4,5]. In normal atrial tissue, fibrous tissue accounts for 2%-4% of my interstitial content, and clinical atrial fibrosis is defined as the content of collagen fibrous tissue exceeding 10% of atrial tissue. Clinically, the occurrence of atrial fibrosis is a common pathological feature of atrial tissue in patients with atrial fibrillation [6-8]. The results of several studies have shown that patients with atrial fibrillation have significant atrial fibrosis, while the opposite is true in patients with sinus rhythm. Studies have found that many cytokines are involved in atrial fibrosis [9]. Platelet-derived growth factor D (PDGF-D) is a member of PDGF. PDGF-D plays a pivotal role in tissue repair, fibrosis, immune response, cell proliferation and other processes [10,11]. However, there are few studies on the correlation between PDGF-D and right atrial fibrosis in patients with atrial fibrillation [12,13]. In this study, the right atrial tissue, blood and basic clinical data of patients with valvular atrial fibrillation were collected, and the relationship between PDGF-D and atrial fibrosis in patients with valvular atrial fibrillation was discussed at the histological level by applying biochemical and molecular biological techniques.
Materials and Methods
Patient registration and data collection
In this study, 84 patients with rheumatic valvular heart disease were admitted to the Department of Cardiac Surgery of the First Affiliated Hospital of Xi'an Jiao tong University, Xining Hospital and Shaanxi Provincial People's Hospital from May 28, 2012 to August 13, 2012 for heart valve replacement surgery. There were 39 cases in permanent AF group and 45 cases in SR group. Baseline demographic, physical, routine laboratory, echocardiogram, and other clinical data were available to all patients prior to surgery. The inclusion criteria for this study were that subjects with a significant history of atrial fibrillation and ECG showed atrial fibrillation for more than 6 months were considered eligible for inclusion. The control group of the experiment was set as a sinus rhythm patient without a history of AF. There was no history of antibiotic use in the last 2 weeks.
The exclusion criteria are: Age 65 years or older, coronary atherosclerotic heart disease, liver and kidney failure, nephrotic syndrome, diabetes, hyperthyroidism, hypertension, cardiomyopathy, chronic pulmonary heart disease, autoimmune disease, heart failure exceeding New York Heart Function Grade 3, EF to less than 40%, rheumatic fever with acute activity, chronic inflammation, with significant acute infection, tumor patients, and patients who had taken aldosterone receptor antagonists, angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors within five half-lives were excluded from this study.
Before the trial, each patient who participated in the trial provided written informed consent that was obtained from each enrolled patient or his or her family. All informed consent forms and procedural agreements were authorized by the First Affix Ethics Committee at Xi'an Jiao tong University Hospital. This survey is in line with the principles outlined in the Declaration of Helsinki.
Collection and preservation of human heart tissue
Before cardiopulmonary bypass was established in all patients during cardiac surgery, right atrial tissue (0.3-0.5 cm 3 ) was obtained before cardiopulmonary bypass intubation and after the right atrium was opened. After removing blood and adipocytes, each atrial tissue was cut into three pieces. The first piece was placed in liquid nitrogen bottle and stored at -80 °C for protein extraction and Western blot detection. The second piece was put into EP tube for collagen content and mRNA separation; the third block was fixed with 4% formaldehyde solution and used for paraffin embedding section and immunostaining.
Masson's trichrome staining and collagen volume fraction assay
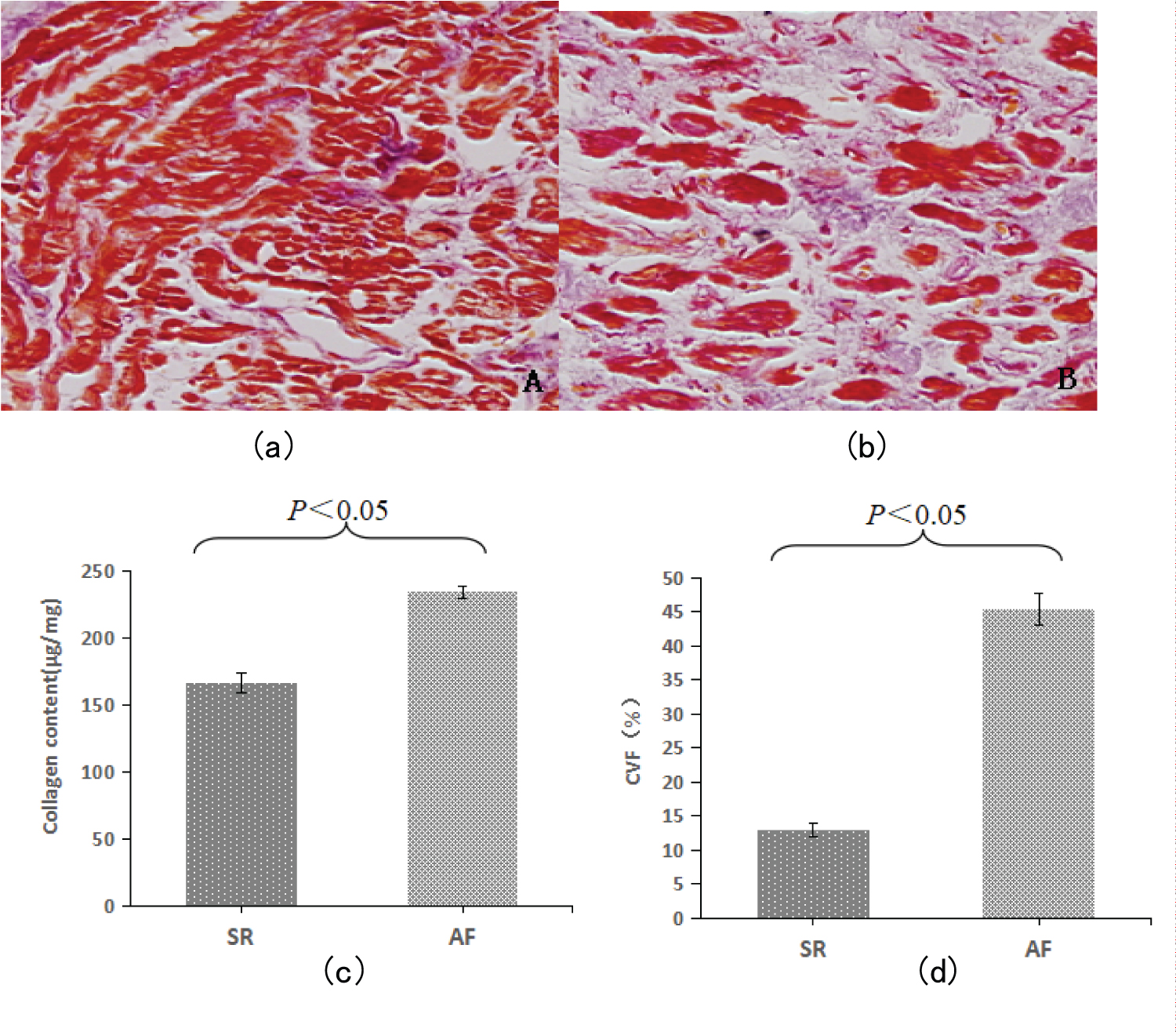
The samples were fixed with 4% par formaldehyde, dehydrated with ethanol, and then embedded in paraffin. These were serially sectioned at 4μmand Masson's trichrome staining was performed to highlight collagen fibers. The collagen fibers were dyed blue and the muscle tissue was dyed red. Collagen volume fraction (CVF) was determined by right atrial section.Masson’strichrome a were obtained from Boster Biological Engineering Corporation (Wuhan, China). Two slides were randomly selected for each sample and observed under a polarizing microscope. Each section was randomly selected under the microscope with 6 high-magnification fields. The multifunctional microscope and automatic image processing and analysis system (Leica, Germany) were used to photograph each section, and Collagen volume fraction (collagen area/measured visual field area × %) was expressed as mean ± standard deviation (± S) for all data.
Detection of positive for PDGF-D by immunofluorescence staining
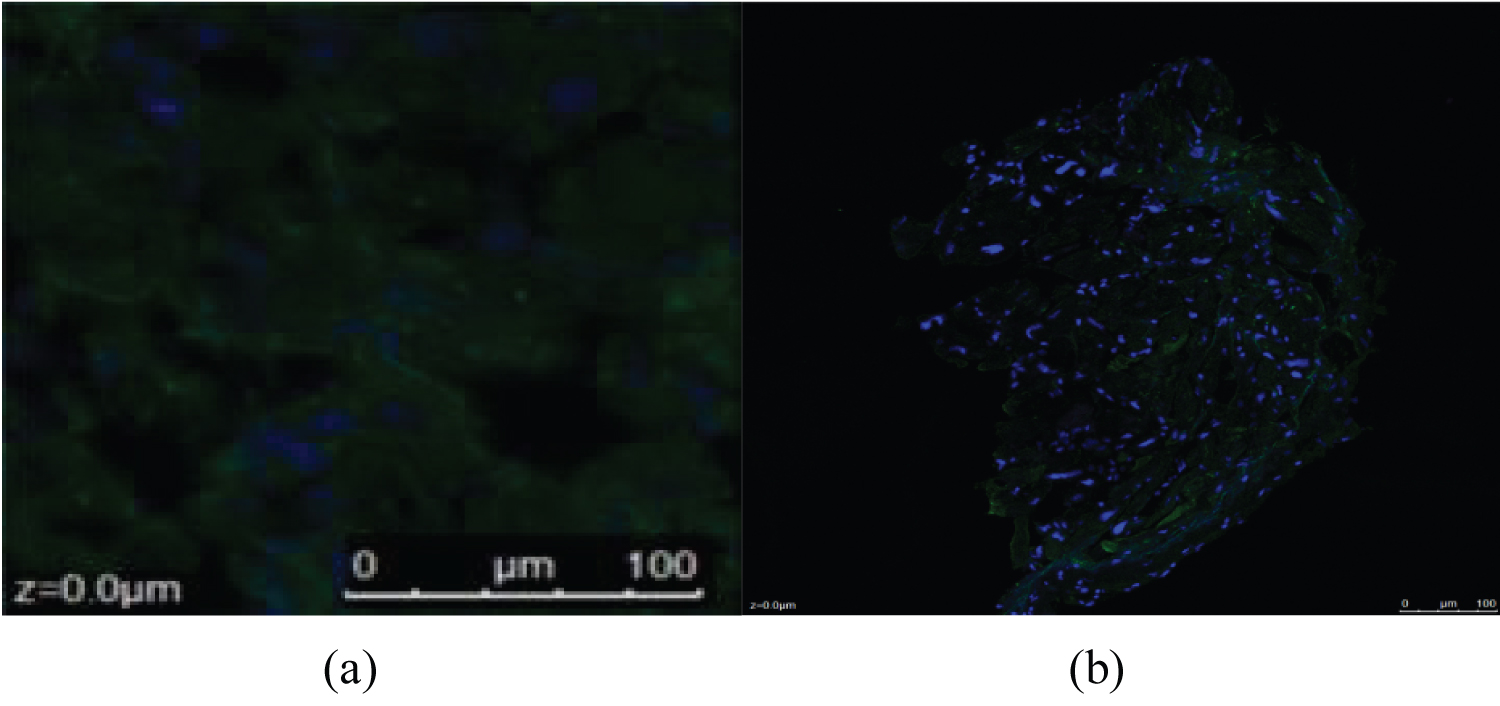
After embedding in frozen section embedding agent, 7 μm slices were obtained on polylysine coated slides. The slides were treated with acetone for 10 minutes at 4 degrees Celsius and then with PBS. Normal bovine serum was added and sealed for 40 min, then rinsed with 0.01 mol/L PBS for 10 min × 1 time; Then the working solution of primary antibody was added (PDGF-D primary antibody 1:200 dilution; PDGFR-α monoclonal antibody in 1:150 dilution), incubated at 37 °C for 1 h, and overnight at 4 °C. After that, it was rinsed with 0.01 mol/L PBS for 10 min × 3 times. Fluorescent secondary antibody working solution (PDGF-D and PDGFR-α, sheep anti-mouse /FITC, both in 1:100 dilution) was added, and incubated at 37 degrees Celsius for 1 hither, they were washed with 0.01 mol/L PBS for 10 min × 3 times. DAPI dye working solution was added (1:100 dilution) and incubated at room temperature for 15 min. Rinse with 0.01 mol/L PBS for 10 min × 3 times; Then seal with buffer glycerin; After the above process, The contents of the sections were observed under a fluorescence microscope and photographed (× 200).
Detection the expressions of mRNA of PDGF-D and type I, III collagen by RT PCR
Simply, the sample was thawed and homogenized on ice, and the separation and quantification of Total RNA was done according to the company's instructions using the RNA-Simple Total RNA Kit (Tianen Biotechnology, China). Using the ReverTra Ace qPCR RT Kit (Tiangen Biotechnology), the RNA was then reverse-transcribed into cDNA as per the manufacturer's instructions. cDNA amplification was performed on a thermal cycler (Applied Biosystems Step One Plus System) The obtained cDNA was amplified on a thermal cycler (Applied Biosystems Step One Plus System). The volume of the reaction mixture was a total of 20 μl, which was then smoothly amplified according to the merchant's description (SYBR ® Premix Ex Taq™II PCR kit and Applied Biosystems First Step Addition system). Eight tubes were placed into a PCR apparatus for amplification, and the process was 40 cycles of predenaturation at 95 °C for 30 s, 95 °C for 5 s, and 60 °C for 30 s. At the end of the reaction, the CT value, dissolution and amplification curves of each reaction well were read and analyzed. The CT value (Cycle threshold) was obtained by IQ5 software. The relative expression 2-△△CT was calculated using the following formula: -△△CT=CT (SR group) -CtGAPDH (SR group) -Ct (AF group) + CTGAPDH (AF group). Oligonucleotide probes were as following (Table 1).
Western blotting analysis
For Western blot analysis, frozen right atrial tissue samples were used for protein isolation. Protein extraction was performed according to the instructions of the total protein extraction kit (applied gen Technologies Inc., China). SDS-PAGE electrophoresis was used to isolate the protein (10 µg) extracted above. It was then transferred to the nitrocellulose membrane (Stratagene, USA). Ponceau S solution (Sigma, USA) stains it. 5% skim milk was used to block the membrane and detect rabbit polyclonal anti-human PDGF-D antibodies (Abcam, USA). Rabbit Anti-mouse or anti-Rabbit IgG conjured to equine radish peroxidase (1:5000, Santa Cruz Biotechnology) as a secondary antibody. Incubation was performed under the ECL Western Blot assay kit (Amersham, Netherlands). The amount of protein selected was within the range of the linear immune response signal. The immunoreactive signals were exposed to Kodak film for 5 min, scanned and analyzed with a gelpro analyzer. Discovery Series TM image analysis software (Bio-Rad, USA) was used for normalization with the corresponding β-actin values. Experiments were repeated three times and averaged.
Statistical analysis
The experimental results were anal deviation was uyzed using SPSS16.0 statistical software package. Mean ± standards for measurement data, and normality test was performed by (± S), and the data in this study were in line with normal distribution. The t test was used to compare the means between the two groups, and the X 2 test was used to compare the rates. A difference of 0.05 was considered statistically significant.
Results
Clinical characteristics
Details of enrolled patients are shown in Table 2. In general, AF group is similar to SR group in many aspects. Left and right atrial diameters, as indicated by echocardiography, were significantly larger in the AF group than in the SR group. All drugs were discontinued at least 12 hours before surgery.
Collagen content and distribution
The results of Masson staining of Arial tissue between the two groups are shown in figure. In the sinus rhythm control group, a small amount of collagen fibers were observed in the atrial tissue (Figure 1a). In the AF group, there was a significant proliferation of purple-blue collagen fibers in the atrial tissue (Figure 1b). In the subendocardium, collagen deposition in the interstitium was significantly increased, and the muscle bundles were separated by a large number of cord-like collagen fibers.The semi-quantitative results of Masson staining of atrial tissue by image analysis also showed that the CVF of AF group was significantly higher than that of SR group (P < 0.05). Hydroxyproline assay kit was used to detect the content of this amino acid, and the content of collagen in atrial tissue (μg/mg) could be obtained from this. The results showed that the collagen content of AF group was significantly higher than that of SR group (P < 0.05).
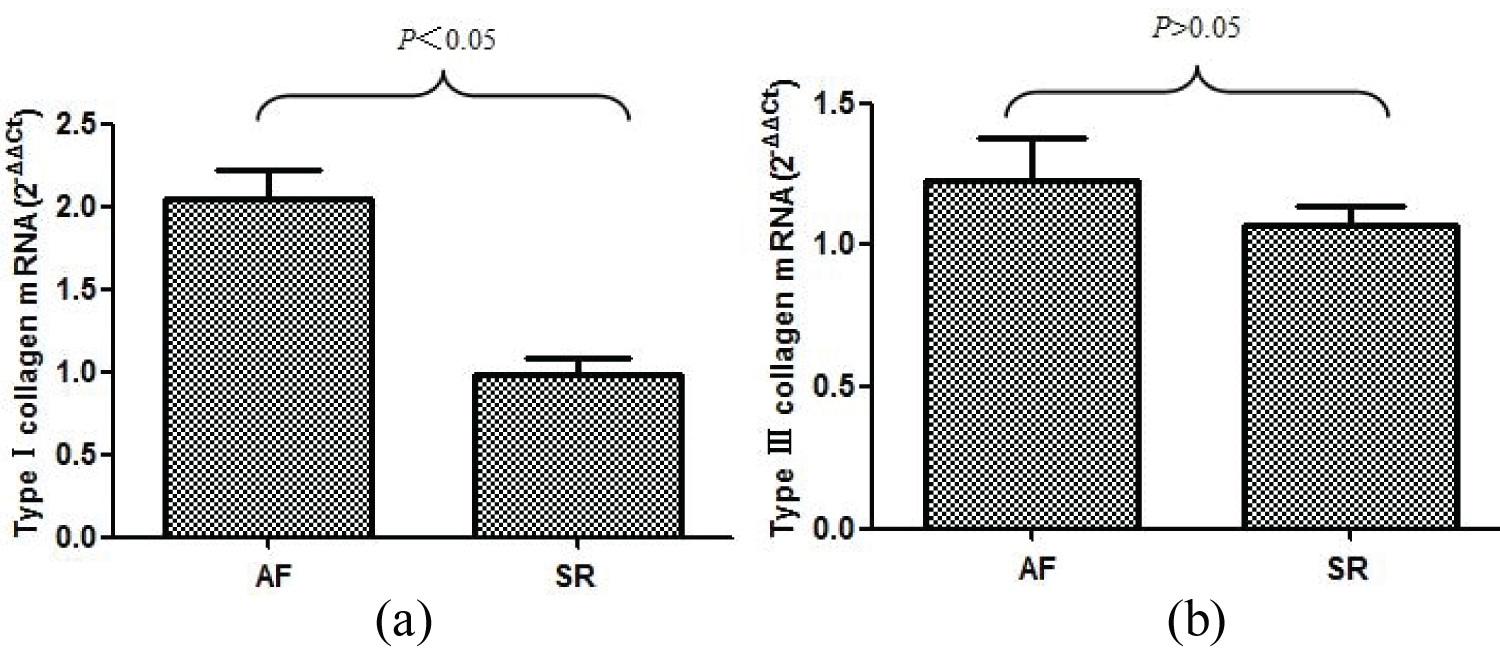
mRNA expression of type I collagen and type III collagen
The relative expression of collagen type I and collagen type III mRNA (2-△△Ct value) in the specimens of enrolled patients was determined by real-time fluorescence quantitative PCR. The relative expression levels of type I collagen mRNA and type III collagen mRNA in AF and SR groups (2-△△Ct value) were 2.042 ± 0.177 vs. 0.988 ± 0.099, P & lt; 0.05; 1.228 ± 0.151 vs. 1.067 ± 0.068, P > 0.05. The results showed that the mRNA expression level of type I collagen in AF group was significantly higher than that in SR group, and the difference was statistically significant. The mRNA expression of type III collagen was only slightly higher than that of SR group, and there was no statistical significance between the two groups (Figure 2).
The expression of PDGF-D increased in patients with AF
The expression of PDGF-D protein was positive in SR group and AF group, and it was located in the cell membrane near the nucleus of myocardium or fibroblast nucleus. The expression of PDGF-D protein in the SR group was weak, while the fluorescence intensity and density of the expression in the AF group were significantly stronger (Figure 3).
Amplification and dissolution curves
The resolution curves of real-time fluorescence quantitative PCR showed a single peak, indicating that the amplification reaction was specific. The inflection point of each mRNA amplification curve was clear, the exponential phase and plateau phase were obvious, and the slope of the exponential phase was large, indicating that the amplification efficiency was high. The curves were well parallel, indicating similar amplification efficiencies. The results showed that the expression of PDGF-D mRNA (2-△△Ct value) in AF group was significantly higher than that in sr group (2.062 ± 0.184 vs. 0.991 ± 0.062, P < 0.05) (Figure 4).
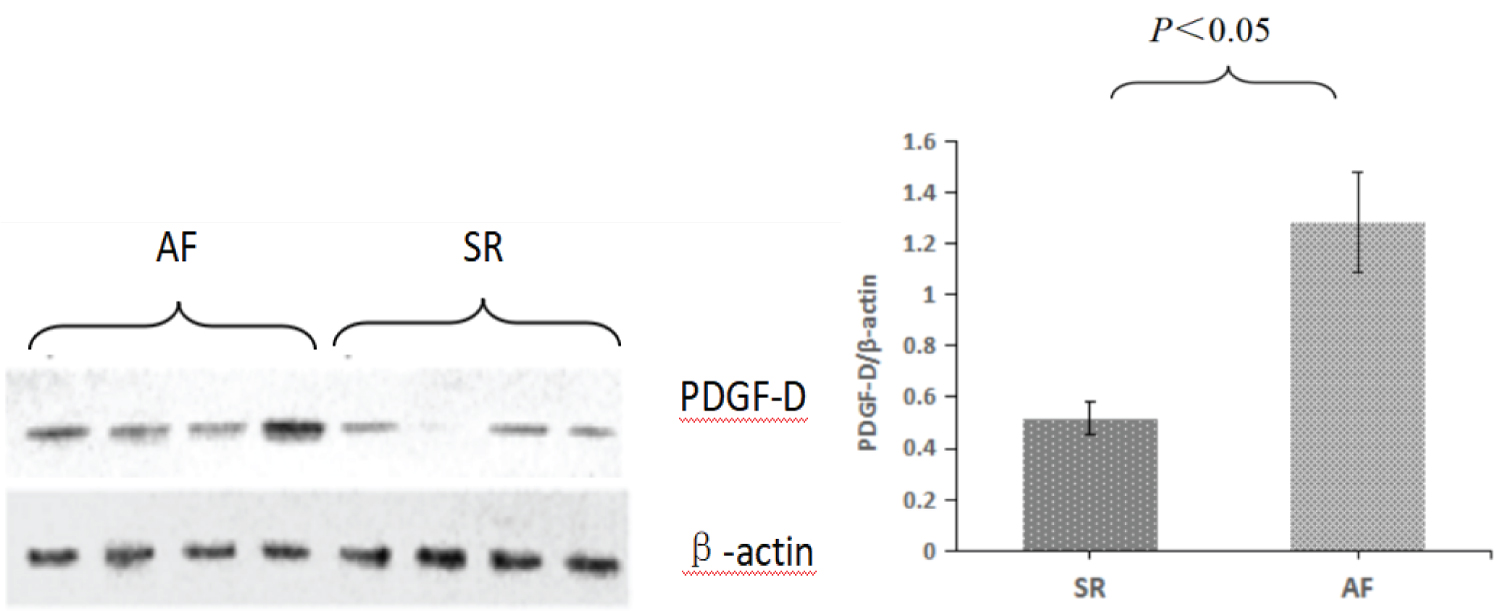
Measurement of PDGF-D protein levels in atrial tissue
The relative expression of PDGF-D protein in atrial tissue samples of patients with sinus rhythm and atrial fibrillation was detected by Western-Blot technique. Image processing was performed by scanning the images and recording the results using Gel Doc100 imaging system. The target protein was semi-quantitatively analyzed by the ratio of the absorbance area integral of the target band to that of the reference β-actin band. The relative expression level of PDGF-D protein in AF group and SR group was 1.282 ± 0.193 vs. 0.517 ± 0.067 (P < 0.05), indicating that AF related protein was more expressed (Figure 5).
Discussion
Platelet-derived growth factor is a 24 ku cationic glycoprotein, which is mainly found in platelet α granules, but also in damaged endothelial cells, migrating macrophages, smooth muscle cells, fibroblasts and other cells [14]. These cells release PDGF in large quantities and mediate endothelial-mesenchymal interactions in various tissues [15]. PDGFs is the main mitogen in the origin cell population of neuroectoderm, a cytokine that can promote the mitosis and chemotaxis of fibroblasts and vascular smooth muscle cells, regulate the content of collagen, fibronectin, proteoglycan, hyaluronase and collagen protease in the extracellular matrix, and It also plays a pivotal role in the remodeling of connective tissue [16,17]. The polypeptide chain of PDGF-D is composed of 196 and 211 amino acid residues, which are highly expressed in the heart, pancreas and skeletal muscle tissues. It can bind to specific receptors on the cell membrane, and thus transmit information into the cell's interior, producing a response through a series of actions [18,19]. PDGF is mainly distributed in cells of interstitial or glial origin: Fibroblasts, osteoblasts, chondrocytes, smooth muscle cells and glial cells [20,21].
At present, the research on the relationship between atrial fibrillation and cardiac collagen expression has become a hot topic. Many studies have shown that AF is associated with excessive collagen deposition, and the duration of atrial fibrillation is positively correlated with the expression of collagen in the extracellular matrix. The uniform conduction of atrial activation is closely related to the structural and functional integrity of cardiomyocytes and extracellular matrix [22,23]. At the same time, the enlargement of atrial cavity can accommodate more reentrant wavelet, which is more likely to induce atrial fibrillation, and provides the pathological basis for the occurrence and maintenance of atrial fibrillation [24,25]. In addition, interatrial interstitial fibrosis can also cause changes in the normal gap junctions between atrial myocytes and the density of ion channels in the cell membrane, which leads to atrial electrical remodeling and promotes the occurrence and maintenance of atrial fibrillation [26,27].
The results showed that compared with the general data of enrolled patients, AF group was obviously larger than SR group in terms of right atrial diameter. Masson staining showed that atrial fibrosis was related to collagen content. The CVF and collagen content in the enrolled patients were compared, and it was found that the SR group was significantly less than the AF group. RT-PCR showed that the mRNA relative expression level of type I collagen in AF group was significantly higher than that in SR group (P < 0.05), and the mRNA expression level of type III collagen was slightly higher than that in SR group (P > 0.05). The consistency of morphological, semi-quantitative and quantitative measurements of collagen showed that the degree of atrial fibrosis in persistent AF group was significantly higher than that in sinus rhythm group, which may be closely related to the occurrence and maintenance of atrial fibrillation.
Studies have confirmed that PDGF-D plays an important role in the development of myocardial fibrosis. RT-PCR was used to detect the relative expression level of PDGF-D mRNA in atrial tissue samples of SR and AF patients. The relative expression level of PDGF-D mRNA in atrial tissue samples of enrolled patients was higher in AF, and vice versa in SR patients. The relative expression of PDGF-D protein in atrial muscle tissue samples of patients with sinus rhythm group and atrial fibrillation group was measured by Western-Blot technique. The analysis showed that the relative expression of PDGF-D protein in atrial muscle tissue samples of patients with AF was also significantly higher than that in the SR group. Combined with the content of the previous study, the correlation analysis of PDGF-D mRNA and collagen type I and III mRNA showed that the mRNA and protein levels of PDGF-D were positively correlated with the mRNA levels of collagen type I and III. This further demonstrated the correlation between PDGF-D and atrial fibrosis in patients with atrial fibrillation from gene expression and protein expression levels.
Conclusions
There is significant atrial remodeling in patients with chronic AF. PDGF-D is highly expressed in the right atrium of patients with atrial fibrillation, and is closely related to the occurrence of atrial fibrillation. PDGF-D may be involved in the development of atrial fibrosis by up-regulating the expression of type I collagen.
Declaration of Conflicting Interest
The authors declare that there is no conflict of interest.
Funding Information
Financial supports from the Natural Science Foundation of Shaanxi Province, China (Grant No.: 2020JM-652); Cultivation Project of Xi’an Health Committee (Grant No.: 2020MS02); Xi’an Science and Technology Bureau Xi'an Innovation Ability Strong Foundation Plan (Grant No.:21YXYJ0018).
References
- Harris MA, Johnson TR, Weinberg PM, et al. (2007) Delayed-enhancement cardiovascular magnetic resonance identifies fibrous tissue in children after surgery for congenital heart disease. J Thorac Cardiovasc Surg 133: 676-681.
- Liu Y, Niu XH, Yin X, et al. (2018) Elevated circulating fibrocytes is a marker of left atrial fibrosis and recurrence of persistent atrial fibrillation. J Am Heart Assoc 13: e008083.
- Wang FF, Han YF, Liang XY, et al. (2022) Tang bp. aging-induced atrial fibrosis in if current change and its effect on atrial fibrillation in dogs. Ann Noninvasive Electrocardiol 27: e12951.
- Abe I, Teshima Y, Kondo H, et al. (2018) Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm 15: 1717-1727.
- Borkham-Kamphorst E, Meurer SK, Van de Leur E, et al. (2015) PDGF-D signaling in portal myofibroblasts and hepatic stellate cells proves identical to PDGF-B via both PDGF receptor type α and β. Cell Signal 27: 1305-1314.
- Ishizaka N, Matsuzaki G, Saito K, et al. (2006) Expression and localization of PDGF-B, PDGF-D, and PDGF receptor in the kidney of angiotensin II-infused rat. Lab Invest 86: 1285-1292.
- Cooley N, Cowley MJ, Lin RC, et al. (2012) Influence of atrial fibrillation on microRNA expression profiles in left and right atria from patients with valvular heart disease. Physiol Genomics 13: 211-219.
- Iwasaki YK, Sekiguchi A, Makabe R, et al. (2022) Effects of aging and hypertension on the antithrombotic function of atrial endocardium in rats. Int Heart J 63: 141-146.
- Qi X, Xu H, Liu Q, et al. (2022) Prospective cohort study of characteristics and sex differences in elderly patients with degenerative valvular disease. BMJ Open 12: e060882.
- Gu R, Yang JQ, Zhao XL, et al. (2023) Impact of non-valvular atrial fibrillation on global cognitive function and executive function. Zhonghua Xin Xue Guan Bing Za Zhi 24: 32-37.
- Agnihotri K, Charilaou P, Voruganti D, et al. (2022) Impact of atrial fibrillation on in-hospital outcomes among hospitalizations for cardiac surgery: an analysis of the National Inpatient Sample. J Investig Med 70: 899-906.
- Kazlauskas A (2017) PDGFs and their receptors. Gene 614: 1-7.
- Chen X, Zhang W, Wang Q, et al. (2016) Eplerenone inhibits atrial fibrosis in mutant TGF-β1 transgenic mice. Science China 10: 1042-1047.
- Wu H, Xie J, Li GN, et al. (2015) Possible involvement of TGF-β/periostin in fibrosis of right atrial appendages in patients with atrial fibrillation. Int J Clin Exp Pathol 8: 6859-6869.
- Pietras K, Pahler J, Bergers G, et al. (2008) Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med 5: e19.
- Fan B, Ma L, Li Q, et al. (2013) Role of PDGFs/PDGFRs signaling pathway in myocardial fibrosis of DOCA/salt hypertensive rats. Int J Clin Exp Pathol 7: 16-27.
- Pandey P, Khan F, Upadhyay TK, et al. (2023) New insights about the PDGF/PDGFR signaling pathway as a promising target to develop cancer therapeutic strategies, Biomedicine & Pharmacotherapy 161: 114491.
- Li L, Wu D, Qin X, et al. (2022) Prodomain Differentially Inhibits the Biological Activities of PDGF-D and PDGF-B. J Mol Biol 434: 167709.
- Wang Z, Kong D, Li Y, et al. 2009 PDGF-D signaling: a novel target in cancer therapy. Curr Drug Targets 10: 38-41.
- Moore K, Fulmer D, Guo L, et al. (2021) PDGFRα: Expression and Function during Mitral Valve Morphogenesis. J Cardiovasc Dev Dis 8: 28.
- Horikawa S, Ishii Y, Hamashima T, et al. (2015) PDGFRα plays a crucial role in connective tissue remodeling. Sci Rep 5: 17948.
- Kallergis EM, Manios EG, Kanoupakis EM, et al. (2008) Extracellular matrix alterations in patients with paroxysmal and persistent atrial fibrillation: Biochemical assessment of collagen type-I turnover. J Am Coll Cardiol 52: 211-215.
- Xu GJ, Gan TY, Tang BP, et al. (2009) Differential expression of collagen and matrix metalloproteinases between left and right atria in patients with chronic atrial fibrillation. Sheng Li Xue Bao 61: 211-216.
- Bodagh N, Williams MC, Vickneson K, et al. (2023) State of the art paper: Cardiac computed tomography of the left atrium in atrial fibrillation. J Cardiovasc Comput Tomogr 17: 166-176.
- Tops LF, Bax JJ, Zeppenfeld K, et al. (2006) Effect of radiofrequency catheter ablation for atrial fibrillation on left atrial cavity size. Am J Cardiol 97: 1220-1222.
- Zou T, Chen Q, Chen C, et al. (2022) Moricizine prevents atrial fibrillation by late sodium current inhibition in atrial myocytes. J Thorac Dis 14: 2187-2200.
- Krishnan A, Chilton E, Raman J, et al. (2021) Are interactions between epicardial adipose tissue, cardiac fibroblasts and cardiac myocytes instrumental in atrial fibrosis and atrial fibrillation? Cells 21: 2501.
Corresponding Author
Junqiang Pan, Department of Cardiology, Xi'an Central Hospital, Xi'an, China.
Copyright
© 2023 Zhao R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.