The Impact of Gender on In-Hospital Outcome after Elective Percutaneous Coronary Intervention: A Comparative Single Center Audit
Abstract
Background: In the literatures, there is still argument regarding whether female gender is correlated with worse outcome following percutaneous coronary intervention (PCI). In recent years, using of PCI has steadily increased in Yemen. However, the impact of gender on the clinical outcome following PCI with 2 nd generation drug-eluting stents is still unknown in Yemeni patients.
Aims : To compare in-hospital outcome according to gender in Yemeni patients with coronary artery disease who underwent elective PCI at Military hospital, Sana’a City, Yemen.
Methods and results : We analyzed data from 316 consecutive patients who underwent elective PCI procedures from January 2021 to December 2021 at the Military hospital in Sana’a City, Yemen. Data were collected retrospectively through medical records, and computerized database of the hospital. An overall of 45 (45.9%) were women. Men were nearly 3 years younger than women (56.7 ± 11 vs. 60 ± 9.3 years respectively; p-value, 0.005). The prevalence of smoking and previous IHD was more common in men than women (p-value, < 0.005) and in men more stents were implanted. By contrast, women were more frequently had obesity, dyslipidemia, diabetes mellitus, and hypertension (p-value, < 0.005). Likewise, incidence of coronary artery dissection, bleeding, and renal insufficiency following PCI was more common in women than men (p-value, < 0.005). Using multivariate logistic regression analysis, we found that female gender (adjusted odd ratio (AOR), 2.26; 95% confidence interval (CI), 1.05-5.43; p-value, 0.032) and dyslipidemia (AOR, 2.22; 95% CI, 1.05-4.66; p-value = 0.036) were independent predictors of in-hospital bleeding.
Conclusion : Our study sheds additional light on the gender advantage enjoyed by males in PCI compared to females. Women were more likely to more experience in-hospital bleeding, coronary artery dissection and renal insufficiency complications following PCI. Female gender and dyslipidemia were independent predictors of in-hospital bleeding. It is important to evaluate each patient's individual risk factors for bleeding before undergoing PCI, including dyslipidemia, and to take appropriate measures to minimize bleeding complications, especially in women.
Keywords
Coronary artery diseases, Percutaneous coronary intervention, Gender diversities, Sex differences, Yemen
Introduction
With an increase in the burden of coronary artery disease (CAD), the percutaneous coronary intervention (PCI) has emerged as one of the most effective treatments for CAD [1]. Globally, it has been successfully utilized in clinical application for more than 40 years, and it has undergone ongoing improvement [2].
Recently, due to the ongoing development of drug-eluting stents, drugs, equipment, and interventional technology, treatment indications have steadily expanded. Nowadays, for high-risk indicated patient sessions, many heart centers are able to handle full revascularization, and the efficacy has considerably improved.
Several recent research have revealed that there is gender-related disparity in the clinical prognosis of patients with CAD following PCI, which are affected by many associated risk factors of gender diversities [2-6]. However, several reports have been inconsistent in demonstrating a reduction in the gender gap in hospital outcome after PCI. Increased mortality [5] and significantly worse early and late outcome [2,4,7,8] are associated with female gender.
The cause of female sex specific high risk is supposed to be multifactorial. Women undergoing PCI are frequently delayed in diagnosis and treatment, are older age, have larger burden of co morbidities, and have atypical clinical presentation [9-15]. In addition, the smaller size of coronary arteries in females makes percutaneous procedures more challenging in general [16].
In this study, we aimed to compare in-hospital outcome after elective PCI in men and women admitted to a military hospital, Sana’a City, Yemen.
Patients and Methods
Study population
This study was single-center, retrospective, observational study. From the hospital chart, we identified all consecutive patients with CAD who underwent elective PCI procedures at military hospital in Sana’a City, Yemen, between January 2021 and December 2021. We included ischemic heart disease (IHD) patients whom aged > 20 and ≤ 85 years and underwent elective PCI. Patients who underwent only diagnostic coronary artery angiography, referred to coronary artery bypass grafting, underwent primary PCI, with sepsis, active cancer, and Covid-19, cardiac arrest prior to PCI, or those with a missing hospital chart were excluded from analysis.
Data source and variables
From computerized database and medical records of the hospital, we identified all consecutive patients with CAD who underwent an elective PCI ate military hospital in Sana’a City, Yemen. We collected all data including demographic-related (gender and age), associated risk factors (obesity as body mass index (BMI) > 30 kg/m 2 , and smoking), co morbidities (diabetes mellitus (DM), hypertension (HTN), peripheral vascular disease (PVD) and chronic kidney disease (CKD), biochemical investigations in both pre and post procedure (hemoglobin (HB), white blood cells (WBC), platelets (PLT), and serum creatinine (sCr), as well as ejection fraction (EF), parameters of angiography (number of coronary artery narrowed, and number of stents implanted), and complications (major adverse cardiac events (MACE), cardiogenic shock, hypotension, new myocardial infarction (MI), arrhythmia, coronary dissection, bleeding, contrast allergy, renal insufficiency, and in hospital mortality.
Interventional procedures and antiplatelet therapy
At a military hospital, all cardiac Catha interventions are carried out by highly skilled, qualified and experienced cardiologists. Patient preparation begins the day before procedure by investigation and medication like loading dose Clopidogrel (600 mg) and Aspirin (325-500 mg). In all our patients, coronary angiography was performed with phlips Catha Lab Equipment. A minimum of two orthogonal views were obtained for each vessel. Visual coronary analysis was used for quantification of coronary lesions. All the angiograms were evaluated by two separate cardiologists and all the discrepancies were sorted out before finalizing the reports. The angiographic pattern of coronary arteries was classified according to the number of major coronary arteries involved. It was called single, double, triple vessel disease when one, two or three major coronary arteries were with > 50% luminal stenos is, respectively. The arterial segments were seen in such views that have minimal overlapping or foreshortening of the lesions. In all cases, drug eluting stents (zotarolimus eluting stents) were tried to be implanted, and the arteriotomy site was the femoral artery and trans-femoral Intervention was the access route of choice.
Operational definitions
We defined an elective procedure as the absence of MI in the two weeks prior to the intervention. “History of smoking is defined as being a smoker of any amount of any type (cigarette, water pipe, cigar, and pipe) before index PCI” [17]. Dyslipidemia is defined based on concentrations of low-density lipoprotein cholesterol (LDL-C) and non-high-density lipoprotein cholesterol (non-HDL-C) (130 and 160 mg/dL, or 3.4 and 4.1 mmol/L, respectively) [18], and patients who used lipid-lowering agent were considered as hyperlipidemic patients. Patients were considered hypertensive if systolic blood pressure (SBP) ≥ 140 and/or diastolic blood pressure (DBP) ≥ 90 mmHg or if they were taking antihypertensive(s) or changing their lifestyle to manage HTN [19]. These criteria set the following as values that are indicative of DM: Glycosylated hemoglobin (HbA1C) ≥ 6.5%, fasting blood sugar (FBS) ≥ 126 mg/dl (7.0 mmol/l), 2-hours plasma glucose ≥ 200 mg/dl (11.1 mmol/l) during an oral glucose tolerance test, random plasma glucose ≥ 200 mg/dl (11.1 mmol/l), or receiving anti-hyperglycemic agents [20]. The Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries (GUSTO) criteria were used to define incidence of bleeding [8]. MACE included procedure-related deaths, renal failure, MI, stent thrombosis, bleeding complications, ischemic target vessel revascularization, and stroke.
Statistical analysis
The statistical package for the social sciences (SPSS) version 28.0 was used for data analysis (SPSS, Inc., Chicago, Illinois, USA). A Shapiro-Wilk test was used for normality test of the continuous data. Person’s x 2 test was used to compare between categorical variables; whenever any of the expected values were less than 5, Fisher’s exact test was used instead. Continuous normally distributed variables were expressed as mean ± standard deviation (SD) and Student’s t-test was used for comparison, while non-normal distributions were represented by the median associated with the range (minimum-maximum) and Mann-Whitney U test was used for comparison. Previous research have indicated that, up to the age of 50 to 60, women are more likely than men to experience worse in-hospital outcomes [4,21,22]. Therefore, for gender comparisons, logistic regression was used to calculate age-adjusted odds ratio (OR) with 95% confidence interval (CI) as a linear variable. Then again, the Person’s x 2 test was used to calculate OR for in-hospital bleeding. In order to determine independent predictors of in-hospital bleeding, multivariate logistic regression was then performed. Based on univariate p-values of < 0.05, included variables in multivariate logistic regression were selected. These variables included age, gender, smoking status, dyslipidemia, BMI > 30, HTN, DM, PVD, and CKD. The results were regarded as significant when the p-value was ≥ 0.05.
Results
Demographic data, co morbidities and associated risk factors are presented in the Table 1. We included a total of 316 consecutive patients, 171 (54.1%) were male’s and 145 (45.9%) were females. Mean age was 58 ± 10.3 years. HTN was the most common risk factors in 165 (52.2%) patients, followed by smoking in 153 (48.4%) patients, dyslipidemia in 151 (47.8%) patients, previous history of IHD in 143 (45.3%) patients, DM in 132 (41.8%) patients and obesity in 70 (22.2%).
Regarding the comparison between both genders, men were nearly 3 years younger than women. In men, smoking, and previous history of IHD was more common than in women. By contrast, women were more frequently had obesity, dyslipidemia, DM, and HTN. There were no significant differences between both genders with respect to the PVD and CKD.
As shown in the Table 2, both pre and post HB were significantly higher in men (p-value, < 0.001). On the other hand, pre and post sCr were significantly higher in women (p-value, 0.000 and 0.005 respectively). However, there were no significant between men and women in other variables.
Procedural data and in-hospital outcome are presented in the Table 3. Men were more likely to have had higher number of stents implanted per patient (p-value, 0.001). By contrast, women were more likely to have had higher coronary artery dissection (p-value, 0.028), bleeding (p-value, 0.013), and renal insufficiency (p-value, 0.049). Number of coronary artery narrowed, overall complications, MACE, cardiogenic shock, hypotension, new MI, arrhythmia, contrast allergy and mortality in hospital were not different between both genders (p-value ≥ 0.05).
The results of age-adjusted logistic regression analysis are shown in the Table 4. Women were 2.3 (CI, 1.09-4.81; p-value, 0.031), 5.9 (CI, 1.21-29.37; p-value, 0.029) and 2.3 (CI, 1.08-4.78; p-value, 0.030) times more likely to have coronary artery dissection, and renal insufficiency complications respectively during hospitalization.
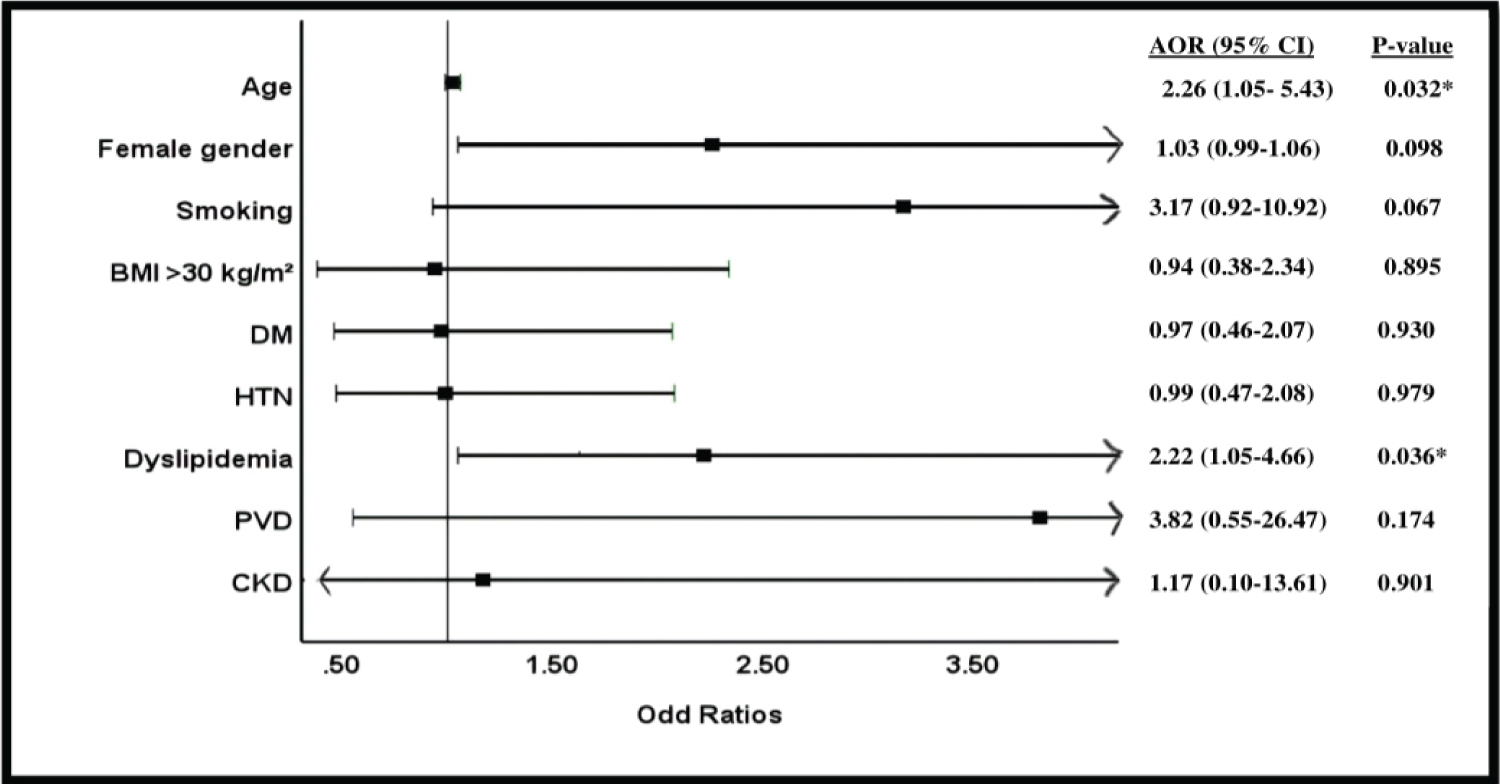
The results of multivariate logistic regression analysis on in-hospital bleeding are shown in the Table 5 and Figure 1. After adjustment for potential confounding factors, which included demographics, associated risk factors, and comorbidities variables in the total our cohort, we found that female gender (adjusted odd ratio (AOR), 2.26; 95% CI, 1.05-5.43; p-value, 0.032) and dyslipidemia (AOR, 2.22; 95% CI, 1.05-4.66; p-value = 0.036) were independent predictors of in-hospital bleeding.
Discussion
Apparently, our study is the first study examining the impact of gender on in-hospital outcome after an elective PCI in Yemeni populations. Our study came to two important findings. First, with the exception of no gender-related differences in the incidence of MACE and overall complications, the higher incidence of in-hospital bleeding, coronary artery dissection and renal insufficiency complications in women were observed. Second, female gender, and dyslipidemia were independent predictors of bleeding complications in hospital.
In fact, women patients with CAD vary from men patients. In agreement with our study, several previous earlier research revealed that compared to men, women were older, and had a higher prevalence of co morbidities such as HTN, and DM [4,7,16,23-27]. The diversities in the epidemiological data and the progression of IHD between men and women have inspired many endeavors to explain this phenomenon. The delayed development of IHD in women may be explained by the protective action of estrogens (by 10 years on average) [28]. A recent systematic review and meta-analysis of six studies with a total of 6515 people found that the difference was by 2.8 years [2]. In our patients, women were older than men with a difference of 3.3 years. This finding corroborates with the other recent studies done in different settings in the world [6-8,23-26]. The higher reported difference was 8.2 years in South Korea, whereas the lower reported difference was 2 years in Poland as showcased in [7,24] respectively. This diversity is explained by psychological variability and women's greater emotional symptom display. It is also possible that females were referred for PCI at that time later than males were, following the start of symptoms. Moreover, the effects of baseline risk factors for IHD vary between women and men (tobacco use, DM, HTN, low HDL-C levels, and higher triglyceride levels all have a more pronounced effect on women) [16].
Not surprisingly, men in our study had higher average of stents implanted per patient, as this result was reported by several previous research from different geographical regions in the world [21,27].
In concordance with other studies, incidence of bleeding [4,7,8,23] and renal insufficiency [4,27,29] in women was higher than their men counterparts. Notably, access route was femoral in all of our patients. Jibran, et al., revealed that false aneurysm can lead to a higher incidence of bleeding [3]. Another study found that femoral pseudo aneurysm was reported more frequently in women patients [30]. The increased risk of bleeding in women has been attributed to possible anticoagulant or antiplatelet overdoses, variations in platelet biology, and other factors [31-33].
The patient's weight determines the glomerular filtration rate, which is often lower in women than in men, despite the same serum creatinine levels; pharmacokinetic disparities between both genders may be impacted by reduced renal function. Therefore, the effect of contrast using may have more damaging in women than in men. According to Alexander, et al., a higher risk of in-hospital bleeding in women is related to more likely of them to receive excess doses of glycoprotein IIb/IIIa inhibitors and antithrombotic agents [31,33].
Although drugs of glycoprotein IIb/IIIa inhibitors (e.g. tirofiban and eptifibatide) are not available in Yemen, other anticoagulants and antiplatelets have comparable pharmacological effects [31,33,34]. Of note, during the procedure, all patients in our study underwent PCI with a uniform regimen of anticoagulants and antiplatelets. Therefore, this uniform regimen of anticoagulants and antiplatelets in both sexes may impact disparities in bleeding during hospitalization.
Likewise, our study revealed that the incidence of coronary artery dissection was significantly higher in women than men, which is in line with study findings done in Japan [4]. In terms of anatomy, body size and arteries in women are smaller than men's [35]. As seen in our study, smaller arteries may contribute to increased rates of vascular injury and bleeding complications, such as coronary artery dissection [21,36]. The endothelial layer of blood arteries in women patients is more vulnerable than that in men patients, which is an essential factor in the higher rates of vascular events in patients who underwent PCI [21]. During the procedure, women are more likely to experience vascular injury, due to difficult processing stenting to treat coronary artery dissections, which can occasionally lead to a serious situation with no blood or slow flow in the coronary artery that injured [4].
In agreement with other studies, our study showed that there were no significant differences between both genders towards MACE [4,23,25,26] and overall complications in-hospital [23,24].
In our study, we also used analysis of logistic regression to identify independent predictors of in-hospital bleeding. We observed that female gender [4,7,8,23,37], and dyslipidemia were independent predictors of in-hospital bleeding. There is some evidence to suggest that there is an association between dyslipidemia and bleeding following PCI. However, the findings are not consistent, and the current evidence is inconclusive. A study in Japan found that in univariate analysis there was an association between bleeding and dyslipidemia, but this association has disappeared in multivariate analysis, although this study did find that other factors such as age, female gender, previous PCI, and previous HF were independent predictors of bleeding [37]. By contrast, a recent study published in the European Heart Journal found that a threshold value of LDL-C < 70 mg/dL was associated with an increased risk for major bleeds [38]. Dyslipidemia can cause changes in platelet function, coagulation, and fibrinolysis, which can increase the risk of bleeding. Therefore, is important for healthcare providers to carefully evaluate each patient's individual risk factors for bleeding and to take appropriate measures to minimize bleeding complications. Overall, the relationship between dyslipidemia and bleeding following PCI is not entirely clear, and more research is needed to fully understand the relationship between these factors.
Finally, we have observed an alarming prevalence of associated risk factors of CAD, such as HTN, smoking, DM, and dyslipidemia, which is similar to what was previously reported in Yemen [39].
Limitations
It must be acknowledged that our study has several limitations. Firstly, it is a retrospective of a single center study, therefore has an inherent selection bias due to its observational design. Secondly, due to the nature of the review-based study, and we relied on the hospital chart, records of cardiac catheterization, and laboratory, our study may miss independent adjudication of outcomes during hospitalization. Thirdly, we included only elective procedures, limiting our findings generalizability to both rescue and emergent procedures. Lastly, extremely low events in hospital restricted generating the survival curve in our study. It is necessary to do a sizable, prospective, and multicenter study, and this is our endeavor in the future.
Conclusion
Our study sheds additional light on the gender advantage enjoyed by males in PCI compared to females. Despite no gender-related disparities in the incidence of MACE and overall complications were observed, women were more likely to more experience bleeding, coronary artery dissection and renal insufficiency complications following PCI. Female gender and dyslipidemia were independent predictors of in-hospital bleeding. It is important to evaluate each patient's individual risk factors for bleeding before undergoing PCI, including dyslipidemia, and to take appropriate measures to minimize bleeding complications. The use of closure devices, a preference for radial artery access, and avoiding the improper use of glycoprotein inhibitors are all bleeding prevention strategies that should be taken into consideration, particularly in women.
Information Disclosure
Obtaining informed consent was not necessary since the data collection was based on retrospective chart review. Besides, and in accordance with the Declaration of Helsinki, our study was approved by administration and Research Ethic Committee of the military hospital and head of cardiac department and clinical service director of the hospital gave their consent for access to patient charts. Every effort was made to maintain confidentiality throughout the study's duration and after it was over by de-identifying and storing all other personal and medical data separately. Additionally, only the investigators handled the data obtained throughout the study, and data were analyzed in aggregates.
Acknowledgment
The authors extend their appreciation to the military hospital administration, Sana’a City, Yemen represented in Dr. Abbas Najemaldeen and Dr. Ali Alshami, who facilitated data collection process. In addition, the authors appreciate the time and assistance provided by the study participants, data collectors, and hospital record room staff in facilitating the data collection process. The authors also thank Professor Ahmed Kaid Salem for his expertise and careful advising.
References
- Yaya G, YanPing B, Yan G, et al. (2020) Gender differences in clinical outcomes of patients with coronary artery disease after percutaneous coronary intervention. Cardiac Diseases. Rijeka: IntechOpen.
- Thandra A, Jhand A, Guddeti R, et al. (2021) Sex differences in clinical outcomes following percutaneous coronary intervention of unprotected left main coronary artery: A systematic review and meta-analysis. Cardiovascular Revascularization Medicine 28: 25-31.
- Jibran R, Khan JA, Hoye A, et al. (2010) Gender disparity in patients undergoing percutaneous coronary intervention for acute coronary syndromes-does it still exist in contemporary practice. Ann Acad Med Singapore 39: 173-178.
- Numasawa Y, Kohsaka S, Miyata H, et al. (2015) Gender differences in in-hospital clinical outcomes after percutaneous coronary interventions: An insight from a Japanese multicenter registry. PLoS One 10: e0116496.
- Ndrepepa G, Kufner S, Mayer K, et al. (2019) Sex differences in the outcome after percutaneous coronary intervention–a propensity matching analysis. Cardiovascular Revascularization Medicine 20: 101-107.
- Lee SH, Choi J, Chang YJ, et al. (2021) Sex difference in long-term clinical outcomes after percutaneous coronary intervention: A propensity-matched analysis of National Health Insurance data in Republic of Korea. Catheterization and Cardiovascular Interventions 98: E171-E80.
- Kim H-L, Jang J-S, Kim M-A, et al. (2019) Gender differences of in-hospital outcomes in patients undergoing percutaneous coronary intervention in the drug-eluting stent era. Medicine 98: e15557.
- Long J, Zeng F, Wang L, et al. (2020) Gender-specific cardiovascular outcomes in patients undergoing percutaneous coronary intervention in Chinese populations. BMC Cardiovascular Disorders 20: 1-7.
- Epps KC, Holper EM, Selzer F, et al. (2016) Sex differences in outcomes following percutaneous coronary intervention according to age. Circulation: Cardiovascular Quality and Outcomes. 9: S16-S25.
- Kanic V, Vollrath M, Tapajner A, et al. (2017) Sex-related 30-day and long-term mortality in acute myocardial infarction patients treated with percutaneous coronary intervention. Journal of Women's Health 26: 374-379.
- Berry NC, Kereiakes DJ, Yeh RW, et al. (2018) Benefit and risk of prolonged DAPT after coronary stenting in women: results from the DAPT Study. Circulation: Cardiovascular Interventions 11: e005308.
- Potts J, Sirker A, Martinez SC, et al. (2018) Persistent sex disparities in clinical outcomes with percutaneous coronary intervention: Insights from 6.6 million PCI procedures in the United States. PLoS One 13: e0203325.
- Cenko E, Yoon J, Kedev S, et al. (2018) Sex differences in outcomes after STEMI: Effect modification by treatment strategy and age. JAMA Internal Medicine 178: 632-639.
- Raphael CE, Singh M, Bell M, et al. (2018) Sex differences in long-term cause-specific mortality after percutaneous coronary intervention: Temporal trends and mechanisms. Circulation: Cardiovascular Interventions 11: e006062.
- Chichareon P, Modolo R, Kerkmeijer L, et al. (2020) Association of sex with outcomes in patients undergoing percutaneous coronary intervention: A subgroup analysis of the GLOBAL LEADERS randomized clinical trial. JAMA Cardiology 5: 21-29.
- Nowakowska-Arendt A, Grabczewska Z, Kozinski M, et al. (2008) Original article Gender differences and in-hospital mortality in patients undergoing percutaneous coronary interventions. Cardiologic Polska (Polish Heart Journal) 66: 632-329.
- Mohammadi SS, Zibaeenezhad MJ, Sayadi M, et al. (2021) The Impact of Smoking on Clinical Outcomes after Percutaneous Coronary Intervention in Women Compared to Men. Journal of Interventional Cardiology 2021: 6619503.
- Civeira F, Arca M, Cenarro A, et al. (2022) A mechanism-based operational definition and classification of hypercholesterolemia. Journal of Clinical Lipidology 16: 813-821.
- Williams B, Mancia G, Spiering W, et al. (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). European Heart Journal 39: 3021-3104.
- Benzadón M, Forti L, Sinay I, et al. (2014) Actualización en el diagnóstico de la diabetes. Medicina (Buenos Aires) 74: 1.
- Argulian E, Patel AD, Abramson JL, et al. (2006) Gender differences in short-term cardiovascular outcomes after percutaneous coronary interventions. The American Journal of Cardiology 98: 48-53.
- Abramson JL, Veledar E, Weintraub WS, et al. (2003) Association between gender and in-hospital mortality after percutaneous coronary intervention according to age. American Journal of Cardiology 91: 968-971.
- Jarrah MI, Hammoudeh AJ, Al-Natour DB, et al. (2017) Gender differences in risk profile and outcome of Middle Eastern patients undergoing percutaneous coronary intervention. Saudi Medical Journal 38: 149-155.
- Skorupski W, Grygier M, Grajek S, et al. (2020) Is female gender associated with worse outcomes after percutaneous coronary intervention of left main coronary artery? European Heart Journal 41: ehaa946. 3168.
- Guo L, Lv H, Zhong L, et al. (2019) Gender differences in long-term outcomes of medical therapy and successful percutaneous coronary intervention for coronary chronic total occlusions. Journal of Interventional Cardiology 2019: 2017958.
- Josiah A, Farshid A (2019) Gender is not a predictor of mortality or major adverse cardiovascular events in patients undergoing percutaneous coronary intervention for acute coronary syndromes. Heart, Lung and Circulation 28: 727-734.
- Lempereur M, Magne J, Cornelis K, et al. (2014) Impact of gender difference in hospital outcomes following percutaneous coronary intervention. Results of the Belgian Working Group on Interventional Cardiology (BWGIC) registry. Euro Intervention: Journal of EuroPCR in Collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 12: e216-e223.
- Barrett-Connor E (1997) Sex differences in coronary heart disease: why are women so superior? The 1995 Ancel Keys Lecture. Circulation 95: 252-264.
- Desai R, Singh S, Fong HK, et al. (2019) Racial and sex disparities in resource utilization and outcomes of multi-vessel percutaneous coronary interventions (a 5-year nationwide evaluation in the United States). Cardiovascular Diagnosis and Therapy 9: 18-29.
- Erol F, Arslan S, Yüksel IÖ, et al. (2015) Determinants of iatrogenic femoral pseudoaneurysm after cardiac catheterization or percutaneous coronary intervention via the femoral artery. Turk Kardiyol Dern Ars 43: 513-519.
- Alexander KP, Chen AY, Newby LK, et al. (2006) Sex differences in major bleeding with glycoprotein IIb/IIIa inhibitors: results from the CRUSADE (Can Rapid risk stratification of unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) initiative. Circulation 114: 1380-1387.
- Ahmed B, Dauerman HL (2013) Women, bleeding, and coronary intervention. Circulation 127: 641-649.
- Alexander KP, Chen AY, Roe MT, et al. (2005) Excess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromes. JAMA 294: 3108-3116.
- Cho L, Topol EJ, Balog C, et al. (2000) Clinical benefit of glycoprotein IIb/IIIa blockade with abciximab is independent of gender: Pooled analysis from EPIC, EPILOG and EPISTENT trials. Journal of the American College of Cardiology 36: 381-386.
- Numasawa Y, Kawamura A, Kohsaka S, et al. (2014) Anatomical variations affect radial artery spasm and procedural achievement of transradial cardiac catheterization. Heart and Vessels 29: 49-57.
- Ashby DT, Mehran R, Aymong EA, et al. (2003) Comparison of outcomes in men versus women having percutaneous coronary interventions in small coronary arteries. American Journal of Cardiology 91: 979-981.
- Numasawa Y, Kohsaka S, Ueda I, et al. (2017) Incidence and predictors of bleeding complications after percutaneous coronary intervention. Journal of Cardiology 69: 272-279.
- Yang Q, Sun D, Pei C, et al. (2021) LDL cholesterol levels and in-hospital bleeding in patients on high-intensity antithrombotic therapy: Findings from the CCC-ACS project. European Heart Journal 42: 3175-3186.
- Al-Furasi H, Alzendany A, Salem AK, et al. (2021) Predictors of Major Adverse Cardiac Events After Percutaneous Coronary Intervention in Sana’a City-Yemen (Single center study). Al-Razi University Journal for Medical Sciences.
Corresponding Author
Abdulhafeedh Al-Habeet, Shaphaco Pharmaceutical Industries, Sana'a City, Yemen.
Copyright
© 2023 Almaimony T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.