Acute Spontaneous Pericardial Hemorrhage Causing Tamponade 1 Week after COVID-19 Vaccination: A Case Study
Abstract
A 74-year-old male with history of paroxysmal atrial fibrillation started on Apixaban in 2017, hypertension, coronary artery disease without angiography, left ventricular hypertrophy, obesity and gout presented to the emergency department after several episodes of dyspnea and abdominal pain. He obtained his Johnson and Johnson COVID-19 vaccination 1-week prior and directly before symptoms began. Upon presentation, he was in hypoxemic respiratory failure requiring non-invasive positive pressure ventilation with Bi-level positive airway pressure. A point-of-care bedside echocardiogram demonstrated acute pericardial effusion complicated by cardiac tamponade physiology. An emergent pericardiocentesis was completed. Roughly 1 liter of sanguineous fluid was obtained. Anticoagulation was discontinued until follow up and the patient made a complete recovery without recurrence.
Cardiac tamponade is a cardiovascular emergency which requires prompt therapeutic intervention. Spontaneous hemopericardium leading to tamponade has been recorded when utilizing direct oral anticoagulants (DOAC). However, it has not been recorded after obtaining a first dose of the COVID-19 mRNA vaccination. Our patient underwent an initial dose of COVID-19 mRNA vaccination one week prior to presenting to the emergency department with cardiac tamponade from spontaneous hemopericardium.
Our case represents a possible medical anomaly. With the effects of COVID-19 on the hematologic processes and its extensive cytokine storm, COVID-19 causes a rapid coagulopathy. The vaccine may have an effect within the coagulation cascade which has yet to be discovered. More dedicated research to understanding miniscule details within the cascade or platelet function may be required to truly diagnose an acute hemopericardium secondary to a COVID-19 vaccination.
Introduction
Cardiac tamponade is the increase of intrapericardial pressure greater than intracardiac pressure, progressing to reduced diastolic ventricular filling and thus reduced stroke volume and cardiac output [1]. Cardiac tamponade is a cardiovascular emergency which requires prompt therapeutic intervention [1]. The more common etiology stems from pericarditis (infectious and non-infectious), iatrogenic from cardiac procedures, and neoplasms [2]. However, spontaneous hemopericardium leading to tamponade has been recorded when utilizing direct oral anticoagulants (DOAC) [3]. However, it has not been recorded after obtaining a first dose of the COVID-19 mRNA vaccination. Our patient underwent an initial dose of COVID-19 mRNA vaccination one week prior to presenting to the emergency department with cardiac tamponade from spontaneous hemopericardium.
Case
A 74-year-old male with history of paroxysmal atrial fibrillation started on Apixaban in 2017, hypertension, coronary artery disease without angiography, left ventricular hypertrophy, obesity and gout presented to the emergency department after having several episodes of shortness of breath and abdominal pain. Over a 1-week period he had noted increased abdominal distension with shortness of breath and mild dyspnea on exertion. Patient also complained of reduced urine output for 2 days prior to presentation. Of note, he obtained his Johnson and Johnson COVID-19 vaccination 1-week prior to presentation and directly before symptoms began. He denied prior bleeding complications.
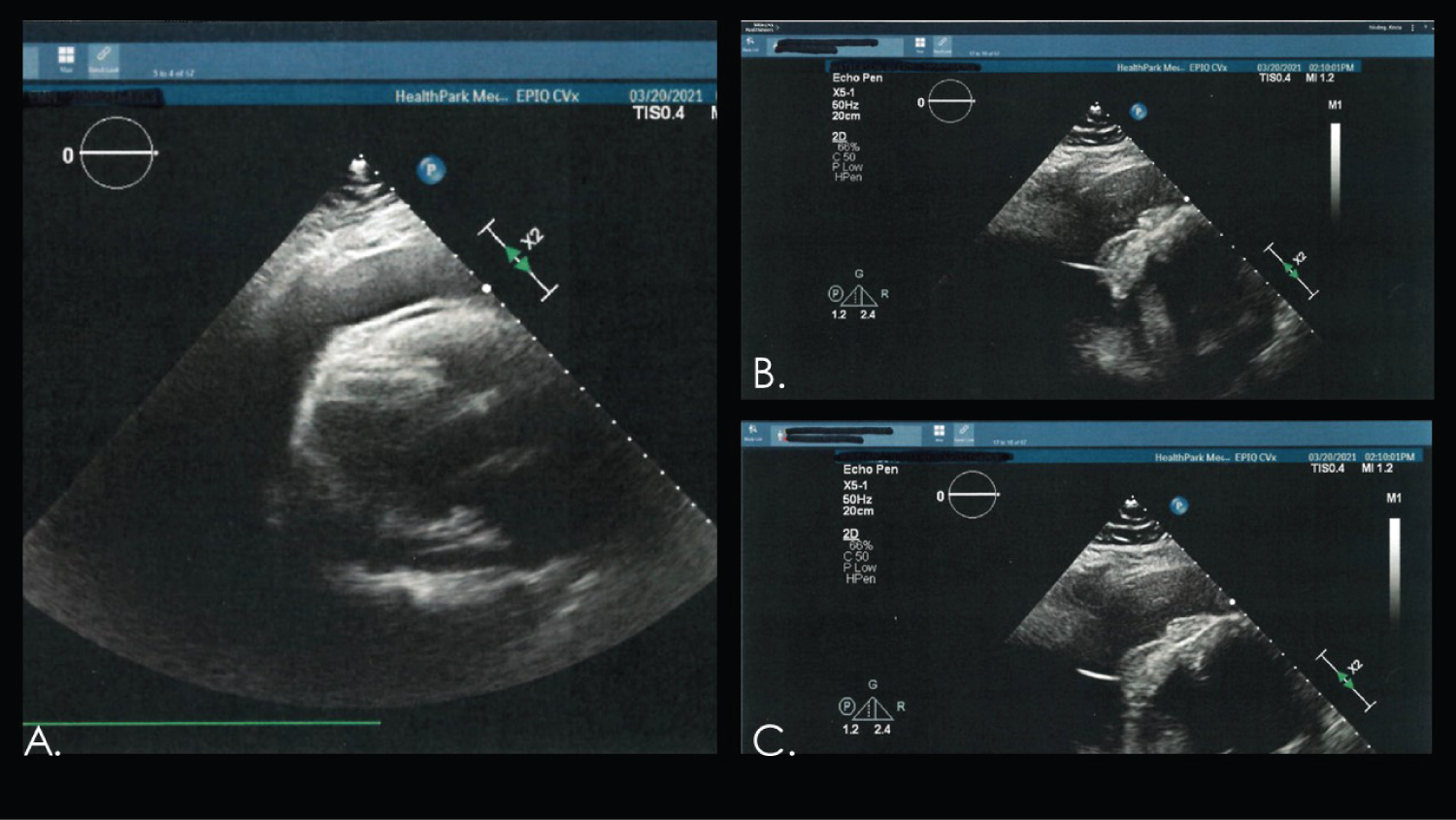
Upon presentation the patient was hemodynamically stable. However, he was in hypoxemic respiratory failure requiring non-invasive positive pressure ventilation with Bi-level positive airway pressure. Initial laboratory studies and chemistries were obtained. An electrocardiogram was obtained, however was unable to found upon chart review. A point-of-care bedside echocardiogram demonstrated acute pericardial effusion with tamponade physiology. An emergent pericardiocentesis ensued (Figure 1). Roughly 1 liter of sanguineous fluid was obtained. Patient required temporary hemodialysis for hyperkalemia and acute pre-renal azotemia. Anticoagulation was discontinued until follow up patient’s cardiologist.
The expected outcome is complete resolution of transmural gradient after pericardial decompression with pericardiocentesis. After repeating the echocardiogram, patient requires close monitoring and transition to another anticoagulant.
Our patient made a complete recovery. He was discharged from the hospital without anticoagulation until follow up with his cardiologist. Patient is being considered for left atrial appendage closure device and is continuing his rhythm control strategy with amiodarone. Repeat echocardiogram on April 8, 2021, demonstrated no recurring pericardial effusion or tamponade physiology. No further hemorrhagic events have occurred.
Discussion
Anticoagulation is the standard of care treatment for thromboembolic diseases and disorders [3]. DOACs and warfarin have been shown to reduce morbidity and mortality when treating these conditions [3]. However, DOAC class has been shown to reduce the risk of major bleeding over vitamin K inhibitors (warfarin) by up to 31% and is now considered superior in most cases [3,4]. Spontaneous hemopericardium has been noted with the utilization of apixaban [4].
Spontaneous hemopericardia or mediastinum is a rare but known complication of direct oral anticoagulation therapy [5]. Though anticoagulants are necessary to multiple disease and disorder processes, they carry the risk of increased hemorrhage [3]. Managing patients with spontaneous hemorrhage while taking DOAC is complex as there are no tests to determine the degree of which the DOAC is affecting the patient [3]. In patients taking DOAC, the rate of fatal hemorrhage ranges from 0.06% to 0.30% for non-major bleed and from 1.1% to 4% in major bleeding events [3]. The pathophysiology of spontaneous hemorrhage stems from the interference of the normal hemostatic pathway as DOACs inhibit factor Xa [3,6]. In addition to DOAC use itself, there are many patient-based risk factors which contribute to spontaneous hemorrhage [3]. Some of these risk factors are increased age, comorbidities (renal failure, liver failure, malignancy), and use of other medications [3]. The more common etiologies of hemopericardium or pericardial effusion stems from pericarditis, neoplasms, infections, acute myocardial infarctions or ischemia, aortic dissection, cardiac surgery, and trauma [4].
There is about a 2.5% to 11% chance of spontaneous hemopericardium in patients taking DOAC therapy [5]. Multiple case reports have demonstrated spontaneous hemopericardium occurred within the first 4 weeks of initiation of DOAC, one progressing to cardiac tamponade [6,7]. In another case report, a patient who had significant non-ischemic cardiomyopathy with left ventricular ejection fraction of 25% and permanent atrial fibrillation on Rivaroxaban for over 2 years presented with an acute hemopericardium and tamponade [8]. Among 5 randomized control trials, the incidence of pericardial hemorrhage with DOAC therapy was 0.05%. A systematic review by Asad, et al. confirmed this is a rare complication and clinical scenario with DOAC therapy [9]. This demonstrates there is no ideal timeline in which a hemopericardium could occur while on DOAC therapy.
Though DOAC therapy is associated with spontaneous hemopericardium, there have been no case reports of spontaneous hemopericardium after COVID-19 vaccination, even if current medications include DOAC therapy. The Pfizer and Moderna SARS-CoV-2 vaccinations are mRNA vaccines. The mechanism of the vaccine is to code for a specific spike protein which will be recognized by antigen presenting cells to create antibodies to that protein. The antibodies will attack that specific surface antigen on the outer membrane of the SARS-CoV-2 virus [10]. However, the Johnson and Johnson COVID-19 vaccine differs from the two priors as it utilizes the DNA of the adenovirus. The adenovirus DNA is modified to produce key parts of the SARS-CoV-2 virus in which the host immune system will be able to recognize and develop a subsequent response [11]. These vaccines and antibodies produced have not had cross-reactivity to other organ systems but have had cross-reactivity to other human strains of Coronavirus [12]. There is also no current documentation, during my literature review, which demonstrated an increased risk of COVID-19 mRNA or adenovirus modified DNA vaccination causing hemopericardium in patients on DOAC therapy. Thus, after just one week of obtaining dose one of an modified adenovirus DNA vaccine for COVID-19, our patient was found to have either a spontaneous hemopericardium or a hemopericardium secondary to the vaccine.
Conclusion
While more data is required to directly state the vaccine was indeed the culprit of the hemopericardium, this etiology should not be discarded lightly. Our case represents a possible medical anomaly. With the effects of COVID-19 on the hematologic processes and its extensive cytokine storm, COVID-19 causes a rapid coagulopathy [13]. The vaccine may have an effect within the coagulation cascade which has yet to be discovered. More dedicated research to understanding miniscule details within the cascade or platelet function may be required to truly diagnose an acute hemopericardium secondary to a COVID-19 vaccination.
Disclosures
None.
Funding
None.
References
- Ball JB, Morrison WL (1997) Cardiac Tamponade. Medical Emergencies. Postgrad Med J 73: 141-145.
- Jensen JK, Poulsen SH, Molgaard H (2017) Cardiac Tamponade: A clinical challenge. E-Journal of Cardiology Practice.
- Gunasekaran K, Rajasurya V, Devasahayam J, et al. (2020) A review of the incidence, diagnosis, and treatment of spontaneous hemorrhage in patients treated with direct oral anticoagulants. J Clin Med 9: 2984.
- Cinelli M, Uddin A, Duka I, et al. (2019) Spontaneous hemorrhagic pericardial and pleural effusion in a patient receiving Apixaban. Cardiol Res 10: 249-252.
- Mazziotti A, Bangash M, Maun JW (1986) Spontaneous mediastinal hemorrhage secondary to oral anticoagulation. Cardiovascular Medicine and Surgery. The Texas Heart Institute Journal.
- Mehta A, Burkland D, Mathuria N (2019) Isolated Hemopericardium after Initiation of Rivaroxaban: Implications and potential mechanism. Clinics and Practice 9: 1096.
- Kham NM, Song M (2016) Spontaneous, life-threatening hemorrhagic cardiac tamponade secondary to rivaroxaban. Am J Ther 23: e1128-e1131.
- Surani A, Quintero BM, Brual D, et al. (2019) Spontaneous hemopericardium as an adverse effect of rivaroxaban administration. Int J Clin Cardiol 6: 161.
- Singh A, Verma V, Chaudhary R (2021) Direct Oral Anticoagulants Associated Hemopericardium. ACC.
- Shahzamani K, Mahmoudian F, Ahangarzadeh S, et al. (2021) Vaccine and delivery approaches for COVID-19. Int Immunopharmacol 100: 108086.
- Livingston EH, Malani PN, Creech CB (2021) The Johnson & Johnson Vaccine for COVID-19. JAMA 325: 1575.
- Grobben M, van der Straten K, Brouwer PJ, et al. (2021) Cross-reactive antibodies after SARS-CoV-2 Infection and Vaccination. ELife 10: e70330.
- Jose RJ, Manuel A (2020) COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir Med 8: e46-e47.
Corresponding Author
Jeffrey Foley, DO, Department of Electrophysiology, University Hospitals, 704 Kobus St. Bowling Green, KY 42104, USA.
Copyright
© 2023 Foley J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.