High Density Lipoprotein as a Biomarker, Potential Therapeutic Target and Therapy
HDL, LDL and how they correlate within acute/chronic phase response
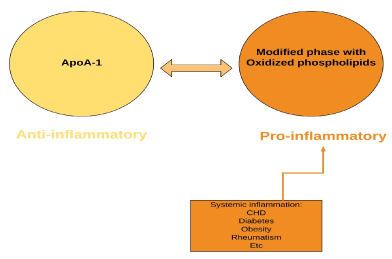
HDL is a plasma lipoprotein with the function of reverse cholesterol transport and inflammation modulation. Studies suggest that HDL can function as both anti inflammatory and proinflammatory depending on absence or presence of inflammation and chronic disease. Functions of HDL may not always match its cholesterol levels. LDL on the other hand is one of the main lipoproteins in plasma which is in charge of extracellular lipid transportation. With its major protein group being apolipoprotein B, LDL is capable of binding to extracellular matrix molecules within subendothelial spaces of arteries, causing a secretion of chemotactic agent, monocyte chemotactic protein 1 (MCP-1), which in turn triggers an inflammatory response of the type seen in atherosclerosis, all of these occur due to LDL lipid oxidation [1-3]. Such responses can be abolished by HDL due to HDL's anti inflammatory properties which prevent LDL oxidation [4].
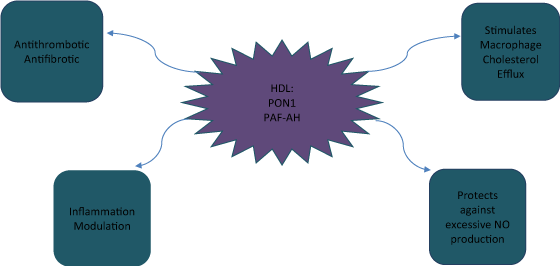
HDL's anti inflammatory properties are beneficial, however during an acute phase response, such abilities are altered since the HDL itself becomes pro inflammatory. Our group conducted a research using two groups of HDL lipoproteins from the same humans and normal rabbits; one isolated before an elective surgery and the other at the peak of an acute phase response. HDL from both groups was tested by their inflammation response on cultures of human artery wall cells. The research on HDL showed not only a shift from anti to proinflammatory during acute phase, but also a decrease in two HDL enzymes, paraoxonase-1 (PON1) and platelet activating acetylhydrolase (PAF-AH) [5]. It was concluded that these enzymes are partly responsible for inhibiting proinflammatory features of HDL [6-8].The Ability of HDL to prevent LDL induced lipid oxidation found to be a major factor in determining HDL anti-inflammatory properties [9,10] (Figure 1).
Can proinflammatory HDL be found in chronic disease?
It has been stated that a vast number of chronic disease exhibit a chronic acute phase response [11]. These responses are associated with persistent elevation of acute phase agents such as C-reactive proteins [12-14]. Examples of these diseases are diabetes, visceral abdominal obesity and a number of autoimmune diseases.
There are numerous studies regarding HDL's inflammatory properties in chronic diseases. Hedrick, et al. [15], found that under hyperglycaemic conditions in diabetes, glycation of HDL and its enzymePON1 occurs. This leads to HDL's failure to inhibit the inflammatory response induced by LDL oxidation within endothelium. The subjects studied by these investigators were individuals with either type 1 or type 2 diabetes with coronary artery disease. Comparing subjects with either abnormal glucose intolerance test or well controlled diabetes or poorly controlled diabetes with normal control group, showed that the first group had 40% reduction of PON1 activity as well as HDL's loss of anti-inflammatory abilities. It must be noted that HDL cholesterol levels were not significantly different amongst the different groups [15]. A study on south Asian immigrants with high incidence of metabolic syndrome and with a high risk of coronary heart disease, showed a correlation between HDL inflammatory properties and the thickness of carotid intima-media [16].
Persegol, et al. have studied subjects with abdominal obesity and also subjects with diabetes (both type 1 and 2) in separate studies [17-19]. They found that in comparison to normal subjects, the HDL from diabetics was unable to prevent LDL oxidation likely due to inflammation. Regardless of the type of diabetes, both types 1 and 2 had dysfunctional HDL [19,20]. A research by Cancello, et al. [21], proved a significant increase in the numbers of macrophages within omental adipose tissue when compared to subcutaneous adipose tissue. This was correlated with fasting glucose, insulin levels and insulin sensitivity as well as hepatic fibro inflammatory lesions [18]. It is concluded that macrophage cytokines play a role in insulin resistance and also in alterations in HDL inflammation properties.
An article by Roberts et al. also claims that HDL cholesterol levels cannot be reliable when it comes to detecting HDL's inflammatory properties [20]. Subjects of their studies were obese men with features of metabolic syndrome that underwent a three week diet treatment and an aerobic exercise program. These subjects showed an improvement in HDL inflammatory properties in spite of a decline in HDL cholesterol levels [20].In patients with familial hypercholesterolemia, LDL apheresis improved HDL inflammatory properties whereas HDL cholesterol levels had fallen due to this treatment [21]. HDL was found to be proinflammatory in 44.7% of women with systemic lupus erythematosus, 20.1% of women with rheumatoid arthritis (RA) and only in 4.1% of healthy women. These properties were significantly decreased in patients with RA who had taken atorvastatin with a daily dose of 80 mg [22,23].
HDL's response to inflammation can also be altered in patients with coronary heart disease (CHD). During a study, HDL from patients with CHD was found to be dysfunctional in preventing MCP-1 production and LDL oxidation in cultures of human artery cell wall compared to HDL from normal subjects. None of the subjects had abnormal lipid levels or diabetes [24].
What is HDL inflammatory index and how is it related to chronic diseases?
HDL inflammatory index (HII) was originally introduced by our group [25]. We measured the ability of LDL from normal subjects to induce MCP-1 both in the absence or in the presence of HDL in cultures of human aortic wall cell. The value in presence of LDL and absence of HDL was normalised to 1.0. The values measured in presence of both LDL and HDL were divided by the values obtained in the presence of LDL and absence of HDL. This new value was named HII. A sample with HII above 1.0 would be considered pro-inflammatory and one with HII below 1.0 would be classified as anti-inflammatory. HII of plasma samples from 26 subjects with CHD and with normal plasma lipid was calculated before and after treatment witha daily dose of 40mg simvastatin for 6 weeks [26]. HII before the medications was 1.38 plus minus 0.91 while HII after treatment was 1.08 plus-minus 0.71. A healthy Control group had HII of 0.38 plus-minus 0.14. In conclusion, HII showed a significant improvement after taking statin albeit still categorized as proinflammatory. (Above 1.0) Ansell, et al. also stated that inflammatory/anti-inflammatory properties of HDL can be more reliable in distinguishing patients from control subjects in comparison to HDL cholesterol levels [25].
Chronic renal disease is associated with chronic acute phase response, therefore 189 patients on hemodialysis were followed for 30 months and their HII was measured [26]. Patients with HII above 1.0 had a significantly higher mortality despite experiencing no difference in total cholesterol levels, LDL cholesterol levels, triglyceride levels or HDL cholesterol levels [27].
Leprosy exhibits cellular events that are similar to atherosclerosis. A study by Cruz, et al. [28], reported that HDL from patients with leprosy was less effective in promoting the conversion of monocytes into CD1B+ dendritic cells and they were found to be proinflammatory (HII > 1.0) when the results were compared to normal anti inflammatory HDL.
The HDL inflammatory properties was tested on inbred strains of mice and rabbits by our group [29,30]. It was found that HDL from atherosclerosis resistant mice was anti inflammatory which was in contrast to HDL from atherosclerosis susceptible mice [29]. We also detected a significant correlation between levels of both HII and serum amyloid A -an acute phase reactant- and with the lesion area in rabbits [29,30] (Figure 2).
HDL, Apo-J and oxidative stress
In a study, oxidised phospholipids were injected into atherosclerosis resistant or into atherosclerosis susceptible mice [31]. The susceptible group had a significant increased HDL-associated acute phase reactant, apo-J, and a significant decline in PON1 levels. There was no change in HDL properties in the resistant group [31].
According to Bhattacharyya, et al. [32], the oxidative stress enzyme, myeloperoxidase, which is present at sites of inflammation is associated with HDL and its enzyme PON1and causes oxidative damage to apoA-I. This damage results in reduced cholesterol efflux by HDL [33].
A specific tyrosine residue is found to be the preferred target for this oxidative modification [34]. Apo-E is found within HDL of CHD patients [35]. In the past, statin and niacin combination appeared to reduce the Apo-E content of HDL in CHD patients and to contribute to an HDL proteome similar to that of healthy individuals [36].
Determination of the inflammatory properties of HDL
In the absence of hemolysis, a small amount of haemoglobin (Hb) is present outside of erythrocytes and in the plasma. Based on a research conducted by Watanabe, et al. [37], an atherogenic diet resulted in association of plasma Hb with HDL in mice. Such HDL was found to be proinflammatory in mice and in humans whereas there was no increase in the concentration of plasma Hb [37,38]. The main protein responsible for such association is haptoglobin (Hp) which increases during the acute phase response. The same atherogenic diet on mice with no Hp did not develop proinflammatory HDL. Humans have 2 alleles and 3 genotypes for Hp which are 1-1, 1-2 and 2-2 [39]. Hp 2-2 paired with diabetic patient (present in 40% of diabetics) increases the risk for CHD [39]. In contrast to humans, mice only have 1 genotype for Hp which is 1-1. Levy and colleagues [39], genetically engineered mice to express 2-2 genotype. The result was that HDL impaired reverse cholesterol transport. It was reported that in both 2-2 diabetic humans and 2-2 diabetic mice HDL was unable to promote cholesterol efflux from macrophages when compared to Hp 1-1 subjects [40].
Is HDL cholesterol level a reliable predictor of risk for atherosclerosis and CHD?
For decades HDL cholesterol level was assumed to be a strong predictor of clinical events associated with atherosclerosis [41]. However many studies reported that subjects with normal levels of HDL cholesterol presented with coronary atherosclerosis and related events [29]. Briel, et al. [42], reported that the risk of CHD events and total deaths would not always be decreased simply by increasing the amounts of HDL cholesterol levels. This can be true in patients with apoA-1 Milano [43]. This mutant apoA-1 leads to decreased HDL cholesterol levels even though the patients are not in higher risk for CHD [43]. With all this taken into account, we can see that the composition, functionality and inflammatory properties of HDL are as important as its cholesterol levels when it comes to determining risk for CHD.
Can HDL be used as a therapy and therapeutic target?
In various studies, HDL and apoA-1 have been efficacious in the treatment of atherosclerosis in both animal models as well as human beings, even in diabetic subjects [44-47]. In a study, infusion of recombinant HDL particles increased AMP-activated protein kinase in skeletal muscles, increased plasma insulin and decreased plasma glucose in type 2 diabetic subjects [48]. ApoA-1 is a large protein with 243 amino acids, which makes it a difficult target for large scale production. While millions of atherosclerotic and diabetic patients would need long term treatment with apoA-1, the cost of such therapy makes it economically prohibitive.
ApoA-1 mimetic peptides may be used as a treatment for atherosclerosis
Segrest and Anantharamaiah designed an 18 amino acid residues peptide with a class A amphipathic helix which it had in common with apoA-1. This peptide was named 18A. This name was updated to 2F when 2 phenylalanine residues were added on the hydrophobic face in order to increase stability and ability to bind non oxidized lipids. The 2F failed to improve atherosclerosis in a mouse model [49-52] since oxidized lipids derived from LDL play a major role in atherosclerosis and 2F could not bind them. A number of amino acids were substituted in 2F to enhance its effectiveness. As a result, a peptide with 5 phenylalanine residues on the hydrophobic face was generated (and thus termed 5F) which significantly improved atherosclerosis in a mouse model [52,53]. A peptide with 4 phenylalanine (4F) was also effective in mouse models to improve atherosclerosis lesions [54]. After these findings, many additional studies were conducted in animal models to indicate 4F efficacy in improving inflammation-based disease such as influenza A pneumonia [55], Type 1 and 2 diabetes, and obesity [56-59]. The 4Fpeptide increased survival in septic rats by inhibiting the inflammatory response [60]. The 4F peptide was found to be synergic with statin resulting in regression of atherosclerotic lesions in mouse models [61]. Moreover, 4F was capable of increasing HDL anti inflammatory properties in the plasma from end-stage renal disease patient [62]. In all the above studies, HII was significantly improved. The 4F peptide binds oxidized lipids 5 million fold better than human apoA-1 [63]. This feature makes 4F extremely capable of removing oxidized lipids from inflamed tissue hence leading to resolution of the inflammatory changes [64].
Four F peptide administration
At first, it was observed that only peptides containing D-amino acids are effective in an oral dose since L-amino acids would be degraded by intestinal proteases [60]. Nonetheless, if oral L-amino acid peptides are combined with niclosamide [61], they will be efficacious [61]. In acidic PH,niclosamide forms a complex with 4F, protecting it from degradation [61]. If the 4F peptide was to be given by injection, it would not matter whether it is made out of the L or the D amino acid [30].
Summary
In conclusion, relying merely on cholesterol levels of HDL does not provide accurate understanding of its composition, functionality and anti inflammatory properties. Currently, there are no tests widely available to measure these features, however, it seems they are directly related to conditions inducing a chronic acute phase response (e.g., diabetes, visceral obesity CHD). Studies on HDL mimetics as therapeutic agents are in progress. Our therapeutic approach must continue to emphasise lifestyle modification, to control diseases such as diabetes, obesity, hyperlipidemia and conditions including oral inflammation.
Plus the inclusion of an appropriate use of aspirin, statins, ACE inhibitors and beta blockers for CHD patients are always recommended (Figure 3).
References
- Navab M, Berliner JA, Watson AD, et al. (1996) The yin and yang of oxidation in the development of the fatty streak: a review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler Thromb Vasc Biol 16: 831-842.
- Navab M, Berliner JA, Subbanagounder G, et al. (2001) HDL and the in?ammatory responses induced by LDL-derived oxidized phospholipids. Arterioscler Thromb Vasc Biol 21: 481-488.
- Berliner JA, Watson AD (2005) A role for oxidized phospholipids in atherosclerosis. N Engl J Med 353: 9-11.
- Navab M, Imes SS, Hama SY, et al. (1991) Monocyte transmigration induced by modi?cation of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotacticr protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest 88: 2039-2046.
- Van Lenten BJ, Hama SY, de Beer FC, et al. (1995) Anti-in?ammatory HDL becomes pro-in?ammatory during the acute phase response: Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest 96: 2758-2767.
- Watson AD, Navab M, Hama SY, et al. (1995) Effect of platelet activating factor-acetylhydrolase on the formation and action of minimally oxidized low density lipoprotein. J Clin Invest 95: 774-782.
- Watson AD, Berliner JA, Hama SY, et al. (1995) Protective effect of high density lipoprotein associated paraoxonase: Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest 96: 2882-2891.
- Watson AD, Leitinger N, Navab M, et al. (1997) Structural identi?cation by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem 272: 13597-13607.
- Navab M, Hama SY, Cooke CJ, et al. (2000) Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: Step 1. J Lipid Res 41: 1481-1494.
- Navab M, Hama SY, Anantharamaiah GM, et al. (2000) Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: Steps 2 and 3. J Lipid Res 41: 1495-1508.
- Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to in?ammation. N Engl J Med 340: 448-454.
- Ridker PM (2007) In?ammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: Implications for longevity. Nutr Rev 65: S253-S259.
- Bruno G, Fornengo P, Novelli G, et al. (2009) C-reactive protein and 5-year survival in type 2 diabetes: The Casale Monferrato Study. Diabetes 58: 926-933.
- Ridker PM (2009) C-reactive protein: Eighty years from discovery to emergence as a major risk marker for cardiovascular disease. Clin Chem 55: 209- 215.
- Hedrick CC, Thorpe SR, Fu MX, et al. (2000) Glycation impairs high-density lipoprotein function. Diabetologia 43: 312-320.
- Dodani S, Kaur R, Reddy S, et al. (2008) Can dysfunctional HDL explain high coronary artery disease risk in South Asians? Int J Cardiol 129: 125-132.
- Perse'gol L, Verge's B, Gambert P, et al. (2007) Inability of HDL from abdominally bese subjects to counteract the inhibitory effect of oxidized LDL on vasorelaxation. J Lipid Res 48: 1396-1401.
- Persegol L, Foissac M, Lagrost L, et al. (2007) HDL particles from type 1 diabetic patients are unable to reverse the inhibitory effect of oxidised LDL on endothelium-dependent vasorelaxation. Diabetologia 50: 2384-2387.
- Persegol L, Verge's B, Foissac M, et al. (2006) Inability of HDL from type 2 diabetic patients to counteract the inhibitory effect of oxidized LDL on endothelium-dependent vasorelaxation. Diabetologia 49: 1380-1386.
- Roberts CK, Ng C, Hama S, et al. (2006) Effect of a short-term diet and exercise intervention on in?ammatory/anti-in?ammatory properties of HDL in overweight/obese men with cardiovascular risk factors. J Appl Physiol 101: 1727-1732.
- Opole IO, Belmont JM, Kumar A, et al. (2007) Effect of low-density lipoprotein apheresis on in?ammatory and nonin?ammatory high-density lipoprotein cholesterol. Am J Cardiol 100: 1416-1418.
- McMahon M, Grossman J, FitzGerald J, et al. (2006) Proin?ammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum 54: 2541-2549.
- Charles-Schoeman C, Khanna D, Furst ED, et al. (2007) Effects of high-dose atorvastatin on antiin?ammatory properties of high density lipoprotein in patients with rheumatoid arthritis: a pilot study. J Rheumatol 34: 1459-1464.
- Navab M, Hama SY, Hough GP, et al. (2001) A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res 42: 1308-1317.
- Ansell BJ, Navab M, Hama S, et al. (2003) In?ammatory/antiin?ammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 108: 2751-2756.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001) Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285: 2486-2497.
- Kalantar-Zadeh K, Kopple JD, Kamranpour N, et al. (2007) HDL-in?ammatory index correlates with poor outcome in hemodialysis patients. Kidney Int 72: 1149-1156.
- Cruz D, Watson AD, Miller CS, et al. (2008) Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J Clin Invest 118: 2917-2928.
- Navab M, Ananthramaiah GM, Reddy ST, et al. (2005) The double jeopardy of HDL. Ann Med 37: 173-178.
- Van Lenten BJ, Wagner AC, Navab M, et al. (2007) Lipoprotein in?ammatory properties and serum amyloid A levels but not cholesterol levels predict lesion area in cholesterol-fed rabbits. J Lipid Res 48: 2344-2353.
- Navab M, Hama-Levy S, Van Lenten BJ, et al. (1997) Mildly oxidized LDL induces an increased apolipoprotein J/paraoxonase ratio. J Clin Invest 99: 2005-2019.
- Bhattacharyya T, Nicholls SJ, Topol EJ, et al. (2008) Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 299: 1265-1276.
- Nicholls SJ, Zheng L, Hazen SL (2005) Formation of dysfunctional high-density lipoprotein by myeloperoxidase. Trends Cardiovasc Med 15: 212-219.
- Wu Z, Wagner MA, Zheng L, et al. (2007) The re?ned structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat Struct Mol Biol 14: 861-868.
- Vaisar T, Pennathur S, Green PS, et al. (2007) Shotgun proteomics implicates protease inhibition and complement activation in the antiin?ammatory properties of HDL. J Clin Invest 117: 746-756.
- Green PS, Vaisar T, Pennathur S, et al. (2008) Combined statin and niacin therapy remodels high-density lipoprotein proteome. Circulation 118: 1259-1267.
- Watanabe J, Chou KJ, Liao JC, et al. (2007) Differential association of hemoglobin with proin?ammatory high density lipoproteins in atherogenic/hyperlipidemic mice: A novel biomarker of atherosclerosis. J Biol Chem 282: 23698- 23707.
- Watanabe J, Grijalva V, Hama S, et al. (2009) Hemoglobin and its scavenger protein haptoglobin associate with apoA-1-containing particles and in?uence the in?ammatory properties and function of high density lipoprotein. J Biol Chem 284: 18292-18301.
- Levy AP, Hochberg I, Jablonski K, et al. (2002) Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: The Strong Heart Study. J Am Coll Cardiol 40: 1984-1990.
- Asleh R, Miller-Lotan R, Aviram M, et al. (2006) Haptoglobin genotype is a regulator of reverse cholesterol transport in vitro and in vivo. Circ Res 99: 1419- 1425.
- Asleh R, Blum S, Kalet-Litman S, et al. (2008) Correction of HDL dysfunction in individuals with diabetes and the haptoglobin 2-2 genotype. Diabetes 57: 2794-2800.
- Gordon T, Castelli WP, Hjortland MC, et al. (1977) High density lipoprotein as a protective factor against coronary heart disease: The Framingham Study. Am J Med 62: 707-714.
- Briel M, Ferreira-Gonzalez I, You JJ, et al. (2009) Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: Systematic review and meta-regression analysis. BMJ 338: b92.
- Alexander ET, Tanaka M, Kono M, et al. (2009) Structural and functional consequences of the Milano mutation (R173C) in human apolipoprotein A-I. J Lipid Res 50: 1409-1419.
- Badimon JJ, Badimon L, Fuster V (1990) Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest 85: 1234-1241.
- Plump AS, Scott CJ, Breslow JL (1994) Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-de?cient mouse. Proc Natl Acad Sci U S A 91: 9607-9611.
- Nissen SE, Tsunoda T, Tuzcu EM, et al. (2003) Effect of recombinant ApoA-I Milano oncoronary atherosclerosis in patients with acute coronary syndromes: A randomized controlled trial. JAMA 290: 2292-22300.
- Patel S, Drew BG, Nakhla S, et al. (2009) Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-in?ammatory properties and cholesterol ef?ux capacity in patients with type 2 diabetes. J Am Coll Cardiol 53: 962-971.
- Drew BG, Duffy SJ, Formosa MF, et al. (2009) High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation 119: 2103-2111.
- Anantharamaiah GM, Jones JL, Brouillette CG, et al. (1985) Studies of synthetic peptide analogs of amphipathic helix: Structure of complexes with dimyristoyl phosphatidylcholine. J Biol Chem 260: 10248-10255.
- Venkatachalapathi YV, Phillips MC, Epand RM, et al. (1993) Effect of end group blockage on the properties of a class A amphipathic helical peptide. Proteins 15: 349- 359.
- Yancey PG, Bielicki JK, Johnson WJ, et al. (1995) Ef?ux of cellular cholesterol and phospholipid to lipid-free apolipoproteins and class A amphipathic peptides. Biochemistry 34: 7955-7965.
- Datta G, Chaddha M, Hama S, et al. (2001) Effects of increasing hydrophobicity on the physical- chemical and biological properties of a class A amphipathic helical peptide. J Lipid Res 42: 1096-1104.
- Garber DW, Datta G, Chaddha M, et al. (2001) A new synthetic class A amphipathic peptide analogue protects mice from diet-induced atheroscle- rosis. J Lipid Res 42: 545-552.
- Navab M, Anantharamaiah GM, Hama S, et al. (2002) Oral administration of an Apo A-I mimetic peptide synthesized from D-amino acids dramatically reduces atheroscle- rosis in mice independent of plasma cholesterol. Circulation 105: 290- 292.
- Van Lenten BJ, Wagner AC, Anantharamaiah GM, et al. (2002) In?uenza infection promotes macrophage traf?c into arteries of mice that is pre- vented by D-4F, an apolipoprotein A-I mimetic peptide. Circulation 106: 1127-1132.
- Kruger AL, Peterson S, Turkseven S, et al. (2005) D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation 111: 3126- 3134.
- Peterson SJ, Husney D, Kruger AL, et al. (2007) Long-term treatment with the apolipoprotein A1 mimetic peptide increases antioxidants and vascular repair in type I diabetic rats. J Phar- macol Exp Ther 322: 514-520.
- Peterson SJ, Drummond G, Kim DH, et al. (2008) L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J Lipid Res 49: 1658-1669.
- Peterson SJ, Kim DH, Li M, et al. (2009) The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J Lipid Res 50: 1293-1304.
- Zhang Z, Datta G, Zhang Y, et al. (2009) Apolipoprotein A-I mimetic peptide treatment inhibits in?ammatory responses and improves survival in septic rats. Am J Physiol Heart CircPhysiol 297: H866-H873.
- Navab M, Anantharamaiah GM, Hama S, et al. (2005) D-4F and statins synergize to render HDL antiin?ammatory in mice and monkeys and cause lesion regression in old apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol 25: 1426-1432.
- Vaziri ND, Moradi H, Pahl MV, et al. (2009) In vitro stimulation of HDL anti-in?ammatory activity and inhibition of LDL pro-in?ammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int 76: 437-444.
- Van Lenten BJ, Wagner AC, Jung CL, et al. (2008) Anti-in?ammatory apoA-I-mimetic peptides bind oxidized lipids with much higher af?nity than human apoA-I. J Lipid Res 49: 2302-2311.
- Buga GM, Frank JS, Mottino GA, et al. (2008) D-4F reduces EO6 immunoreactivity, SREBP-1c mRNA levels, and renal in?am- mation in LDL receptor-null mice fed a Western diet. J Lipid Res 49: 192-205.
Corresponding Author
Shirin Amini, DDS, Atherosclerosis Research, Division of Cardiology, University of California Los Angeles, USA, Tel: 310-498-6015.
Copyright
© 2021 Amini S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.