Transfusion Strategy the Impact of Oxygen Delivery
Abstract
Cardiac surgery is associated with high risk of perioperative blood loss leading to hemodynamic instability, which frequently warrants fluid replacement, pharmacological therapy or transfusion. Regardless of an association of as little as 1-2 blood units with a dramatic increase in morbidity and mortality, transfusion is still widely administered. Despite recent guidelines there is still little consensus on blood transfusion thresholds and the implementation of multidisciplinary patient blood management programs, has not led to convincing effects as RBC transfusion is still a frequent option in cardiac surgery.
Optimization of the haemoglobin level has been the primary focus; with less attention on the fact that haemoglobin is one of the factors of the simple algorithm that determine oxygen delivery. In situations where oxygen delivery does not match with consumption the compensatory mechanisms is triggered, where decrease in one factor results in a compensatory increase in one of the other.
The risk of impaired oxygen balance is higher in cardiac surgery with potential impact on oxygen delivery as both cardiac output and haemoglobin level may be affected. Following, blood transfusion in a fragile patient with an in principle adequate total haemoglobin, could therefore be fluid resuscitated or treated short time with inotropes. However, when it comes to especially patients with ischaemic hearts, the lower limit for haemoglobin should probably be 7 g/dL to avoid the risk of perioperative ischaemic episodes, but signs of impaired oxygenation should always serve as an important guidance before considering transfusions.
Keywords
Oxygen delivery, Transfusion, Cardiac output, Haemoglobin level
Introduction
Cardiac surgery, due to the complexity of the procedures, is associated with high risk of perioperative blood loss [1-10], especially in patients with advanced age and long cardiopulmonary bypass (CPB) [9,10]. The excessive bleeding, which is typically caused by either impaired haemostasis due to enhanced fibrinolysis, platelet dysfunction, haemodilution, acidosis, hypothermia and consumption of coagulation factors or the surgical trauma alone [11,12] may lead to hemodynamic instability and frequently warrants fluid replacement, pharmacological therapy or re-exploration. According to some researchers, re-exploration appears to be a strong risk factor for an increase in immediate postoperative mortality and morbidity [13,14]. However, newer studies have not demonstrated an association [15] and no general consensus exists on when exploration for postoperative bleeding is indicated, and surgical practice varies considerably in this regard [16]. Further, early re-exploration may decrease the need for blood transfusion. In these situations, despite the fact that transfusion of as little as 1 or 2 units of red blood cells (RBC) has been associated with a dramatic increase in morbidity, mortality and costs in patients undergoing cardiac surgery [8,17], blood transfusion is still performed as rescue therapy before the decision of re-exploration is made .
Remarkably, despite new guidelines [18,19], there is still little consensus on what constitutes a massive blood transfusion and thresholds might be associated with adverse outcomes [20,21] and the issue might be, as pointed out in a following editorial, that there was "Something for Almost Everyone" [22]. This is in contrast to earlier ASA guidelines from 1996 [23], and actual guidelines from the Danish Health and Medicine Authority's [24], which firmly declare lower thresholds for transfusion.
The implementation of a multidisciplinary patient blood management programme, the goal of which is careful surgery and focus on perioperative haemostasis and minimization of blood loss [18,25,26], is the basis for a reduction in transfusion requirements and thus decreased health care costs and improved patient outcomes. However, although the later ASA guidelines handled broader aspects of blood management programs the specific recommendations of RBC transfusion was less visible [25,26] and the overall result of the efforts is not convincing as RBC transfusion is still a frequent option in cardiac surgery [7,27].
Haemoglobin as Triggering Factor
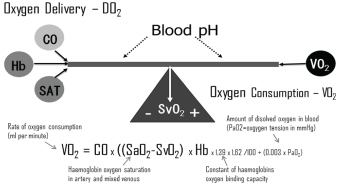
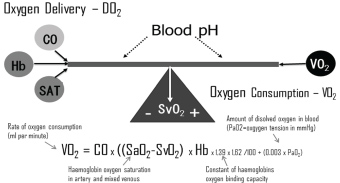
Optimization of the haemoglobin (Hb) level has always been the primary focus while initiating blood transfusions [19,23,28-34] with less attention to the fact that haemoglobin is one of the factors of the relatively simple algorithm determine oxygen delivery, which includes haemoglobin level, cardiac output (CO) and arterial saturation (SAT) (Figure 1).
It has been an observation that most research studies concerning transfusion requirement, predominantly involve patients for non-cardiac surgery with or without co-existing cardiac disease and the question about when to transfuse an individual patient has not been reliably answered. Although, it appears impossible to determine the magic haemoglobin number for RBC transfusion and the newest international guidelines are vague [18,19], there seems to be some consensus that blood transfusions may be indicated if the Hb is below 6.0 g/dL (3.7 mmol/L) [23,28-30]. In doubtful situations, one could follow the ASA guidelines stating that "red blood cell transfusions should be based on the patient's risk of developing complications of inadequate oxygenation" [23], which is typically manifested locally as myocardial ischemia or globally as general haemodynamic instability [30], or an oxygen extraction rate of greater than 50% and a decreased oxygen consumption [29,30].
Overall the evidence-based ASA transfusion guidelines concluded that "blood transfusions should not be dictated by a single haemoglobin transfusion trigger, but instead should be based on the patient's risk of developing complications of inadequate oxygenation". Thus, blood transfusion would generally be indicated, but not mandatory, at Hb levels lower than 6.0 g/dL (3.7 mmol/L) and rarely indicated in patients with haemoglobin higher than 10.0 g/dL (6.2 mmol/L) [23]. In many types of major surgery these recommendations have been followed and the use of transfusions reduced, but despite previously published guidelines, there remains substantial variation in the practice of transfusing patients and physicians often use haemoglobin level to decide when to transfuse [19]. Further, despite institutional efforts to restrain blood transfusions in cardiac surgery, the frequency remains high, and annually more than 3 million patients receive more than 11 million units of blood in the United States [4], and transfusion rates are varying widely, from less than 10% at some institutions to more than 90% at others [17,31]. Thus, the use of blood and blood products is still common clinical practice in cardiac and vascular surgery.
Studies of cardiac surgical patients have shown that correction of the haemodilution following cardiopulmonary bypass with blood transfusions increased mortality [32]. Further, lowering the transfusion threshold in patients undergoing coronary artery bypass surgery (CABG) from 9 to 8 g/dL (5.6-5.0 mmol/L) was neither followed by a higher mortality nor an increase in adverse effects [33], which indicates that patients with coexisting cardiac diseases, including coronary artery disease, tolerate moderate anaemia. A recent study concluded that in patients with a moderate to high risk for death a restrictive strategy (Hb < 7.5 g/dL regarding blood transfusion was equal to a liberal strategy with respect to outcomes [31]. In contrast, one study indicated that blood transfusions may decrease mortality in elderly patients with admission haematocrit (Hct) below 30% and acute myocardial infarction [35]. Further, studies have shown an increased in-hospital mortality in patients with lower preoperative haemoglobin levels [34] and correlation between preoperative haematocrit and 30-day mortality [36]. However, when adjusted and compensated for comorbidity (EuroSCORE [37]) the 30-day mortality was independent of Hb level.
The official Danish guidelines are relatively clearly formulated recommending lower thresholds. Erythrocyte transfusion can be considered (after individual clinical evaluation) when: 1) Haemoglobin concentration < 4.3 mmol/l (7 g/dL) and/or clinical symptoms of anaemia in circulatory stable patients without heart disease; 2) Haemoglobin concentration < 5.0 mmol/l (8.0 g/dL) and simultaneous chronic heart disease and/or clinical symptoms on anaemia in circulatory stable patients with chronic heart disease or 3) Haemoglobin concentration < 5.6 mmol/l (9.0 g/dL) and simultaneous acute coronary syndrome in ischaemic phase or life-threatening bleeding. Therefore, Following, there is in general no indication for RBC transfusion, when haemoglobin is above 5.6 mmol/l (9.0 g/dL).
Haemoglobin and Oxygen Delivery
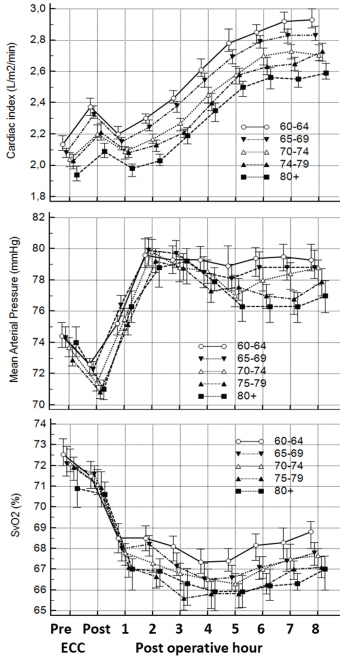
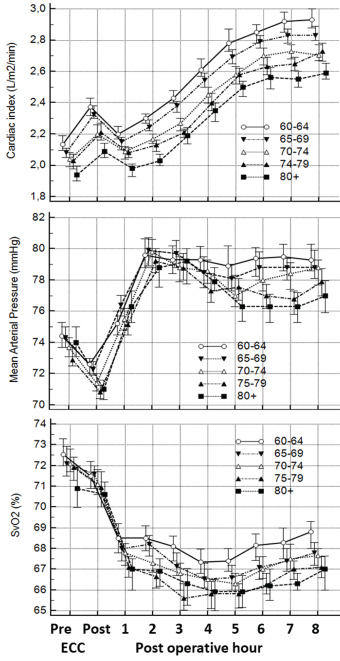
In situations where oxygen delivery does not match with oxygen consumption, the compensatory mechanisms which get triggered are relatively simple (Figure 1), e.g. decrease in haemoglobin level result in a compensatory increase in either cardiac output (CO) or oxygen extraction or a combination. Furthermore, the relation is independent of the fact that CO changes with age [38] and that the ratio of CO to total blood volume changes from roughly 3:1 in small children to 2:3 or lower in the elderly cardiac patient. Besides the age differences the immediate postoperative period is characterized by significant differences (Figure 2).
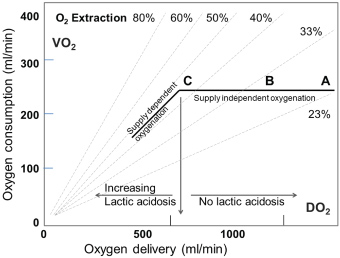
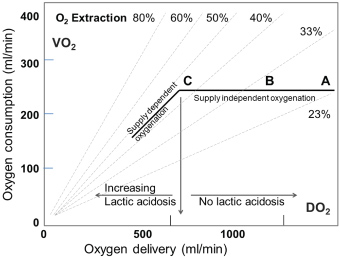
A most valuable parameter in evaluating need for inotropic support or RBC transfusion is whether oxygen consumption is adequately covered by oxygen delivery. In general, oxygen delivery to the cells is higher than actual consumption. When oxygen consumption is high (i.e. during exercise) the increased oxygen requirement is usually provided by an increased cardiac output. However, low cardiac output, low haemoglobin concentration (anaemia) or low haemoglobin saturation may result in an inadequate oxygen delivery, unless a compensatory change occurs in one of the other factors. Alternatively, if oxygen delivery falls relative to oxygen consumption the tissues extract more oxygen from the haemoglobin, the saturation of mixed venous blood (SvO2) falls below 70% ("A-B" in Figure 3). A reduction below point 'C' cannot be compensated by an increased oxygen extraction and results in anaerobic metabolism and lactic acidosis, and oxygen consumption becomes totally dependent on oxygen supply.
The relatively simple oxygen delivery algorithm demonstrates that decision treatment can be substantially supported by measurement of oxygen parameters. In situations with marginal haemoglobin, low SvO2 and increasing lactate, the RBC transfusion may be avoided by administration of inotropes for a shorter period. The haemodynamic data from our institution demonstrate that the first hours in the postoperative period , where the patients normally are sedated, is dominated by lower cardiac outputs but in general a normal SvO2, while later in the awakening period the cardiac output are increasing adequately and SvO2 are marginally lower (Figure 2). In general, the oxygen balances seems adequate as more than 84% of the average SVO2 is higher than 60% (Table 1). However, looking at individual patients 36% has average values below 60% for at least one measurement period indicating a possible short-term problem.
Haemodilution during CBP may constitute a challenge in patients with low haemoglobins and especially low body weight. However, data from our laboratory system indicate that low preoperative Hb levels in connection with cardiac surgery are relatively seldom (Table 2). Further, with careful surgery and focus on perioperative haemostasis the relatively lower postoperative haemoglobin is more determined by haemodilution than actual blood loss.
Conclusion
In general surgery there is still no need for RBC transfusion to patients with haemoglobin above 6 g/dL (3.6 mmol/L). This is further underlined with all the modalities for monitoring and specific supplemental support of the coagulation system. However, when it comes to cardiac surgery, the risk of impaired oxygen balance is higher due to the greater impact on the delivery parameters as both cardiac output and haemoglobin level may be affected, and thus the compensation mechanism troubled. Following, RBG transfusion of a fragile patient with an in principle adequate total haemoglobin, could therefore be fluid resuscitation or short-term inotropes.
Regarding patients with ischaemic hearts, the lower limit for haemoglobin should probably be 7 g/dL (4.3 mmol/L) to avoid the risk of perioperative ischaemic episodes. In the haemoglobin range of 7 to 10 g/dL (4.3-6.2 mmol/L), signs of impaired oxygenation should serve as transfusion indications, and such signs may be reached present at higher haemoglobin values than in healthy patients. However, transfusion for patients with haemoglobins higher than 8 g/dL (5.0 mmol/L) is seldom needed, unless there are special circumstances, or in the unlikely event that impaired oxygenation is present.
References
- Rawn JD (2007) Blood transfusion in cardiac surgery. A silent epidemic revisited. Circulation 116: 2523-2534.
- Koch CG, Li L, Duncan AI, et al. (2006) Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med 34: 1608-1616.
- Andreasen JJ, Dethlefsen C, Modrau I, et al. (2011) Storage time of allogeneic red blood cells is associated with increased risk of severe postoperative infection after coronary artery bypass grafting. European Journal of Cardio-Thoracic Surgery 39: 329-334.
- Engoren MC, Habib RH, Zacharias A, et al. (2002) Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg 74: 1180-1186.
- Koch CG, Li L, Duncan AI, et al. (2006) Transfusion in coronary artery bypass grafting is associated with reduced long-term survival. Ann Thorac Surg 81: 1650-1657.
- Surgenor SD, Kramer RS, Olmstead EM, et al. (2009) The association of perioperative red blood cell transfusions and decreased long-term survival after cardiac surgery. Anesth Analg 108: 1741-1746.
- Jakobsen CJ, Ryhammer PK, Tang M, et al. (2012) Transfusion of blood during cardiac surgery is associated with higher long-term mortality in low risk patients. Eur J Cardiothorac Surg 42: 114-120.
- Karkouti K, Wijeysundera DN, Yau TM, et al. (2004) The independent association of massive blood loss with mortality in cardiac surgery. Transfusion 44: 1453-1462.
- Despotis GJ, Filos KS, Zoys TN, et al. (1996) Factors associated with excessive postoperative blood loss and hemostatic transfusion requirements: A multivariate analysis in cardiac surgery patients. Anesth Analg 82: 13-21.
- Kestin AS, Valeri CR, Khuri SF, et al. (1993) The platelet function defect of cardiopulmonary bypass. Blood 82: 107-117.
- Despotis GJ, Avidan MS, Hogue CW Jr (2001) Mechanisms and attenuation of hemostatic activation during extracorporeal circulation. Ann Thorac Surg 72: S1821-S1831.
- Paparella D, Brister SJ, Buchanan MR (2004) Coagulation disorders of cardiopulmonary bypass: A review. Intensive Care Med 30: 1873-1881.
- Ranucci M, Bozzetti G, Ditta A, et al. (2008) Surgical reexploration after cardiac operations: Why a worse outcome? Ann Thorac Surg 86: 1557-1562.
- Moulton MJ, Creswell LL, Mackey ME, et al. (1996) Reexploration for bleeding is a risk factor for adverse outcomes after cardiac operations. J Thorac Cardiovasc Surg 111: 1037-1046.
- Tambe SP, Kimose HH, Greisen J, et al. (2017) Re-exploration due to bleeding is not associated with severe postoperative complications. Interact Cardiovasc Thorac Surg 25: 233-240.
- Frojd V, Jeppsson A (2016) Reexploration for bleeding and its association with mortality after cardiac surgery. Ann Thorac Surg 102: 109-117.
- Paone G, Likosky DS, Brewer R, et al. (2014) Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann Thorac Surg 97: 87-93.
- Pagano D, Milojevic M, Meesters MI, et al. (2018) 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. Eur J Cardiothorac Surg 53: 79-111.
- Carson JL, Guyatt G, Heddle NM, et al. (2016) Clinical practice guidelines from the AABB red blood cell transfusion thresholds and storage. JAMA 316: 2025-2035.
- Ndrepepa G, Schuster T, Hadamitzky M, et al. (2012) Validation of the bleeding academic research consortium definition of bleeding in patients with coronary artery disease undergoing percutaneous coronary intervention. Circulation 125: 1424-1431.
- Mehran R, Rao SV, Bhatt DL, et al. (2011) Standardized bleeding definitions for cardiovascular clinical trials. A consensus report from the bleeding academic research consortium. Circulation 123: 2736-2747.
- Yazer MH, Triulzi DJ (2016) AABB Red Blood Cell Transfusion Guidelines Something for Almost Everyone. JAMA 316: 1984-1985.
- (1996) Practice guidelines for blood component therapy: A Report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology 84: 732-747.
- https://stps.dk/da/nyheder/2015/~/media/8147748CEC6140FF9112674B5EF1307B.ashx.
- American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies (2006) Practice guidelines for perioperative blood transfusion and adjuvant therapies: An updated report by the American Society of Anesthesiologists task force on perioperative blood transfusion and adjuvant therapies. Anesthesiology 105: 198-208.
- American Society of Anesthesiologists Task Force on Perioperative Blood Management (2015) Practice guidelines for perioperative blood management. An updated report by the American Society of Anesthesiologists task force on perioperative blood management. Anesthesiology 122: 241-275.
- Arias-Morales CE, Stoicea N, Gonzalez-Zacarias AA, et al. (2017) Revisiting blood transfusion and predictors of outcome in cardiac surgery patients: a concise perspective. F1000Res 6: 168.
- Carson JL, Noveck H, Berlin JA, et al. (2002) Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion 42: 812-818.
- Simon TL, Alverson DC, AuBuchon J, et al. (1998) Practice parameter for the use of red blood cell transfusions: Developed by the red blood cell administration practice guideline development task force of the college of american pathologists. Arch Pathol Lab Med 122: 130-138.
- Spahn DR, Schanz U, Pasch T (1998) Perioperative transfusionskriterien. Anaesthesist 47: 1011-1020.
- Mazer CD, Whitlock RP, Fergusson DA, et al. (2017) Restrictive or Liberal Red-Cell transfusion for cardiac surgery. N Engl J Med 377: 2133-2144.
- Fillinger MP, Surgenor SD, Clark C, et al. (2001) The impact on mortality of managing hemodilutional anemia with RBC transfusion after CABG. Anesthesiology 95: 201.
- Bracey AW, Radovancevic R, Riggs SA, et al. (1999) Lowering the haemoglobin threshold for transfusion in coronary artery bypass precedures: Effect on patient outcome. Transfusion 39: 1070-1077.
- Zindrou D, Taylor KM, Bagger JP (2002) Preoperative haemoglobin concentration and mortality rate after coronary artery bypass surgery. Lancet 359: 1747-1748.
- Wu WC, Rathore SS, Wang Y, et al. (2001) Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med 345: 1230-1236.
- Jakobsen CJ (2007) Strategy of transfusion in cardiac surgery - limits of hematocrit and how much is too low? Future Cardiol 3: 141-151.
- Nashef SA, Roques F, Michel P, et al. (1999) European system for cardiac operative risk evaluation (Euro-SCORE). Eur J Cardiothorac Surg 16: 9-13.
- Kuikka JT, Länsimies E (1982) Effect of age on cardiac index, stroke index and left ventricular ejection fraction at rest and during exercise as studied by radiocardiography. Acta Physiol Scand 114: 339-343.
Corresponding Author
Carl-Johan Jakobse, MD, Department of Anaesthesiology and Intensive Care, Aarhus University Hospital, Aarhus, 8200 Aarhus N, Denmark, Tel: +45-3091-1010.
Copyright
© 2018 Bhavsar R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.