Recent Progress in the Diagnosis and Management of Tako-Tsubo Syndrome
Abstract
Tako-Tsubo Syndrome (TTS), also known as apical ballooning syndrome or "broken heart syndrome", was discovered by Japanese investigators 27 years ago, and was initially assumed to be a rarity. However, TTS is relatively common, especially among ageing women and in the setting of severe physical/emotional stress. TTS represents an aberrant β2-adrenoceptor mediated myocardial inflammatory response to catecholamine stimulation, most commonly seen in the ageing female heart. It remains under-diagnosed in most hospitals. Initially, TTS was essentially a diagnosis of exclusion, typically made after emergency coronary angiography in patients presenting with ST segment elevation who had no relevant coronary artery disease. However, at least 50% of patients with TTS never show ST elevation. We therefore propose a diagnostic algorithm for
(a)Initial tentative diagnosis
(b)Definitive positive diagnosis (i.e. not by exclusion)
(c)Quantitation of severity of attacks of TTS,
Which should expedite the avoidance of catecholamine administration to these patients.
Key aspects to consider are:
1. Clinical presentation (including demographics and prior circumstances).
2. ECG changes.
3. Biomarkers (marked elevation of plasma NT-proBNP levels with minimal troponin elevation).
4. Catheterisation findings (especially for patients with initial ST elevation).
5. Cardiac MRI, which demonstrates oedema, as well as the absence of acute myocardial infarction.
Keywords
Tako-tsubo syndrome, Myocardial inflammation, N-terminal-pro BNP, Acute coronary syndrome
Introduction
Tako-Tsubo Syndrome (TTS), also known as stress cardiomyopathy, apical ballooning syndrome and "broken heart syndrome" is increasing recognized as a relatively common and frequently under diagnosed health problem, particularly in aging women. The name "Tako-Tsubo" reflects the original description of TTS, by Japanese investigators, on the basis of the similarities between the shape of the affected left ventricle and that of a Japanese octopus trap. TTS accounts for about 10% of cases initially diagnosed as ST Elevation Myocardial Infarction (STEMI) in women over 50 years of age [1]. It has been recently reported in two large cohort studies [2,3] that TTS is associated not only with substantial early in-hospital mortality rates (due to lethal arrhythmias and cardiogenic shock), but also with medium-term mortality similar to that of post myocardial infarction. There are also high levels of residual disability for at least 3 months [4], and a risk of recurrence of about 3% per annum [5,6].
Clinically, TTS is characterized by variable combinations of chest pain and dyspnoea, often associated with severe initial hypotension, and sometimes with ventricular tachyarrhythmias. Episodes are often precipitated by severe emotional or physical stress and recently an association with psychiatric disease has been noted [2]. Traditionally, TTS has been diagnosed largely via exclusion of Acute Myocardial Infarction (AMI). Indeed, the diagnosis of TTS is inherently difficult and complicated by selection bias, for example with failure to investigate this potential diagnosis in the very elderly, or in postoperative patients in whom rises in plasma troponin levels are noted. Although it was initially considered that the presence of any coronary disease precluded the diagnosis [7], it is certainly possible to diagnose TTS on the basis of characteristic Left Ventricular (LV) wall motion abnormalities [dissociated from the distribution of Coronary Artery Disease (CAD)] and associated myocardial oedema [8].
While it was initially considered that TTS was a relative rarity, at least in Caucasians, little scientific effort was devoted to either understanding its pathogenesis, developing reliable diagnostic algorithms, or establishing effective short- and long-term treatments. The generalities were that the precipitation of TTS had something to do with a "surge" of catecholamine release or exposure, for which there was ample clinical evidence [9-11]. Therefore, many patients were prescribed ß-adrenoceptor antagonists, on theoretical grounds only. However, as stated above, TTS is not rare, although it is still under diagnosed. The current review will discuss the various clinical categories of TTS ("primary" and "secondary" TTS), an algorithm for their diagnosis, and finally observational and scientific progress towards development of appropriate therapies.
Extent of Problem and Clinical Categories
Concept of "Primary" vs. "Secondary" TTS
In the majority of cases, TTS has been diagnosed in ageing women presenting either with an episode of chest pain and/or dyspnoea occurring either randomly or in the setting of recent physical or emotional stress. This is now termed "primary" TTS. "Secondary" TTS has been described among individuals in whom TTS develops in the setting of another acute medical problem which has led to substantial catecholamine release, such as intracranial hemorrhage [12] or phaeochromocytoma [13]. While the distinction between primary and secondary TTS may be blurred, there is a case for regarding all cases of perioperative, or medication-induced TTS as secondary. Secondary TTS should theoretically be less subject to risk of recurrence than primary, and the reportedly greater mortality rates and higher proportion of males affected by secondary TTS [2,14,15] are not surprising.
Definite vs. Putative TTS
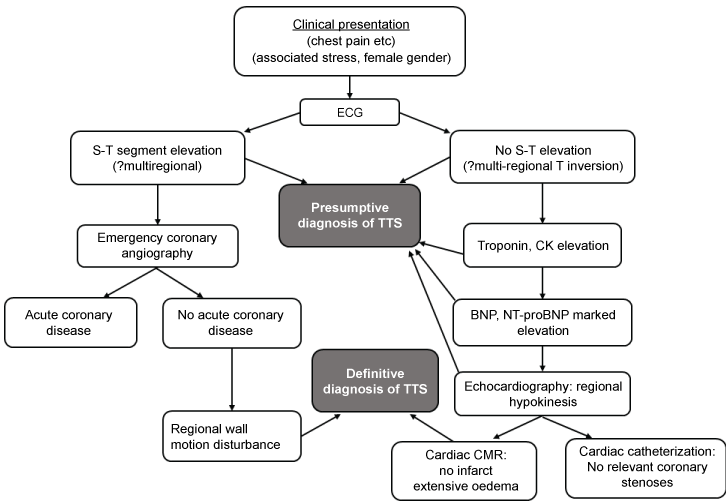
It is in practice impossible to be quite certain of a diagnosis of TTS without performing either a cardiac catheterisation or a Cardiac Magnetic Resonance Imaging (CMR) procedure: indeed there is a strong case for performing both of these procedures in the vast majority of patients. On the other hand there are a number of clinical features and other clinical investigations which can help to make a presumptive diagnosis of TTS prior to performing a definitive investigation, A presumptive diagnosis can assist in planning appropriate therapy early, for example by avoiding catecholamine administration in hypotensive individuals. Of course, diagnosing TTS as a presumptive cause of arrhythmic death remains too hard a challenge at present, and therefore the number of such deaths remains completely uncertain.
The Diagnostic Process: Inherent Difficulties
The diagnosis of TTS is inherently difficult and complicated by selection bias. The condition has usually been recognized, particularly in early series of cases, as a result of cardiac catheterisation of patients presenting with acute chest pain in which the initial diagnosis was an acute coronary syndrome (including a substantial proportion of patients initially diagnosed as STEMI). However, a number of large studies have suggested that in at least 60% of cases of TTS, presentation tends to mimic Non-ST Elevation Myocardial Infarction (NSTEMI), with a variety of associated ECG changes [16]. A final difficulty is that very elderly, frail patients presenting with prolonged chest pain, especially without ST elevation, may not undergo cardiac catheterization, and therefore the diagnosis of TTS may easily be missed.
Consideration on the basis of coronary angiography
By definition, TTC is a disorder characterized by acute development of segmental (usually periapical) LV systolic dysfunction. A number of diagnostic criteria have been proposed by several groups [7,17-20]. The most commonly utilized definition of TTS [8] , while reasonably accurate , reflects the historical practice of making the diagnosis via cardiac catheterisation, and therefore largely by exclusion. The theoretical problems with this are twofold: since TTS has a different pathogenesis from that of "fixed" CAD, and since myocardial ischaemia may induce catecholamine release, there are good reasons why both TTS and CAD may co-exist [21]. In general, if fixed CAD affects the precise region of the LV as that exhibiting hypokinesis, it becomes difficult to diagnose TTS (although not impossible: see below). However, a discrepancy between the regional distribution of the hypokinesis and that of the coronary disease strongly suggest the diagnosis of TTS. There is an additional theoretical difficulty in exclusion of single or multi-vessel coronary spasm as an underlying disorder [22]: in practice total exclusion of spasm is usually impracticable. However, once again, the distribution of hypokinesis in TTS rarely accords with the course of a single coronary artery.
Beyond coronary angiography
As indicated above, the differential diagnosis of TTS includes both myocardial ischaemia and infarction. A number of differences between TTS and acute myocardial infarction have emerged, including findings on routine clinical bio-chemistry. Notably, in TTS there is almost always less myocardial necrosis, with only minimal elevation of troponin and creatine kinase levels, together with far more substantial release of BNP and N-terminal pro BNP [23,24].
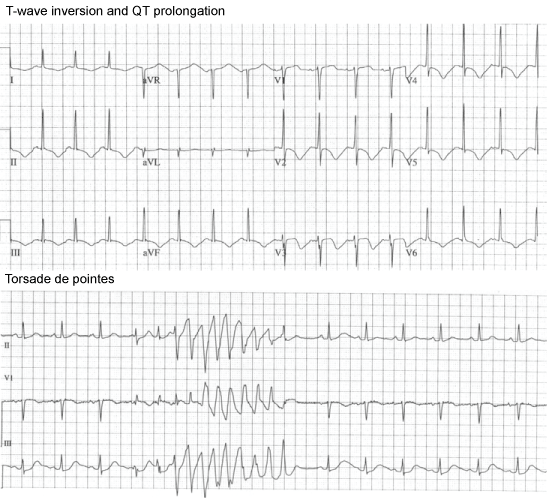
The diagnosis of TTS is also expedited by greater awareness of the epidemiology of the condition, the characteristic "multi-regional" ECG changes (Figure 1), as well as marked elevation of NT-pro-BNP levels and minimal myocardial necrosis [24,25]. Clinical and echocardiography factors should also be taken into account, including gender, recent illness or stress, or administration of drugs which may increase catecholamine levels [26]. Hypokinesis or dyskinesia usually predominantly affects the periapical region of the Left Ventricle (LV), sometimes with sparing of contractility at the very apex, but in between 15% and 25% of cases there may be more marked mid-ventricular changes:- early echocardiography (and indeed ventriculography at the time of cardiac catheterisation) therefore also assist diagnosis [27]. The routine availability of CMR imaging has revolutionized the differential diagnosis of TTS, particularly in patients in whom the diagnosis is not suspected in the first few days post onset of symptoms as described below.
Summary: Diagnostic Algorithm for TTS and for Quantitation of Severity of Episodes
The difficulties inherent in developing presumptive and/or definitive diagnostic criteria for TTS have been addressed previously by Lyon, et al. [18]. In view of recent trends towards a pivotal role for CMR, our view of the position is summarized in (Figure 2) and discussed briefly below. First, it must be remembered that in patients with ST segment elevation on the initial FCG, the diagnostic process effectively begins with emergency coronary angiography.
Presenting features and haemodynamic disturbances
The distinct epidemiological, clinical and biochemical features of TTS need to be considered. TTS is often precipitated by exposure to severe emotional and physical stress. It occurs especially but not exclusively in women aged > 50 years, and often complicate other severe illnesses. TTS is usually characterized symptomatically by chest pain and/or dyspnoea, often associated with hypotension [18]. Clinically, there is a substantial risk of early development of torsade de pointes ventricular tachycardia, and of thrombo-embolism secondary to mural left ventricular thrombosis, although these are not usually the fundamental modes of initial presentation [28]. The most ominous clinical aspect, however, is the early development of severe hypotension, together with peripheral vasoconstriction in the majority of cases [29], and usually without substantial elevation of pulmonary capillary wedge pressure. Occasionally, paradoxical peripheral vasodilatation may occur and also severe acute mitral regurgitation may be present. Apart from avoiding further exposure to catecholamines, there is no established treatment for this shock state, and this therapeutic difficulty may also reflect the uncertain mechanism (s) for its development [18].
It is also widely recognized that TTS tends to be recurrent: a recent meta-analysis has suggested a mean annual recurrence rate of about 3% [30]. Therefore it is important to enquire whether patients have experienced similar episodes in the past. The clinical course of TTS after the first 24 hours is usually one of rapid symptomatic improvement, but without total resolution of malaise and exertional dyspnoea.
ECG
Most patients with TTS develop widespread T-wave inversion and QTc prolongation approximately 24 hours post presentation irrespective of the presence/absence of initial ST segment elevations. Torsade de pointes is an early complication, preceding maximal QT prolongation (Figure 1).
Biomarkers
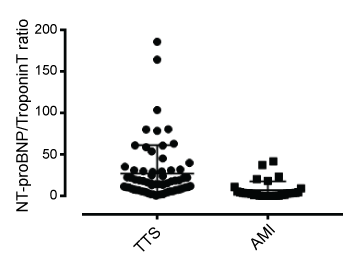
It is clear that release (or administration) of catecholamines initiates many, indeed, probably most attacks [9,10]. Elevated plasma catecholamine levels have frequently been documented in TTS patients, and in our previous studies severity of attacks correlated directly with plasma normetanephrine levels [24,31]. The acute phase of TTS is associated with marked elevation of plasma BNP and NT-proBNP levels [23,24,32]. Both BNP and NT-proBNP concentrations are acutely elevated considerably beyond population norms in all cases of TTS [24]. Extent of elevation of plasma NT-proBNP concentration was directly correlated with impairment of regional LV wall motion, and inversely with LV ejection fraction, as well as with the extent of myocardial oedema on CMR [24,31]. These data therefore suggest that BNP release may result primarily from myocardial inflammation rather than purely from "heart failure" and that the extent of its release into plasma (and consequently also the rise in NT-proBNP concentrations), may be utilized not only as an aid towards diagnosis but also for quantitation of severity of TTS episode severity. Furthermore, the contrast between considerable elevation of NT-proBNP levels and relatively minor elevation of troponin (or creatine kinase) levels can potentially be utilized for provisional differentiation from acute myocardial infarction (Figure 3).
The current role of cardiac catheterisation in the diagnostic process
Immediate cardiac catheterisation remains mandatory for patients presenting with ST segment elevation, and should be considered on an urgent basis for the remainder of suspected TTS cases. Coronary arteries are typically normal but "bystander" CAD can be present. Ventriculography usually shows wall motion abnormalities including apical, mid-wall, or occasionally basal hypokinesis.
Echocardiography/CMR
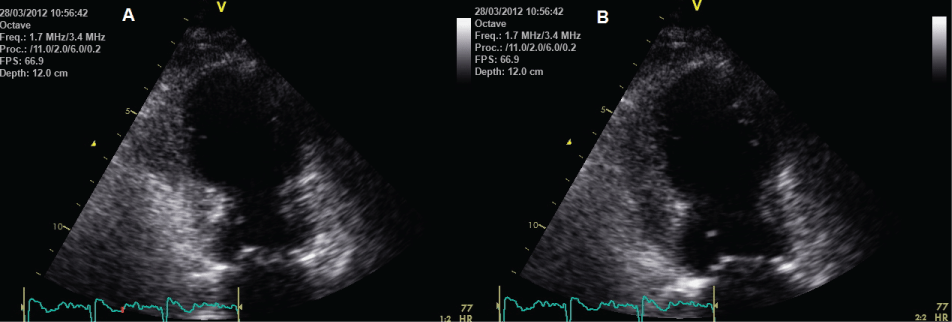
Early echocardiography is an important priority for early investigation, especially in the absence of ST elevation, and usually demonstrates extensive wall motion impairment extending beyond the territory of a single coronary artery. Typically this is mainly apical (Figure 4) or mid-ventricular, with rapid improvement in LV regional wall motion over the next 7 days [33]. Echocardiography with concomitant echo-contrast injection and flash imaging can be utilized to establish the diagnosis of TTS in patients unsuitable for coronary angiography and/or CMR.
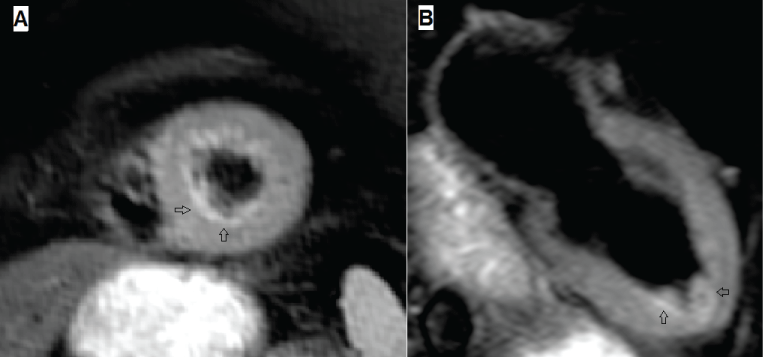
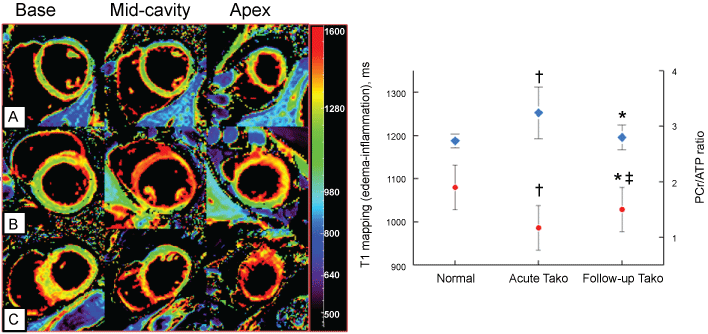
A number of studies utilizing CMR have demonstrated that TTS is associated with intense myocardial inflammation demonstrated by myocardial oedema, not only at the LV apex, but throughout the LV (Figure 5). This oedema resolves slowly, and remains detectable at 3 months after acute attacks [31]. Furthermore, 31P cardiac magnetic resonance spectroscopy, currently only a research technique, demonstrates impaired myocardial energetic, persists for at least 3 months post acute episodes of TTS [34] (Figure 6). It is even possible to diagnose a recent episode of TTS in patients with previous myocardial infarction via CMR, by demonstrating a region of myocardial oedema remote from the site of the previous infarction.
Advantages of Early Diagnosis: Therapeutic Options?
To date, no clinical trials have been undertaken in TTS. Given the association with marked elevation of plasma concentrations of catecholamines, a number of clinicians have discharged patients on β-adrenoceptor antagonists. However, there is no evidence of a beneficial effect of such treatment to date [35]. On the other hand, there may well be a case for the utilization of ACE inhibitors in the long term. While no impact on rate of recovery of left ventricular function has been recorded, it appears that ACE inhibitors may reduce the risk of recurrence of TTC [15].
While there is no consensus regarding the management of cardiogenic shock complicating TTS, catecholamine administration should be avoided, and it has been suggested on the basis of animal experiments that the calcium sensitizer levosimendan may be useful [36,37]. Given the recent demonstration of long-term mortality implications post acute episodes of TTS, the question of monitoring risk of late arrhythmic genesis arises. To date, this possibility has received little attention.
Conclusions
The occurrence of TTS reflects, to a large extent, an abnormal inflammatory reaction of the aging (usually female) hearts to catecholamine exposure. Differentiation of TTS from acute myocardial infarction no longer rests purely on an angiographic exclusion process, but can be expedited by appropriate clinical awareness of the problem, utilization of careful interpretation of ECG and biomarker data, and early performance of both echocardiography and CMR. The importance of early differentiation from acute myocardial infarction currently is limited to avoidance of further catecholamine exposure and the lack of necessity to initiate anti-ischaemic therapy, but will increase as specific treatments for TTS are developed.
Acknowledgements
This work was funded in part by a research grant from the Australian National Health and Medical Research Council. G.J.O. and S.Y.S. were supported by PhD scholarships from the University of Adelaide.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Raman B, Singh K, Zeitz CJ, et al. (2014) Takotsubo cardiomyopathy presenting as S-T elevation myocardial infarction: Not gone but forgotten? International Journal of Cardiology 172: e261-e262.
- Templin C, Ghadri JR, Diekmann J, et al. (2015) Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. The New England Journal of Medicine 373: 929-938.
- Tornvall P, Collste O, Ehrenborg E, et al. (2016) A Case-Control Study of Risk Markers and Mortality in Takotsubo Stress Cardiomyopathy. Journal of the American College of Cardiology 67: 1931-1936.
- Bellandi B, Salvadori C, Parodi G, et al. (2012) Epidemiology of Tako-tsubo cardiomyopathy: the Tuscany Registry for Tako-tsubo Cardiomyopathy . G Ital Cardiol (Rome) 13: 59-66.
- Dib C, Asirvatham S, Elesber A, et al. (2009) Clinical correlates and prognostic significance of electrocardiographic abnormalities in apical ballooning syndrome (Takotsubo/stress-induced cardiomyopathy). Am Heart J 157: 933-938.
- Elesber AA, Prasad A, Lennon RJ, et al. (2007) Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol 50: 448-452.
- Prasad A, Lerman A, Rihal CS (2008) Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 155: 408-417.
- Madhavan M, Prasad A (2010) Proposed Mayo Clinic criteria for the diagnosis of Tako-Tsubo cardiomyopathy and long-term prognosis. Herz 35: 240-243.
- Mosley WJ 2nd, Manuchehry A, McEvoy C, et al. (2010) Takotsubo cardiomyopathy induced by dobutamine infusion: a new phenomenon or an old disease with a new name. Echocardiography 27: E30-E33.
- Silberbauer J, Hong P, Lloyd GW (2008) Takotsubo cardiomyopathy (left ventricular ballooning syndrome) induced during dobutamine stress echocardiography. Eur J Echocardiogr 9: 136-138.
- Wittstein IS, Thiemann DR, Lima JA, et al. (2005) Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 352: 539-548.
- Talahma M, Alkhachroum AM, Alyahya M, et al. (2016) Takotsubo cardiomyopathy in aneurysmal subarachnoid hemorrhage: Institutional experience and literature review. Clin Neurol Neurosurg 141: 65-70.
- Y-Hassan S (2016) Clinical Features and Outcome of Pheochromocytoma-Induced Takotsubo Syndrome: Analysis of 80 Published Cases. The American Journal of Cardiology 117: 1836-1844.
- Nunez-Gil IJ, Almendro-Delia M, Andres M, et al. (2016) Secondary forms of Takotsubo cardiomyopathy: A whole different prognosis. Eur Heart J Acute Cardiovasc Care 5: 308-316.
- Singh K, Carson K, Shah R, et al. (2014) Meta-analysis of clinical correlates of acute mortality in takotsubo cardiomyopathy. Am J Cardiol 113: 1420-1428.
- Sharkey SW, Maron BJ (2014) Epidemiology and clinical profile of Takotsubo cardiomyopathy. Circulation Journal : Official Journal of the Japanese Circulation Society 78: 2119-2128.
- Kawai S, Kitabatake A, Tomoike H (2007) Guidelines for diagnosis of takotsubo (ampulla) cardiomyopathy. Circulation Journal: Official Journal of the Japanese Circulation Society 71: 990-992.
- Lyon AR, Bossone E, Schneider B, et al. (2016) Current state of knowledge on Takotsubo syndrome: a Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 18: 8-27.
- Omerovic E (2011) How to think about stress-induced cardiomyopathy? - Think "out of the box"! Scand Cardiovasc J 45: 67-71.
- Parodi G, Citro R, Bellandi B, et al. (2014) Revised clinical diagnostic criteria for Tako-tsubo syndrome: the Tako-tsubo Italian Network proposal. Int J Cardiol 172: 282-283.
- Previtali M, Repetto A, Panigada S, et al. (2009) Left ventricular apical ballooning syndrome: prevalence, clinical characteristics and pathogenetic mechanisms in a European population. International Journal of Cardiology 134: 91-96.
- Sharkey SW, Windenburg DC, Lesser JR, et al. (2010) Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol 55: 333-341.
- Akashi YJ, Musha H, Nakazawa K, et al. (2004) Plasma brain natriuretic peptide in takotsubo cardiomyopathy. QJM 97: 599-607.
- Nguyen TH, Neil CJ, Sverdlov AL, et al. (2011) N-terminal pro-brain natriuretic protein levels in takotsubo cardiomyopathy. Am J Cardiol 108: 1316-1321.
- Pilgrim TM, Wyss TR (2008) Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: A systematic review. Int J Cardiol 124: 283-292.
- Neil CJ, Chong CR, Nguyen TH, et al. (2012) Occurrence of Tako-Tsubo cardiomyopathy in association with ingestion of serotonin/noradrenaline reuptake inhibitors. Heart, Lung & Circulation 21: 203-205.
- Desmet W, Bennett J, Ferdinande B, et al. (2014) The apical nipple sign: a useful tool for discriminating between anterior infarction and transient left ventricular ballooning syndrome. Eur Heart J Acute Cardiovasc Care 3: 264-267.
- Madias C, Fitzgibbons TP, Alsheikh-Ali AA, et al. (2011) Acquired long QT syndrome from stress cardiomyopathy is associated with ventricular arrhythmias and torsades de pointes. Heart Rhythm 8: 555-561.
- Chong CR, Neil CJ, Nguyen TH, et al. (2013) Dissociation between severity of takotsubo cardiomyopathy and presentation with shock or hypotension. Clin Cardiol 36: 401-406.
- Singh K, Carson K, Usmani Z, et al. (2014) Systematic review and meta-analysis of incidence and correlates of recurrence of takotsubo cardiomyopathy. Int J Cardiol 174: 696-701.
- Neil C, Nguyen TH, Kucia A, et al. (2012) Slowly resolving global myocardial inflammation/oedema in Tako-Tsubo cardiomyopathy: evidence from T2-weighted cardiac MRI. Heart (British Cardiac Society) 98: 1278-1284.
- Nef HM, Mollmann H, Troidl C, et al. (2008) Tako-Tsubo cardiomyopathy: NT-proBNP as a reliable parameter of a favourable prognosis? International Journal of Cardiology 124: 237-238.
- Mansencal N, Abbou N, Pilliere R, et al. (2009) Usefulness of two-dimensional speckle tracking echocardiography for assessment of Tako-Tsubo cardiomyopathy. Am J Cardiol 103: 1020-1024.
- Dawson DK, Neil CJ, Henning A, et al. (2015) Tako-Tsubo Cardiomyopathy: A Heart Stressed Out of Energy? JACC Cardiovascular Imaging 8: 985-987.
- Citro R, Rigo F, D'Andrea A, et al. (2014) Echocardiographic correlates of acute heart failure, cardiogenic shock, and in-hospital mortality in tako-tsubo cardiomyopathy. JACC Cardiovascular Imaging 7: 119-129.
- Karvouniaris M, Papanikolaou J, Makris D, et al. (2012) Sepsis-associated takotsubo cardiomyopathy can be reversed with levosimendan. Am J Emerg Med 30: 832.e5-832.e7.
- Santoro F, Ieva R, Ferraretti A, et al. (2013) Safety and feasibility of levosimendan administration in takotsubo cardiomyopathy: a case series. Cardiovasc Ther 31: e133-e137.
Corresponding Author
John D Horowitz, Department of Cardiology, The Queen Elizabeth Hospital, 28 Woodville Road, Woodville South, SA 5011, Australia, Tel: 61-08-8222-6725, Fax: 61-08-8222-6422.
Copyright
© 2017 Nguyen TH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.