Flow Velocity, Fluid Dynamics and Vascular Pathophysiology
Abstract
Diseases of blood vessels cause more morbidity and mortality than combined impact of any other major non-communicable disease (NCD) including cancer. By and large, these diseases are diagnosed by monitoring known risk factors. The management of the disease is more or less limited to the management of the risk factors. We strongly feel, that development of a therapy system based on the management of disease of the vessel, than management of the risk factors, will yield better results and provide better opportunity for individualized therapy. Framingham Studies provided evidence, to support the role of risk factors such as increased blood pressure and altered blood lipids, for promoting acute cardiovascular (CV) events (https://www.framinghamheartstudy.org). Based on these results, management strategies for these risk factors were developed. Some of the risk score models currently available include; Framingham Risk Score (FRS), Monitoring Changes in Intimal Medium Thickness (IMT), University of Minnesota-10 Point Risk Scoring System (UMSS), Coronary Calcium Scoring (CCS), Magnetic Resonance Imaging (MRI) of Vessels, 3D Ultrasound (US) for Monitoring Carotid Plaque Volume (CPV), 3D Ultrasound for Monitoring Benefits of Therapy, and Pulse Wave Velocity (PWV) Index for monitoring arterial compliance. Recent studies with CCS using CT (calcium scoring for coronary arteries), and MRI (vessel volume measurements) have demonstrated that using conventional methods for monitoring risk (Framingham Risk Score), may exclude more than 30% of the individuals from further screening for high-risk. With rapid progress in emerging technologies, software and analytics, predictive and preventive care will improve tremendously. In this overview, we discuss the importance of monitoring blood flow velocity and fluid dynamics of regional vascular beds for the early diagnosis of vascular disease and its management.
Introduction
A recent study by the Non-Communicable Diseases (NCD) Risk Factor Collaboration, published in the Lancet (2016:387:1513-30), estimates that the global Type-2 diabetes incidence has quadrupled in less than three decades. Obesity, which is closely associated with hypertension and diabetes, is also on the rise (1.5 billion obese individuals worldwide). According to these experts, if these trends continue, the probability of meeting the global target (The Millennium Development Goal) of halting these chronic disorders by 2025 at the 2010 level is unlikely. Atherosclerotic cardiovascular disease, especially coronary heart disease, is the number one cause of death worldwide [1]. In our monograph on coronary artery disease (CAD), we refer to this condition as "vascular disease" rather than heart disease, as ischemia due to poor blood circulation precedes the tissue injury to the heart. This is probably true of most of the clinical complications associated with cardiometabolic diseases, including Type-2 diabetes [2,3]. Since I have been invited to write an overview for the inaugural issue of Scientific Pages of Heart, I have selected a topic that is dear to me, "vascular physiology and pathology and the need for the development of novel non-invasive diagnostic technology". A few years ago, a debate on the topic of our interest, "Predictive and Preventive Health", erupted in the cardiovascular community in the USA, dividing those in favor of risk-factor screening and prevention of CV events on the one side, and those who advocate early screening for the manifestation of the disease itself, on the other side. Drs. Jay Cohn and Daniel Duprez (University of Minnesota) have argued in favor of early identification of the disease through simple screening tests. They have developed a 10 point-test screening algorithm, which they have been using for quite some time for the management of cardiometabolic diseases [4]. Detection of subclinical carotid, coronary or femoral vessel atherosclerosis improves the risk prediction and management of the progression or regression of the disease [5,6]. This approach will also help clinician's manage the clinical complications of Type-2 diabetes, such as nephropathy, retinopathy and neuropathy [7]. However there is no simple non-invasive methodology to monitor the flow velocity of regional vascular beds, calculate fluid dynamics and develop proprietary software and algorithms for risk assessment and management. In the following few paragraphs, we will briefly discuss what is known about the flow velocity and fluid dynamics of the circulatory system and share our views on the development of non-invasive diagnostic technologies, that have been undergoing clinical validation and testing to address these issues.
Heart and the circulatory system constitute our vascular system, which modulates the circulation or distribution of the blood throughout the body. The circulatory system performs varieties of vital functions, such as carrying oxygen and nutrients to the cells and tissues as well as removal of the waste from the body. The heart a four chambered muscular organ beats approximately 70 times a minute and continues to do so, for over half a billion times in its life time. The circulatory system is a complex network of vascular tubes of varying sizes and texture. It is estimated to be the equivalent of 60,000 miles of vessels carrying about five liters of blood throughout the body every minute. The entire length of these vessels is lined with a monolayer of endothelial cells (endothelial cells can cover approximately six tennis courts). Vascular system has three types of arteries; arterioles, muscular arteries and elastic arteries. Morphology varies according to the functional needs. Arterioles are the smallest with narrow lumen and muscular walls. Muscular arteries are responsible for distribution of oxygenated blood throughout the body. Elastic arteries are larger vessels and their walls contain elastin. Presence of these specialized cells as well as the composition of the cellular matrix plays a very important role in the modulation of the flow velocity, fluid dynamics, vascular physiology and function.
The human cardiovascular system is an internal closed flow loop with multiple branches, circulating complex fluid, with variable speed and pressure. Important features of blood flow in arteries are pulsatile in nature, with numerous branches, which cause vessel wall shear stress (VWSS) to be cyclical and non-uniform. Blood flow velocity may change and can create pockets in which, VWSS is low and oscillates in different directions. Atherosclerosis tends to localize in these sites of low VWSS and create narrowing of the vessel or a stenosis. The relationship between vascular injury, inflammation, atherosclerosis and thrombosis is complex, especially in the post stenotic flow field [7]. Examples of Computational Fluid Dynamics (CFD) in biomedical systems as they relate to vascular function include, heart pumps, heart valves, vessel graft implants, cell fluid interface, artificial organ design, vessel wall shear stress and blood viscosity and flow velocity. Computational Fluid Dynamics in engineering applications seems straight forward compared to the biological systems. Walls of all blood vessels, except in the smallest consists of three layers: the tunica intima, tunica media, and tunica externa. The tunica intima reduces friction between the vessel walls and blood; the tunica media controls vasoconstriction and vasodilation of the vessels, and the tunica externa protects, reinforces and anchors the vessel to the surrounding tissues.
When we look at the blood vessel anatomy, average lumen diameter (D) and vessel wall thickness (T) varies considerably. According to published literature, the vessel anatomy is as follows: Elastic artery (D: 1.5 cm, T: 1.0 mm); Muscular artery (D: 6.0 mm, T: 1.0 mm); Arteriole (D: 37:0 um, T 6.0 um); Capillary (D 9.0 um, T: 0.5 um); Venule (D: 20.0 um, T: 1.0 um); Vein (D: 5.0 mm, T: 0.5 mm). Elastic or conducting arteries contain large amounts of elastin, which enables these vessels to withstand pressure fluctuations. Muscular or distributing arteries deliver blood to specific body organs; have greatest proportions of tunica media, and are more active in vasoconstriction. Arterioles are the smallest vessels and allow for exchange of nutrients between the blood and interstitial fluid. Resistance to flow is a measure of the friction between blood and the vessels wall, arises from three sources: blood viscosity, blood vessel length, and blood vessel diameter. Peripheral resistance is the most important factor influencing the regional or local blood flow, because vasoconstriction or vasodilation can alter local resistance, while systemic blood pressure remains unchanged.
Vascular Physiology
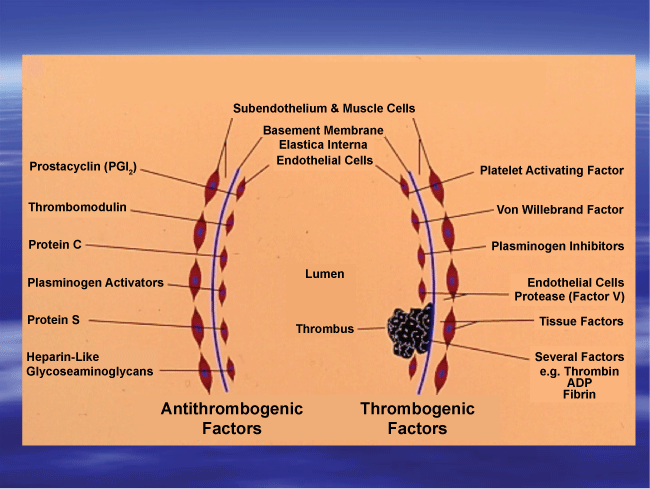
Functional and structural changes in the arterial wall precede the development of atherosclerosis, obstructive coronary artery disease and may even serve as an early marker for the hypertensive disease. Similar to the vessel wall, functional and structural changes in the vascular endothelial cells (EC) are modulated by a variety of thrombogenic factors as well as anti-thrombogenic factors (Figure1). Vasoactive chemicals both endocrine and paracrine, influence blood pressure by acting on vascular smooth muscle or the vasomotor center. Vasodilation directly affects the mean arterial pressure, cardiac output and total peripheral resistance. Some of the endogenous vasodilators include, prostacyclin, Prostaglandin (PG) E1, Prostaglandin E2, nitric oxide, inflammatory chemicals such as histamine, and kinins. Some of the known vasodilators of blood vessels include L-arginine, bradykinin, substance-P, niacin, and adenosine. On the other hand, norepinephrine, epinephrine, serotonin, PG-endoperoxides, thromboxanes, lipid peroxides, endothelium derived factors, angiotensin 11, are potent vasoconstrictors. Alterations in the balance between platelet (blood components) associated vasoconstrictors and EC-derived vasodilators results in vascular dysfunction (endothelial dysfunction). Studies from our laboratory at the University of Minnesota demonstrated the existence of such an imbalance in the production of these vasoactive compounds in cigarette smokers as well as users of hormonal contraceptives and in type-2 diabetic subjects [1,3,7] (Figure 1).
Fluid Dynamics
Computed Fluid Dynamics methods for simulating blood flow in three-dimensional models of arteries have either considered a rigid wall assumption for the vessel or used simplified or reduced geometries. Arteries adapt their diameters so as to maintain wall shear stress in a narrow range of values around 15 dynes/cm2. Current thinking about the role of fluid dynamics in atherosclerosis is that intimal thickening is a normal response to low wall shear stress and this intimal thickening can develop into an early atherosclerotic plaque [8-10]. In terms of risks for developing atherogenesis, the carotid bifurcation, the coronary arteries the intra-renal abdominal aorta and the vessels supplying the lower extremities are at highest risk. These observations have lead to the hypothesis, that local mechanical factors such as VWSS and mural tensile stress potentiate atherogenesis [9,10]. Ability to detect localized atherosclerosis plaques using ultrasound measurements has advanced significantly by investigations into the nature and occurrence of velocity disturbances created by arterial stenosis and diagnosis of carotid and femoral bifurcation disease.
Human blood exhibits shear-thinning behavior. At low shear rates or shear stress the apparent viscosity is high, whereas the apparent viscosity decreases with the increasing shear and approaches a minimum value under high shear forces [11]. In addition to variety of blood constituents (soluble as well as suspended) in the plasma, the cellular components as well as their rheological properties play a major role as determinants of blood fluidity or viscosity. Red blood cells (RBCs) are the major determinants of this effect. Therefore, deformation and orientation are the primary cellular factors affecting blood viscosity at high shear rates [11-13]. In view of these observations, it is important to consider variety of factors that affect the deformability of circulating blood cells. Studies from our laboratory have developed a novel method to follow cellular deformability [14]. We have demonstrated that common drug, aspirin affects the deformability of platelet membranes and promotes adhesion of platelets to vessel walls, whereas, epinephrine reverses this effect of aspirin on platelet membranes [14].
Blood flow velocity and pressure in large arteries are greatly influenced by the deformability of the vessel [15]. Researchers at Stanford University, USA, have developed mathematical formulation to the fluid-solid coupling, membrane formulation, time integration method, boundary and initial conditions. Contrary to earlier studies, where majority of blood flow simulations had assumed rigid walls, in recent years, significant progress have been made in computing blood flow dynamics in deformable walls, using the Arbitrality Lagrangian-Eularian method, the immersed boundary method, space-time finite element method and coupled momentum method (CMM). Xiong et al. have described the first of a kind simulation of blood flow and vessel wall dynamics, employing large scale-3D subject specific models and assigned variable mechanical wall properties [16]. Cellular deformability plays much greater role in smaller arteries and capillary circulation. Wang and Xing have simulated the dynamics of the blood flow and the role of RBCs deformability in capillaries using a numerical approach [17]. Results of their studies suggest that RBCs in narrow capillaries change to parachute shape in the flow fields. The profile of the capillary flow seems to be markedly blunt in comparison to the parabolic one, which characterizes the cell free plasma flow in these capillaries. Since a variety of things can influence the hematocrit, blood constituents and behavior of circulating cells, flow velocity and fluid dynamics of deformable vessels plays an important role, in physiology and pathology of blood vessels. Readers are urged to refer to comprehensive reviews on this subject for additional information [11-13].
The non-optical methods used worldwide for vascular imaging are X-ray/CT, MRI, Ultrasound (US), and Positron Emission Tomography (PET). X-ray imaging technology is based on the attenuation of X-ray energy, inside the body by different tissues. The technique has been in use for many years to examine large blood vessels. An advanced technology, Computed Tomography (CT) allows 3D visualization of the vessels and surroundings. CT scans use multiple x-rays taken at thin cross-sections in the region of interest. Micro-CT (uCT) provides much higher resolution (-1 um) imaging, better than Ultrasound (-30 um), and MRI (-100 um). This technology allows visualization and quantification of microvasculature with contrast agents. Milner et al. have reported a method for monitoring internal carotid artery waveforms with MRI-phase contrast velocity imaging [18]. According to them, "such techniques combined with subject specific MRI measurements of carotid artery plaque thickness and composition, provide tools necessary for entirely non-invasive, prospective, in vivo human studies of hemodynamics and the relationship of hemodynamics to vascular disease" [18].
Doppler Ultrasound (US) is non-invasive real-time method used for monitoring hemodynamics. This technique is based on Doppler Effect Principle that proposes changes in frequency of a wave for an observer (red cells), moving relative to the source of the respective wave (ultrasound transducer). Currently available US machines offer three methods of evaluation (triplex mode). In B-mode US, linear arrays of transducers simultaneously scan though a plane of tissues as a two dimensional gray-scale image. Colored US images of flow (Color or Spectral modes) are obtained from moving of red cells. Pulsed wave Doppler provides information on changing flow velocity throughout the cardiac cycle [19-21]. Synchron X-ray imaging technique uses carbon dioxide (CO2) micro-bubbles as flow tracer for measurement of pulsatile blood flows [20]. Authors claim that this technique could be used for accurately monitoring whole velocity field information of real pulsatile blood flows and has strong potential for hemodynamic diagnosis of cardiovascular disease. According to the Society of Radiologists in Ultrasound Consensus Conference (DOI: http//:doi.org/10.1148/radiol.2292030516), Doppler US is by far the most common imaging examination performed worldwide to aid in the diagnosis of carotid disease.
Positron Emission Tomography (PET) is the gold standard diagnosis tool for visualization of tumor burden, progression or regression. The most common form of nuclear medicine-scan uses a gamma-ray emitting radioisotope bound to a chemical with known physiological properties. After it is administered, single photons emitted by the decaying isotope are detected with a gamma camera. Usually, 2-deoxy-2(18fluorine) fluro-D-Glucose (FDG), an analogue of glucose, is used as a radiotracer; 18FDG and its metabolite 18F-FDG-6-Phosphate are traced in the tumor, which consumes the sugar at a greater rate than the normal tissue. In a recent study, Mazurek et al. have demonstrated the utility of using 18FDG uptake in pericoronary adipose tissue in patients with stable coronary artery disease as an independent predictor of atherosclerotic lesions' formation [22]. Parameters such as flow velocity (FV); fluid dynamics (FD), computed fluid dynamics (CFD) blood viscosity (BV), cellular rheology (CR), vessel wall shear stress (VWSS), hemodynamics, metabolism, proprietary software and analytics have been used in the development of precision diagnostic tools for medical imaging. We have just made a limited attempt to describe few applications in this overview; readers are urged to refer to comprehensive reviews and textbooks for additional information [23-26]. We have discussed some of the many US applications and opportunities for further improvements, in the subsequent paragraphs.
Heart Valves
Since the first heart valve replacement in 1952 (Hufnagel), more than 50 valve designs have been developed [10]. Currently over 290,000 heart valve procedures are performed annually worldwide. It is essential to assess the fluid mechanics of prosthetic heart valves in order to understand clinical valve success or failure. The use of Hufnagel cage valve became obsolete with the introduction of the Starr-Edwards ball-and-cage valve in 1962. Later caged-disc valves were developed (Kay-Shiley). Most significant development in mechanical heart valves was the development of Bjork-Shiley and Lillehei-Kaster tilting-disc valves. St. Judes Medical (SJM) introduced their bileaflet (pyrolitic carbon) valves in 1978. Professor Walter Lillehei at the University of Minnesota developed the St. Judes Medical bi-leaflet heart valve. In this connection, I would like to mention a little known fact, as to where this idea came from? Dr. Lillehei had a surgical resident from Mumbai, India, with him in those days. In one of the casual conversations, this resident mentioned as to how water is controlled in irrigation canals in India by dual acting floodgates. I did hear this story from Dr. Lillehei, when I met him in one of the meetings. ATS Medical of Minneapolis introduced Open Pivot valve in 2000. Both the SJM and ATS heart valves were tested in our laboratory (Blood Biocompatibility Lab, Lillehei Heart Institute, University of Minnesota) for their blood compatibility. We developed extracorporeal circulation model for exposing the heart valves to optimal flow velocities and VWSS.
Vascular Perfusion Systems
Blood Perfusion Systems (BPS) was custom designed to meet the requirements for specific studies. For testing individual carbon leaf lets less than 100 ml of fresh human blood was used and the leaflets exposed to circulating blood in custom developed perfusion chambers. For these studies Watson-Marrow Bredel Pumps (Boston, MA) were used for perfusing blood at an appropriate flow rate. After circulating blood for 20-60 minutes over the test surface the perfusion was stopped, surfaces washed with buffer and fixed for scanning electron microscopy. For testing fully assembled valves, 'MOX'-100 Oxygenator (Waters Instruments, Rochester, MN) was used. Fully assembled heart valves manufactured from different companies were tested in custom designed chambers using freshly obtained human blood as well as animal blood. Desired flow rate of blood was obtained using a diaphragm pump. After perfusing blood over the test valves for 20-60 minutes, the valve-assembly was washed with buffer, fixed with glutaraldehyde for further processing the heart valves for characterization of the surfaces by scanning electron microscopy.
Blood biocompatibility laboratory at the University of Minnesota has evaluated both pyrolytic carbon (PC) leaf lets as well as fully assembled heart valves manufactured by different companies, using customized blood perfusion system. Although pyrolytic carbon is supposed to be very inert, all "PC" surface tested were very reactive and attracted fibrinogen and platelets onto their highly polished surfaces. Blood obtained from donors who had ingested aspirin (350 mgs), Clopidogrel (75 mgs) or Coumadin were less reactive (10-30% less reactive) compared to control (100%). As expected, blood obtained from animals, (sheep, pig) were relatively non-reactive with the valve surfaces. Significant finding from these studies was that application of "force field" current on PC surface virtually eliminated platelet interaction on charged surfaces (less than 5% interaction) compared to the control untreated heart valves ( > 90% reaction).
In spite of the widespread use of artificial heart valve designs, neither mechanical nor bio-prosthetic valves are free from complications. The observed complications are as follows: structural valvular deterioration, non-structural dysfunction, valve thrombosis, embolism, bleeding and endocarditis. Bio prosthetic valve areas were found to develop tissue calcification and tearing. The mechanical valves were associated with hemolysis, platelet activation, and thromboembolic events. Majority of complications are related to non-physiological flow patterns in the vicinity of the heart valves. As mentioned in an earlier paragraph, our studies at University of Minnesota were aimed at determining the extent of platelet activation during non-physiological blood flow over the mechanical heart valves. It is well known that abnormal flow patterns promote blood cell destruction and thus may initiate, thrombus formation as well as tissue calcification [27]. In a study conducted by Chandran et al., the peak turbulent shear stress measured along the centerline plane of the SJM valve at peak systole was in the order of 1500 dyn/cm2 [28]. There are considerable advances made in the application of CFD and we hope, in the near future superior valves will be designed with the use of computational fluid dynamics approaches.
Vascular Stents
According to published literature, 20% of the nearly 1 million stents deployed annually develop restenosis due to neointimal hyperplasia [8]. Researchers have used commercially available software package (CFD-ACE: CFD-RC, Huntsville AL) to perform simulations to test the hypothesis that differences in the geometric design of an implanted stent (eg: number, width, and thickness of struts) influence acute distributions of VWSS predicted with three-dimensional (3D)-CFD modeling and compared these results with those produced by a different stent-to-artery deployment ratio [29]. In these studies, majority of the vessel wall was subjected to VWSS values between 5 and 8 dyn/cm2 when the stent thickness was 0.096 mm. Altering the deployment ratios, did not substantially affect the percent distribution of VWSS in this range. Studies have shown that vascular damage, as well as regions of lower shear stress at the inlets of the stent may be more susceptible to neointimal hyperplasia.
Vascular Assist Devices
The number of people suffering from congestive heart failure in the United Stares of America is estimated to be more than 4.5 million. Majority of them could benefit from a mechanical assist device. The application of computational fluid dynamics principles and models has revolutionized the design of Ventricular Assist Devices (VADs), to shift from large bulky devices, to a variety of smaller devices for patients with different cardiovascular conditions. Many of these devices are continuous flow devices (MicroMed Debakey VAD, HeartMate 11, and Jarvik 2000 CentriMag, HeartMate 111, 50ccLvad). Curtas et al. have described the process of developing a device from prototype to clinical device, using HeartQuest LVAD as an example [30]. The project used three software programs for all of their development work; BladenGen, YurboGrid and YASC flow. In addition to this article one can refer to an exhaustive monograph on this subject for further information [31]. The final pump they developed seemed to perform well and according to the researchers, did not damage the blood and its components. Continuous-flow devices incorporate either axial or centrifugal pump technology that generates high-speed rotation of the blood. For instance, in CentriMag pump researchers found a Pressure at the head of 320 mm HG, at a flow rate of 5.0 L/min and a rotational speed of 4000 rpm [32,33]. In view of the fact that continuous flow devices use axial or centrifugal pumps, which generate high-speed rotation of the blood, there is considerable concern about activation of the coagulation pathway as well as circulating platelets, under increased shear stress. We at the University of Minnesota, evaluated the blood pump compatibility using our extracorporeal circulation model, with freshly obtained human blood. We followed presence of circulating biomarkers (tissue factor, fibrinogen degradation products, platelet micro particles, activated platelets, CD36 expression, E and S-selectin) for activation of these pathways. As a collaborative study with the researchers (Professor Mark Slaughter and his associates) at Christ Hospital, Chicago, we looked at these biomarkers in patients who had permanent LVADs for prevention of heart failure. These patients similar to those who received heart valves were on anti-platelet therapy using Aspirin, Clopidogrel or a combination of both these drugs. Results were inconclusive because of the sample size of these studies (N of 35).
Device induced complications are common in the use of LVADs. CFD principles and programs are extensively used in the design and process of LVADs. However, the best method is elusive and is debatable. The Device induced complications include damage of blood cells, activation of coagulation pathway and platelets, thrombosis and emboli. Many studies have demonstrated that in view of the high shear forces, endothelialization is hampered and suggest that the future pumps be developed with low shear rates to promote optimum endothelialization. Karmonik et al. using image based modeling of the pump, based on computed fluid dynamics (CFD) data, suggest that device outflow graft should be placed in such a way, that the jet of blood is aimed at the lumen of the aortic arch, to avoid turbulences and increases in shear stress [33].
Monitoring Flow Velocity and Fluid Dynamics
Now that we have reviewed some of the CFD applications in the development of vascular stents, heart valves and vascular assist devices, we would like to discuss some possible futuristic applications in following altered flow of blood as well as its effect on vessel wall physiology and pathology. Alterations in the vessel wall physiology and compliance of the vessels and the changes if any, in the blood flow velocity are the earliest stages of vascular dysfunction that could be detected. There are several devices available in the market that can monitor changes in the flow velocity and provide information on endothelial dysfunction. Some of the devices in use include, CV Profilor (Hypertension Diagnostics™ of USA: hypertensiondiagnostics.com), Periscope (Genesis Medical System, Hyderabad, India: genesismedicals.com) and TM-Oxi (LD Technologies, Florida: www.ldteck.com). Majority of the people who suffer heart attacks have no symptoms, making prevention very difficult. However, now with the availability of these devices, we will be able to identify heart disease (vessel wall disease or dysfunction) at its earliest stage and in people with no symptoms. In spite of the advances made in the diagnostic medical device development, we still do not have a simple hand-held point-of-care monitor for diagnosis and management of vascular dysfunction [34].
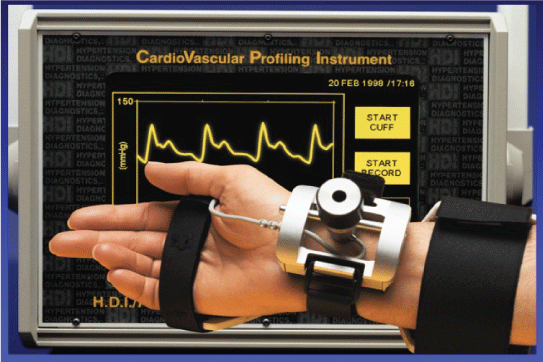
Hypertension Diagnostics (HD) (www.hypertensiondiagnostics.com) of Minneapolis, Minnesota, has developed a method for non-invasively measuring the elasticity of large and small arteries, of which small artery elasticity is the earliest and most sensitive marker of cardiovascular disease (Figure 2). One of the tests that the University of Minnesota uses in their 10-point risk assessment is CV-Profilor [4]. The device collects 30 seconds of blood pressure wave form data from a small artery and a big one, performs analysis of the digitized blood pressure waveforms and generates a report that contains information on the blood pressure, body surface area, body mass index, both C-1 large and C-2 small artery elasticity indices. According to the researchers who have used this device, changes in the small artery elasticity have been highly predictive of cardiovascular disease. Its main product CVProfilor DO-2020, Cardiovascular Profiling System has been approved by the US Food and Drug Administration. Although the manufacturers and some of the users claim that the small artery elasticity is highly predictive of CVD, the device has not been used extensively in population studies, to determine early on set of endothelial dysfunction or vascular disease.
Genesis Medical Systems of Hyderabad (www.genesismedicals.com), India, have developed a simple non-invasive oscillometric device (Periscope) to monitor pulse wave velocity in small arteries. The report generated by this system provides 8-second tracings of ECG, all pressure pulse waveforms and calculated results. Pulse wave velocity is the speed at which the blood pressure pulses travel from the heart to the peripheral artery after the blood rushes out during contraction. This measurement is used for evaluating arterial stiffness. Periscope like CV profiler has been in use for number of years in different laboratories for determining arterial stiffness. Independent clinical validation has not been done with these devices to establish sensitivity or specificity for monitoring endothelial dysfunction in general population or in patient populations. It is our strong belief, that we can develop a cost effective hand-held device with proprietary software and analytics, dedicated to monitor endothelial dysfunction, which is in our opinion and that of others, the earliest sign of vascular pathology. Pulse wave velocity increases with stiffness of the arteries. The pulse wave velocity (PWV) is considered one of the most important clinical parameters for evaluating cardiovascular risk, and therapeutic efficacy [35]. The commercial devices dedicated to PWV measurements estimate a regional assessment, measured between two vessels. However, we feel that a local measurement is more precise for evaluation of the health of the vessels. Peripheral arteries are stiffer than the deeper arteries. The heterogeneity of the structure of arterial wall and its components pose challenge for PWV measurements and computations.
Ultrasonography has been used extensively as a diagnostic imaging modality. Dr. Aaron Fenster and his associates at Robarts Research Institute, London, Canada, have developed three-dimensional ultrasound imaging for improving the visualization and quantification of atherosclerotic plaque in the carotid artery. This technology if available worldwide will be of great help in monitoring the morphology, volume of the plaque, and for the assessment of therapeutic efficacy [35]. There are several types of ultrasound systems for obtaining 3D images. The commonly used options are the Mechanical Linear 3D ultrasound scanning and the sensed free-hand techniques. A 10-year follow-up study by Dr. Andrew Nicolaides and associates on carotid and femoral bifurcation ultrasound screening demonstrated the usefulness of this simple technique in identifying populations with varying degree of risks for CV events, depending upon the progression of arteriosclerotic vascular disease. They were able to classify those with low risk, limited risk, moderate risk and high risk, based on carotid and femoral bifurcation morphology [36].
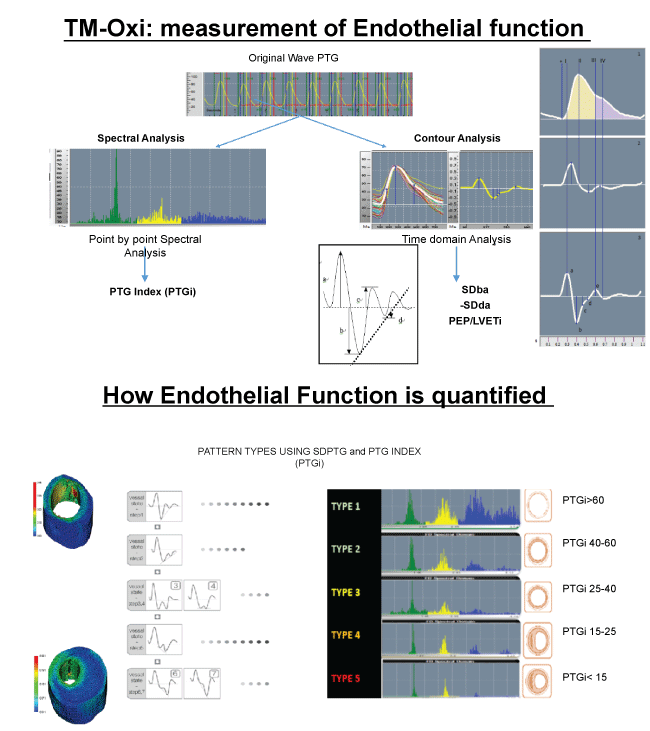
Gandhi and Rao have described a non-invasive method, which uses spectral analysis of photoplethysmography (PTG) to evaluate the cardiovascular risk. The TM-Oxi system developed by the LD Technologies of Miami, Florida, uses a pulse oximeter and an automatic oscillometric blood pressure monitor managed by proprietary software [37]. The PTG provides the measurements of relative blood volume in the fingertip during cardiac events. The first derivatives of PTG peaks are used for heart rate computing and the second derivatives for more accurate recognition of the inflection points of the original beats. In brief, the ratio of the amplitude of this waveform provides information on the relative degree of arterial stiffness [38]. Over thousand patients have been screened at our IPC Clinic in Mumbai, India, using this technology. These studies have confirmed the presence of significant endothelial dysfunction in the diabetic population [7].
As suggested by Albert Maarek et al., in their seminal work on the use of Pulse Oximeter and simple Blood pressure monitors, to detect and measure the endothelial function, we would like to develop simple cost effective pulse wave monitors, to look at the regional flow of blood and compute the altered flow dynamics with proprietary analytics and algorithms [7]. LD Technologies, Miami, FL, USA, specializes in the development of noninvasive Point-Of-Care devices. They have put together a platform called "RISC" (for Life Span, India), which uses ANS tests, HRV tests, sudomotor function tests, and the TM-Oxi system. The diagnostic platform used at the IPC Heart Care (TM-Oxi), Mumbai, India, and the Life Span Kiosks (RISC) in different cities in India, is a combination of many devices and uses photoplethysmography, spectrophotometry, oscillometry, and galvanic skin response technologies and displays data rapidly. The scores for various functional tests are color-coded (green, orange, yellow, and red) and printed out graphically as well as digitally. It generates impressive diagnostic reports with some recommendations. According to the authors who developed this technology, spectral analysis of plethysmography data gave high specificity (96.8%) and sensitivity for detecting CVD (82.5%). Having said that, I would like to clarify that the data are derived by the use of proprietary software and analytics. In addition, the various risk scores computed are not usually used for the management of these diseases in most diabetic clinics. It would have been more useful for following up progression of the vascular disease and for monitoring efficacy of disease management, if the risk scores could be developed for the known risk factors for CVD and for clinical parameters such as blood glucose, HbA1c, plasma insulin, etc. Since the device is modular such diagnostic capabilities could be developed and added to this platform [7,34,37] (Figure 3).
We would like to use either Piezo sensors, or modified pulse oximeters and develop cost effective devices, to obtain pulse waveforms at various pulse pressure points and calculate flow velocity measurements, so that we can develop information about regional blood flow dynamics [7]. The pulse oximeter combines the two technologies of spectrophotometry (which measures hemoglobin oxygen saturation) and optical plethysmography (which measures pulsatile changes in arterial blood volume at the sensor site) [7]. In the proposed studies we will use the photo-plethysmography to compute the flow velocity of arterial blood at different regional beds. We also would like to explore other options such as the use of ultrasound imaging to follow blood flow dynamics in peripheral arteries and veins.
As we mentioned earlier, B-mode ultrasound is in use for several years as a convenient, safe diagnostic tool. This method has been extensively used for monitoring carotid and femoral bifurcation morphology, to detect subclinical atherosclerosis, plaque volume, plaque morphology, plaque texture, obstruction of the arteries (arterial sonography), venous thrombosis (Thrombosonography), venous insufficiency (venosonography), progression of the plaque volume and response to therapies [36]. We have discussed with major manufacturers of ultrasound equipment (Fujifilm Holdings, GE Healthcare, Siemens Healthcare, Phillips Healthcare, Shimadzu Corporation, Toshiba Medical Systems, Carestream Health and Hitachi Medical) the need for a hand-held device. Some of these manufactures already have high-end equipment capable of imaging peripheral arteries and veins. We the members of a Consortium for the development of affordable medical technologies, National Design Research Foundation (NDRF), Bangalore, India, are interested in developing a simple hand-held ultrasound imaging device, which can be interfaced with existing non-invasive diagnostic platforms such as RISC and TM-Oxi, with proprietary software and analytics, so that the imaging of the peripheral arteries and veins could be done at the clinics, to follow flow velocity alterations due to subclinical atherosclerosis or blocks. We are currently exploring calibrating available 2D Ultrasound instruments for monitoring peripheral arteries and veins. Once these devices are clinically validated, we will develop appropriate software and analytics for clinical use.
Conclusions
Many Methods are available for Risk Profiling of cardiovascular disease (CVD) Patients. For a long time Framingham Risk Score (FRS) used to be the Gold Standard. In recent years several groups have used monitoring IMT and consider this method a considerable improvement over FRS. Some newer studies have shown that Calcium CT seems to outscore both the above two methods. Jay Cohn and associates have shown that, University of Minnesota 10 point scoring, to be a better predictor of CVD Risk on out-come based results; over a 6 year follow up. 3D Ultrasound developed by Robarts Laboratory at London, Ontario, seems to provide the much needed ability to monitor plaque volume, plaque progression and plaque regression followed by therapy or life style changes. Ultrasound imaging (screening 10-year follow-up) of subclinical atherosclerosis by Prof. Andrew Nicolaides and associates have demonstrated the usefulness of this technique to determine various stages of the disease progress of the vessel walls. Further studies on the morphology and composition of plaques will provide the ability to predict the nature of the plaque in terms of its stability or vulnerability. There is a great need for the development of ultra small, super sensitive ultrasound systems that can monitor the atherosclerotic plaque in regional vascular beds of both small and medium size vessels, so that the progression of the disease as well as the effective management of the disease with appropriate treatment modalities can be followed.
References
- Rao GHR, V Kakkar (2001) Coronary Artery Disease in South Asians: Epidemiology, Risk Factors and Prevention. Jaypee Medical Publishers, New Delhi, India.
- Rao GHR, S Thanikachalam (2005) Coronary Artery Disease: Risk Factors Pathophysiology and Prevention. Jaypee Medical Publishers, New Delhi, India.
- V Mohan, Rao GHR (2007) Type 2 Diabetes in South Asians: Epidemiology, Risk Factors and Prevention. Jaypee Medical Publishers, New Delhi, India.
- Cohn J, Duprez DA, Grandits GA (2005) Arterial elasticity as part of a comprehensive assessment of cardiovascular risk and drug treatment. Hypertension 46: 217-220.
- Nicolaides A, Panayiotou AG (2016) Screening for atherosclerotic cardiovascular risk using ultrasound. J Am Coll Cardiol 67: 1275-1277.
- Nicolaides A (2010) Screening for cardiovascular risk. Br J Cardiol 17: 105-107.
- Maarek A, Gandhi PG, Rao GHR (2015) Identifying autonomic neuropathy and endothelial dysfunction in Type-2 diabetic patients. EC Neurology 2: 63-78.
- Wooton DM, Ku DN (1999) Fluid dynamics of vascular system, diseases and thrombosis. Ann Rev Biomed Eng 1: 299-329.
- Giddens DP, Zarins CK, Glagov S (1993) Atherosclerosis. J Biomechanical Eng 115: 588-594
- Glagov S, Zarins CK, Giddens DP, et al. (1998) Hemodynamics and atherosclerosis insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med 112: 1018-1031.
- Baskurt OK, Mesielman HJ (2003) Blood rheology and hemodynamics. Sem thromb Hemost 29: 435-450.
- Coley A (1990) Fluid mechanics and biorheology. Clin hemorheology 10: 3-19.
- Merrill EW (1969) Rheology of blood. Physiol Rev 49: 863-888.
- Burris SM, Smith CM, Rao GHR, et al. (1987) Aspirin treatment induces platelet resistance to deformation. Arterioscl Thromb Vasc Bio 7: 385-388.
- Figueroa CA, Vignon-clementel IE, Jansen KE, et al. (2006) A coupled momentum method for blood flow in three-dimensional deformable arteries. Compu Methods in Appl Mech Eng 195: 5685-5706.
- Xiong G, Figueroa CA, Xiao N, et al. (2011) Simulation of blood flow in deformable vessels using subject specific geometry and spatially varying wall properties. Int J Numer Methods Biomed Eng 27: 1000-1016.
- Wang T, Xing Z (2010) Characterization of blood flow in capillaries by numerical simulation. J Med Phys 1: 349-356.
- Milner JS, Moore JA, Ruft BK, et al. (1998) Hemodynamics of human carotid artery bifurcations: computational studies with models reconstructed from magnetic resonance imaging. J Vasc Surg 28: 143-156.
- Abbas AF, Fortuin FD, Schiller, et al. (2003) A simple method of non-invasive estimation of pulmonary vascular resistance. J Am Coll Cardiol 41: 1021-1027.
- Park H, Yeom E, Seo S, et al. (2015) Measurement of real pulse blood flow using X-ray PIV technique with CO2 micro-bubbles. Sci Rep 5: 8840.
- Jennings D, Raghunand N, Gilles RJ (2008) Imaging Hemodynamics. Cancer Metastasis Rev 27: 589-613.
- Majurek T, Kobylecka M, Zelenkiewicz M, et al. (2016) PET/CT evaluation of 18F-FDG uptake in pericoronary adipose tissue with stable coronary artery disease: Independent predictor of atherosclerotic lesions' formation? J Nulc Cardiol.
- Francombe T, Krzysztof Iniewski (2013) Medical Imaging: Technology and Applications. CRC Press, Vancouver, Canada.
- Smistad E, Falch TL, Bozorgi M, et al. (2015) Medical Imaging Segmentation on GPUs: A comprehensive review. Med Image Anal 20: 1-18.
- Suetens P (2002) Fundamentals of Medical Imaging. Cambridge University Press, London.
- Dasi LP, Simon HA, Sucosky P, et al. (2009) Fluid mechanics of artificial heart valves. Clin Exp Pharmacol Physiol 36: 225-237.
- Chandy T, Vasudev SC, Rao GHR (1998) Changes in pericardial calcification due to anti-platelet agents: in vitro studies. Artif Organs 22: 666-671.
- Chandran KB, Rittgers SE, Yoganathan AP (2006) Biofluid Mechanics: The Human Circulation. CRC Press Boca Raton.
- LaDisa JF Jr, Olson LE, Guler I, Hettrick DA, Audi SH, et al. (2004) Stent design properties and deployment ratio influence index of wall shear stress: a three-dimensional computational fluid dynamics investigation within a normal artery. J Appl Physiol 97: 424-430.
- Curtas AR, Wood HG, Allaire PE, et al. (2002) Computational fluid dynamics modeling of impeller designs for the HeartQuest left ventricular assist device. ASAIO J 48: 552-561.
- Goldstein DJ, Mehmet Oz (2000) Cardiac Assist Devices. Futura Publishing Company, Armonk, New York.
- Zhang J, Gellman B, Koert A, et al. (2006) Computational and experimental evaluation of the fluid dynamics and hemocompatibilty of the CentriMag blood pump. Artif Organs 30: 168-177.
- Karmonik C, Partovi, S Loebe M, et al. ( 2014) Computational fluid dynamics in patients with continuous-flow left ventricular assist device support show hemodynamic alterations in the ascending aorta. J Thorac Vasc Surg 147: 1326-1333.
- Rao GHR, Gandhi PG (2014) Need for a non-invasive diagnostic platform for early detection and management of cardiometabolic disorders. J Clinical and Preventive Cardiology 3: 93-98.
- Pereira T, Correira C, Cardoso J (2015) Novel methods of pulse wave velocity measurement. J Med Biol Eng 35: 555-565.
- Belcaro G, Nicolaides AN, Ramaswami G, et al. (2001) Carotid and femoral ultrasound morphology screening and cardiovascular events in low risk subjects: a 10-year follow-up study (the CAFES-CAVE study (1)). Atherosclerosis 156: 379-387.
- Gandhi PG, Rao GHR (2014) The spectral analysis of photoplethysmography to evaluate an independent cardiovascular risk factor. Int J Gen Med 7: 539-547.
- Lewis JE, Lantigua L, Atlas SE, et al. (2014) A cross-sectional assessment to detect type-2 diabetes with endothelial and autonomic nervous system markers using a novel system. J Diabetes and Met Dis 13: 118.
Corresponding Author
Gundu HR Rao, Emeritus Professor, Laboratory Medicine and Pathology, Lillehei Heart Institute, University of Minnesota, Minnesota, USA, Tel: 301-444-4545.
Copyright
© 2016 Rao GHR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.