Parotid Acinic Cell Carcinoma with Intracranial Metastasis: A Case Report and Literature Review
Abstract
Introduction: Acinic cell carcinoma is a rare salivary gland neoplasm frequently associated with local recurrence. While distal metastasis occurs in approximately 19% of cases, intracranial metastasis has been rarely reported in the literature and both preoperative patient characteristics and postoperative prognoses are not currently well-characterized.
Methods: In this study, we conducted a systematic review of all relevant studies in the Pubmed database and presented a case series of salivary gland acinic cell carcinomas with intracranial metastasis found in the literature. All relevant studies were reviewed, and information related to the number of patient(s) described, their sex and age, presenting symptoms, location of metastasis, treatment, and time intervals from initial diagnosis and intracranial metastasis and from treatment for intracranial metastasis to last reported follow-up were recorded.
Results: Seven studies matching the inclusion criteria were included for data collection and further analysis. The mean age of presentation was 48.9 ± 11.9 years, with intracranial metastasis occurring, on average, 8.7 ± 10.3 years following the initial diagnosis of acinic cell carcinoma. All patients with reported treatment modalities received either surgical resection and/or radiation therapy. The minimum postoperative follow-up for these patients occurred at 11 months, with no postoperative deaths reported in any cases previously published in the literature.
Conclusion: Parotid acinic cell carcinoma is a frequently recurring salivary gland neoplasm with metastatic potential, often many years following initial diagnosis. Though intracranial metastasis has only rarely been reported in the literature and associated with increased management complexity, patients demonstrate a positive prognosis following treatment.
Keywords
Acinic cell carcinoma, Intracranial, Metastasis, Parotid gland, Salivary gland
Abbreviations
ACC: Acinic Cell Carcinoma; ACTH: Adrenocorticotropin; DOG1: Discovered on Gastrointestinal Tumor Protein 1; EBV: Epstein-Barr Virus; EMA: Epithelial Membrane Antigen; NCDB: National Cancer Data Base; NR4A3: Nuclear Receptor 4A3; PAS: Periodic-Acid Schiff; SBRT: Stereotactic Body Radiation Therapy
Introduction
Salivary gland carcinomas are divided into tumors arising from cells of the major intraoral glands (parotid, submandibular, and sublingual glands) and those from the numerous minor intraoral glands lining the upper respiratory tract. Acinic cell carcinoma (ACC) is a rare primary salivary gland malignancy composed of serous cells that usually has a favorable prognosis following surgical resection [1]. While local recurrence of salivary gland ACC is common, distant metastases to visceral organs in the body are infrequently reported in the literature [2]. Even rarer are reports of intracranial metastases involving the brain, which significantly increase the complexity of patient management. In this study, we review the literature regarding intracranial metastatic ACC and present an illustrative case of an older-aged female patient with a long history of recurrent and metastatic ACC who underwent neurosurgical resection of a newly found frontoparietal lobe metastatic lesion.
Methods
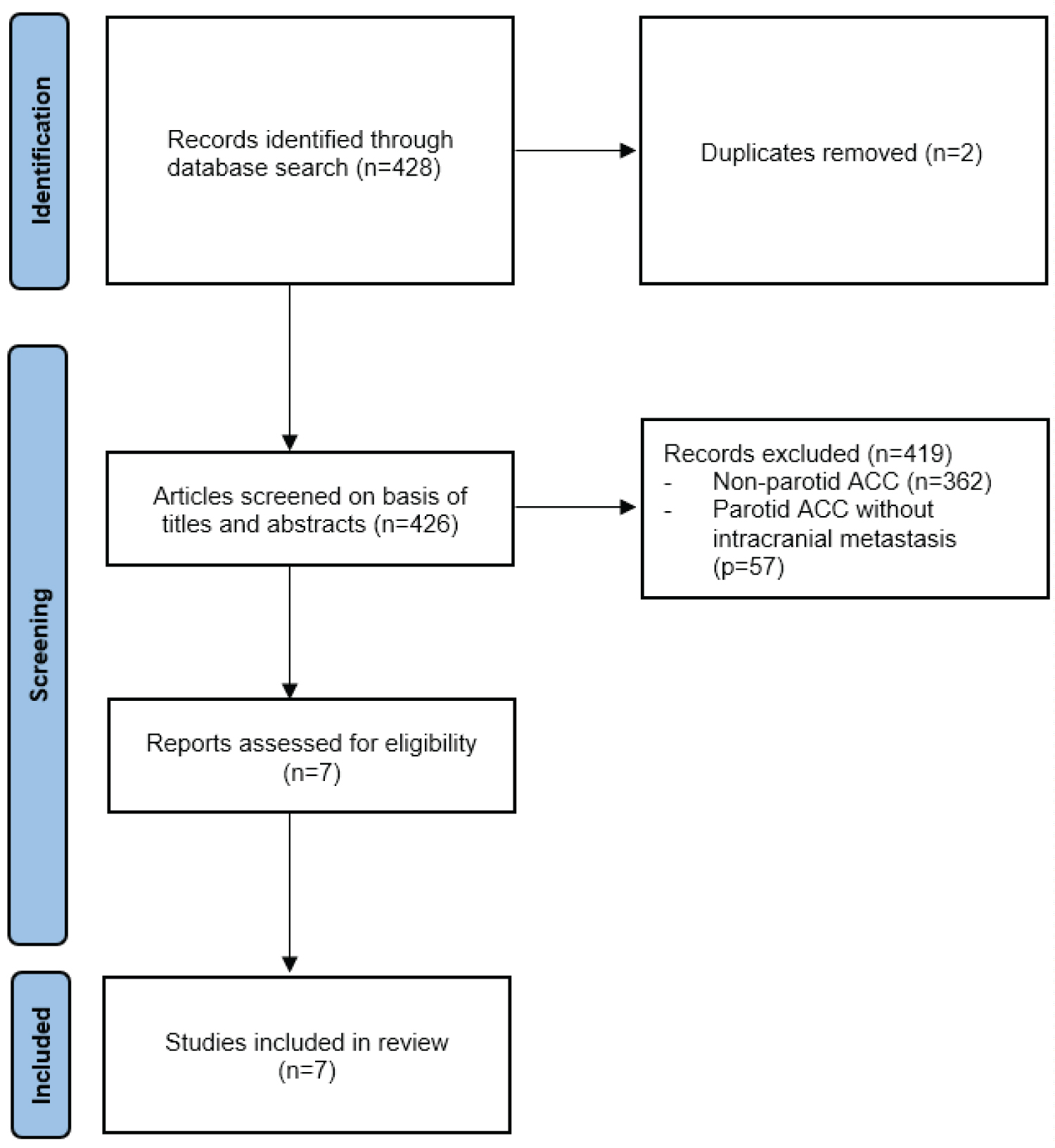
We conducted a systematic search using the Pubmed database for case reports or case series describing patients with intracranial metastatic ACC arising from the salivary glands. Search terms included "acinar cell carcinoma," "metastatic," "parotid," and/or "salivary gland." A study was included in our review if the patient(s) included in the case report or series had a confirmed diagnosis of ACC on histological analysis. Systematic reviews referencing other case reports or case series as part of their study were excluded from our search due to possible duplication of patient information. All relevant studies were reviewed, and information related to the number of patient(s) described, their sex and age, presenting symptoms, location of metastasis, treatment, and time intervals from initial ACC diagnosis and intracranial metastasis and from treatment for intracranial metastasis to last reported follow-up were recorded. The search strategy used for study inclusion is represented by the PRISMA flow diagram in Figure 1 [3].
Results
Four-hundred and twenty-eight results were provided by the PubMed database search. Of those 428 articles, 421 results were excluded due to duplicated search findings (n = 2), studies describing primary ACC not emerging from the salivary glands (n = 362), and studies describing salivary gland ACC without intracranial metastasis (n = 57). Seven studies matching the inclusion criteria described in the Methods were included for data collection and further analysis. The mean age of presentation for all other cases of intracranial metastatic ACC reported in the literature was 48.9 ± 11.9 years, with intracranial metastasis occurring, on average, 8.7 ± 10.3 years following the initial diagnosis of ACC. Four of six patients (66.7%) with reported treatment modalities underwent surgical resection, and all patients with reported treatment either received surgery and/or radiation. For patients in which a last follow-up appointment was reported (n = 5), patients were noted to be in good health at a minimum of 11 months following treatment, with no postoperative deaths reported in any cases previously published in the literature. The results are shown in Table 1 [4-10].
Case Presentation
A 67-year-old Caucasian female with past medical history of hypothyroidism and metastatic acinic cell carcinoma of the left parotid gland (initially diagnosed in 1995) presented for elective left craniotomy for resection of a left-sided frontoparietal lobe lesion. Her past surgical history was significant for left-sided parotidectomy in 1995, and multiple resections since that time for local recurrence and distant metastases to the ribs (status post cryoablation), right frontal bone, left temporal dome, right parietal bone, hip (status post radiation therapy), liver (status post three radiofrequency ablation treatments), left sacrum (status post cryoablation), and right temporalis bone. The patient also had chronic dysphagia and was unable to fully close her mouth due to long-standing left-sided facial paresis, and chronic pain resulting from progressive radiation osteonecrosis of the right mandible. Monitoring PET-CT performed in late 2021 confirmed increased FDG uptake and lytic osseus lesions in the sacrum, ileum, and pubic ramus, as well as multiple intracranial lesions concerning for metastatic intracranial disease. Three months after completing stereotactic body radiation therapy (SBRT) to the brain and sacrum in early 2022, follow-up imaging noted tumor progression of a left frontal operculum lesion, which measured 32 × 24 × 23 mm. The patient was subsequently referred to our neurosurgery department for surgical resection.
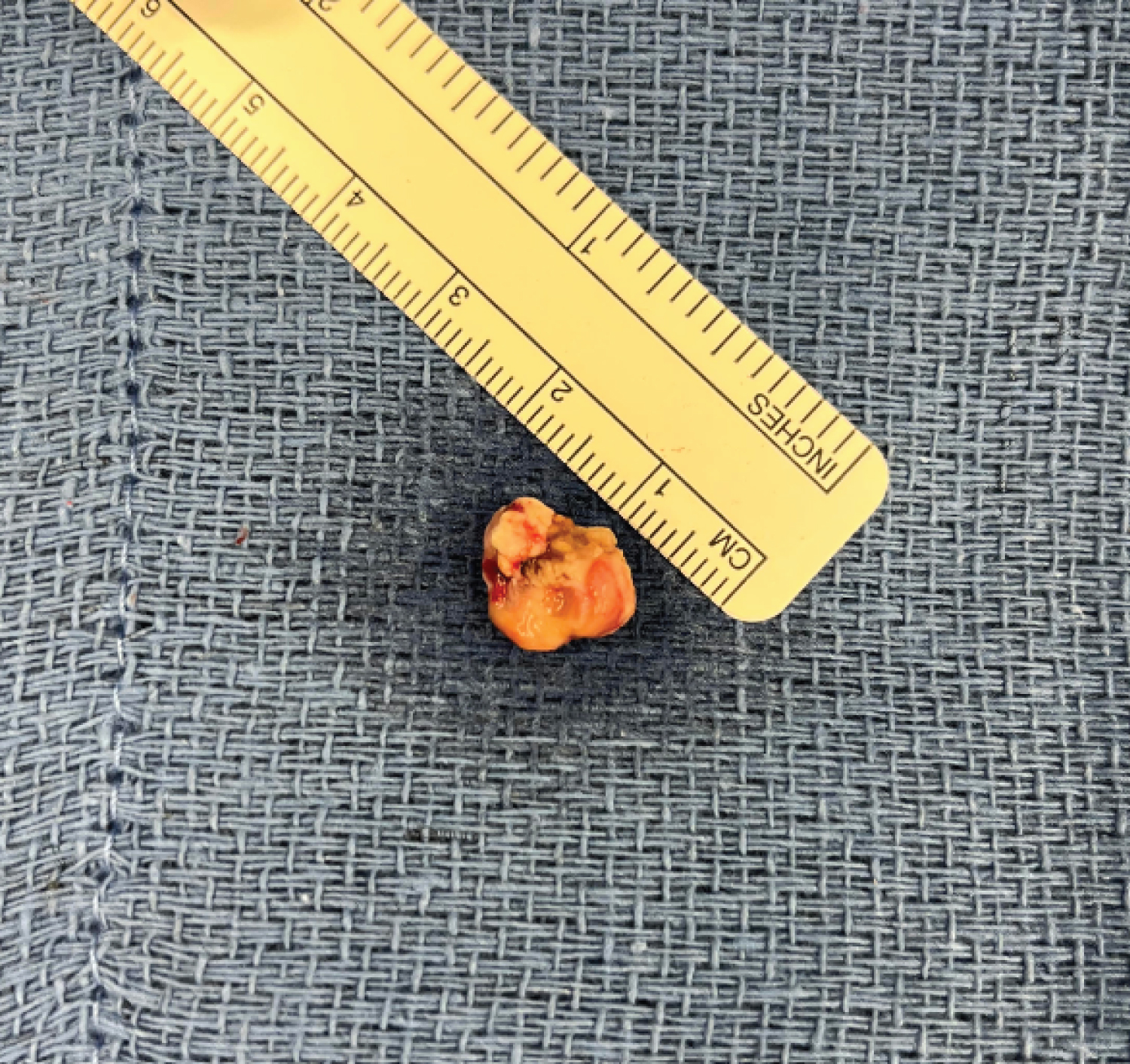
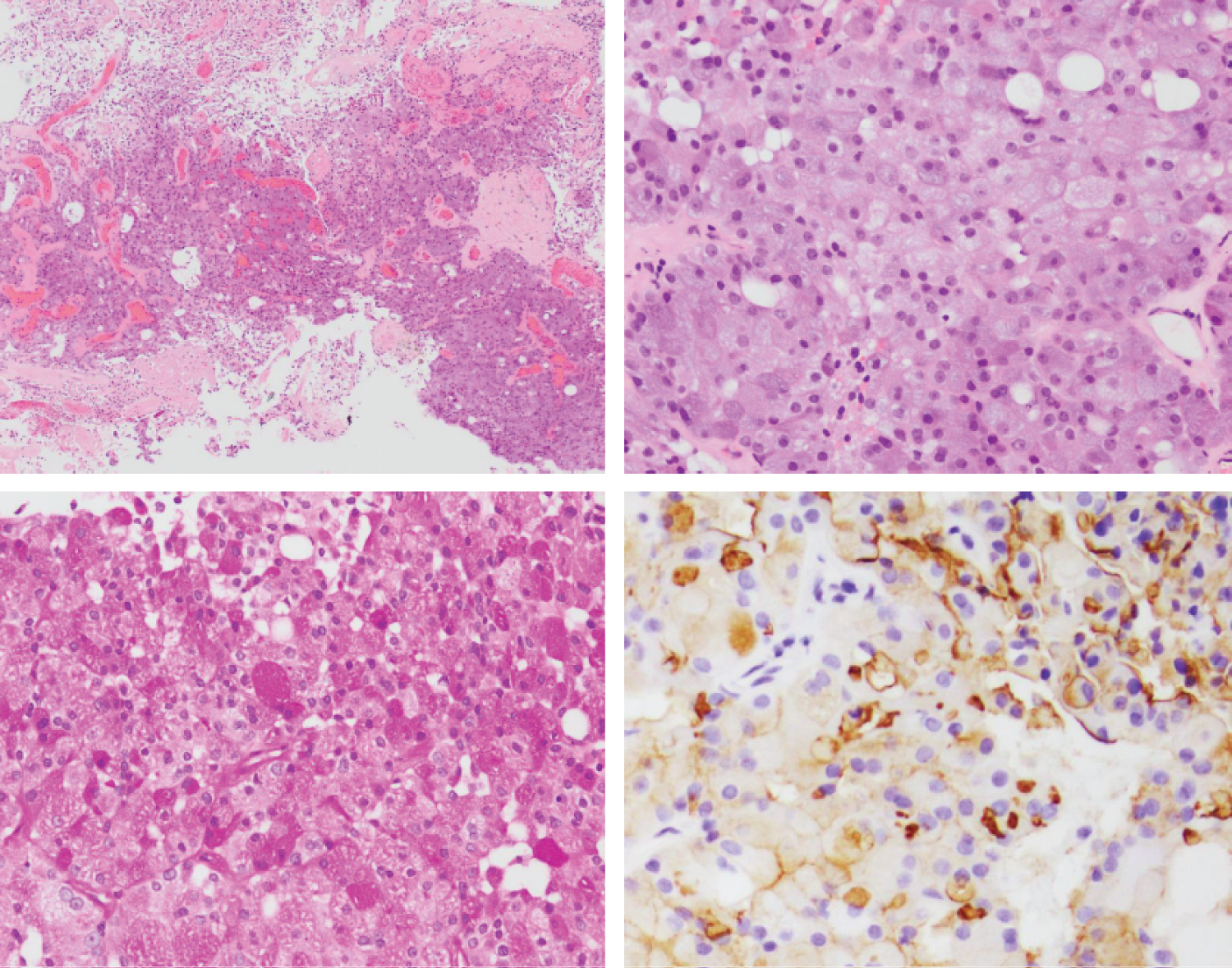
At the time of surgery, left-sided facial paresis was noted but the patient was otherwise neurologically intact. The patient was positioned supine with the head rotated 15 degrees to the right. A left frontal incision was made using previous surgical scars to minimize risk for wound healing and the scalp was retracted anteriorly. A frontoparietal craniotomy was performed using a high-speed pneumatic drill and a curvilinear durotomy was completed using a No. 15 blade scalpel and microscissors. Arachnoid dissection along the surface of the inferior parietal lobe revealed a cystic lesion just deep to the pial surface consisting of grayish fluid on aspiration. Approximately 3.5 mL of fluid were sent for pathological examination and determined to be primarily saliva. A 1.2 cm solid component of the cyst wall was located and dissected from the surrounding brain parenchyma using bipolar electrocautery and microscissors. The gross sample of the lesion is presented in Figure 2. Tissue samples sent for further analysis included the frontal cyst wall and the left frontal tumor and confirmed the presence of infiltrative tumor cells with abundant basophilic, granular cytoplasm and positive DOG1 staining (Figure 3).
Following resection, the tumor cavity was irrigated and lined with absorbable hemostatic material after achieving hemostasis. The dura was closed with tack-up sutures to the skull and the bone flap was plated onto native calvarium. The scalp was then closed in anatomic layers with good tissue apposition. No changes were noted to neuromonitoring parameters throughout the case and the patient was extubated without issue.
Postoperatively CT brain showed no evidence of intracranial hemorrhage or hematoma, with otherwise normal postoperative changes. The patient had an uncomplicated postoperative course and was discharged from the hospital on post-operative day 3.
Discussion
Acinic cell carcinoma is a rare, slow-growing, epithelial salivary gland neoplasm that arises from glandular cells in the oral cavity. Salivary gland tumors in general are rare (comprising 0.6% of all cancer diagnoses) and ACC in particular occurs in only 6-8% of all salivary gland neoplasms [1]. While an estimated 81-98% of ACC occurs in the parotid gland, approximately 3-12% of ACC arise from minor intraoral salivary glands located in the palate [11]. ACC has an incidence of 0.13 cases per 100,000 patients per year and the majority of cases have been found to occur in patients over the age of 40 [12]. Large-scale case series demonstrate a female to male predilection of 1.47:1 and an increased incidence in patients of Caucasian descent (85.03% of cases) [12].
While ACC generally presents as a low-grade tumor and is associated with a 20-year survival of 89.74% [12], a retrospective study of 1353 cases of ACC from the National Cancer Data Base (NCDB) found that poorer prognoses were associated with patients presenting with high-grade tumors, age greater than or equal to 30 years, and metastasis [13]. Patients with poorly differentiated and distant metastases are reported to have significantly decreased rates of 20-year survival (38.06% and 21.99%, respectively) [12]. Tumor recurrence in ACC also occurs in 35-44% of cases and rates of metastasis are reported in 19% of patients [12], both of which can occur up to 30 years following initial treatment of the disease [11]. Metastasis tends to occur through hematogenous rather than lymphatic pathways, and commonly affects the lungs, bones, liver, and other visceral organs [1,14-16]. Along with increasing the complexity of (or precluding) complete surgical management, metastatic ACC has rarely also been associated with paraneoplastic syndromes such as ectopic adrenocorticotropin hormone (ACTH) release [17,18] with significantly increased morbidity and mortality. Intracranial metastasis is thought to be exceedingly rare and only reported a few times in the medical literature (as presented in our Results section).
On gross appearance, ACC tumors present as a solid, encapsulated lesion with a smooth or lobulated (more commonly in recurrent ACC) surface and may include calcified or cystic components [11]. They are rarely bilateral or multifocal. Tumors have a median diameter of 2 cm [13] and, consistent with most intraoral salivary tumors, rarely grow to be larger than 3 cm [2]. Histologically, ACC has been reported to have four distinct architectural patterns: Solid, papillary-cystic (macro-cystic), micro-cystic, and follicular [1], though tumors often contain a mix of solid and cystic features [2,19,20]. While ACC has previously been identified on histology by the presence of intracellular zymogen secretory granules, psammomatoid bodies, and micro-calcifications consistent with acinar cell differentiation [1,11], more recent studies have found the involvement of other cell types, including intercalated ductal, myoepithelial, and basal cells [2].
Due to the rarity of cases, risk factors for the development of ACC are currently unknown. It has been suggested that prior radiation exposure and family history of ACC are strong predisposing factors [11], though large-scale studies have not been conducted to demonstrate a significant association. While the Epstein-Barr Virus (EBV) has been implicated in the etiology of various forms of oropharyngeal carcinoma, a history of EBV infection has not been shown to be correlated with the development of ACC lesions [21]. Additionally, in vitro studies have shown that tumor cells are capable of expressing estrogen, progesterone, or androgen receptors, suggesting that tumor growth can be hormonally driven in certain cases of ACC [21]. In the most recent edition of the World Health Organization Classification of Head and Neck Tumors, ACC has been reclassified genetically as lesions involving mutations of the NR4A3 (86% prevalence) and MSANTD3 (4% prevalence) genes in the 9q31 and 19q31.1 chromosomal regions [22]. Immunohistochemical studies have demonstrated that acinar cells stain positive for keratins CK7 and CAM5.2 and epithelial membrane antigen (EMA), and negative for p63, myeloid markers, and keratin CK20 [23]. Notably, the nuclear receptor 4A3 (NR4A3) and Discovered on Gastrointestinal Tumor Protein 1 (DOG1) have been shown to be highly expressed by ACC tumor cells and can be used as immunohistochemical markers to distinguish ACC from other forms of salivary gland tumors such as secretory carcinoma, mucoepidermoid carcinoma, and other oncocytic tumors [24,25].
Due to the slow-growing nature of these tumors, patients are frequently asymptomatic until they present with a late diagnosis when more advanced disease is present. Tumors are usually successfully treated with complete surgical resection and total or subtotal parotidectomy. While adjuvant radiotherapy has previously been reserved for patient with positive surgical margins, recurrent disease, involvement of the facial nerve, and high-grade features [26], incomplete excision with adjuvant radiotherapy or radiotherapy alone has become increasingly utilized as a treatment option for tumors not amenable to surgical resection [11]. However, in a case series of 45 patients, adjuvant radiotherapy was not found to result in a significant survival benefit in patients who had previously undergone close margin resection [27]. While platinum-based medication regimens have traditionally been indicated for the treatment of metastatic salivary gland tumors [1], chemotherapy has been found to have a limited role to play in the management of ACC, as the slow metabolism of ACC tumor cells precludes many chemotherapeutic options. One case report documented a patient who showed consistent disease progression of metastatic ACC despite treatment with Carboplatin, Paclitaxel, Doxorubicin, and Pembrolizumab [1], and the most common chemotherapeutic regimen (Cyclophosphamide, Doxorubicin, and Cisplatin) has been reported to have a response rate of less than 50% [1]. While mortality in cases of ACC is rare, patient death is almost always associated with distant metastasis [26]. Due to the high tendency for ACC to recur, often many years following initial treatment, long-term follow-up is necessary for these patients.
Conclusions
Parotid acinic cell carcinoma is a frequently recurring salivary gland neoplasm with metastatic potential. Though intracranial metastasis has only been reported in isolated cases in the literature, it is associated with increased complexity of surgical management and must remain on the differential for these patients who require long-term follow-up.
Author Contributions
Jeff F Zhang BS: Drafting the manuscript, editing the manuscript, approving the manuscript, accountable for manuscript integrity; Bayard Wilson MD: Providing operative details, editing the manuscript, approving the manuscript; Hilda Mirbaha MD: Providing histopathologic analysis, editing the manuscript, approving the manuscript; Karam Han MD: Providing histopathologic analysis, editing the manuscript, approving the manuscript; Khashayar Mozaffari BS: Editing the manuscript, approving the manuscript; Negar Khanlou MD: Providing histopathologic analysis, approving the manuscript; Isaac Yang MD: Editing the manuscript, approving the manuscript.
Funding and Disclosures
The authors of this manuscript declare no conflicts of interest and received no funding from any sources internal or external for any of the materials or findings related to this manuscript. Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy. All data supporting the findings of this study will be made available from the corresponding author upon request.
References
- Khelfa Y, Mansour M, Abdel-Aziz Y, et al. (2016) Relapsed acinic cell carcinoma of the parotid gland with diffuse distant metastasis: Case report with literature review. J Investig Med High Impact Case Rep 4: 2324709616674742.
- Omlie JE, Koutlas IG (2010) Acinic cell carcinoma of minor salivary glands: A clinicopathologic study of 21 cases. J Oral Maxillofac Surg 68: 2053-2057.
- Page MJ, Moher D, Bossuyt PM, et al. (2021) PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 372: n160.
- Buiret G, Ceruse P, Ramade A, et al. (2010) Acinic cell carcinoma of the parotid gland with skull base invasion: Case study, managed by exclusive external 3d radiation therapy. Eur Ann Otorhinolaryngol Head Neck Dis 129: 111-114.
- Lesniak MS, Tihan T, Olivi A (2002) Solitary central nervous system metastasis from acinic cell carcinoma of the parotid gland. J Otolaryngol 31: 38-41.
- Magliulo G, Iannella G, Alessi S, et al. (2013) Acinic cell carcinoma and petrous bone metastasis. Otol Neurotol 34: e18-19.
- Saleh TA, Hakin KN, Davidson MJ (2003) Metastasis of acinic cell carcinoma of the parotid gland to the contralateral orbit. Arch Ophthalmol 121: 1783-1786.
- Spencer ML, Neto AG, Fuller GN, et al. (2005) Intracranial extension of acinic cell carcinoma of the parotid gland. Arch Pathol Lab Med 129: 780-782.
- Francis Thottian AG, Gandhi AK, Ramateke PP, et al. (2018) Acinic cell carcinoma of parotid gland with cavernous sinus metastasis: A case report. J Cancer Res Ther 14: 1428-1430.
- Yildirim N, Oksuzoglu B, Vural M, et al. (2005) Case report: Cavernous sinus metastasis of the parotid carcinoma: A very unusual case. J Neurooncol 73: 181-183.
- Al-Zaher N, Obeid A, Al-Salam S, et al. (2009) Acinic cell carcinoma of the salivary glands: A literature review. Hematol Oncol Stem Cell Ther 2: 259-264.
- Patel NR, Sanghvi S, Khan MN (2014) Demographic trends and disease-specific survival in salivary acinic cell carcinoma: An analysis of 1129 cases. Laryngoscope 124: 172-178.
- Hoffman HT, Karnell LH, Robinson RA, et al. (1999) National Cancer Data Base report on cancer of the head and neck: Acinic cell carcinoma. Head Neck 21: 297-309.
- Schwentner I, Obrist P, Thumfart W, et al. (2006) Distant metastasis of parotid gland tumors. Acta Otolaryngol 126: 340-345.
- Al-Otaibi SS, Alotaibi F, Al Zaher Y, et al. (2017) High-Grade transformation (dedifferentiation) of acinic cell carcinoma of the parotid gland: Report of an unusual variant. Rep Otolaryngol 2017: 7296467.
- Vidyadhara S, Shetty AP, Rajasekaran S (2007). Widespread metastases from acinic cell carcinoma of parotid gland. Singapore Med J 48: e13-e15.
- Shenoy VV, Lwin Z, Morton A, et al. (2011) Ectopic adrenocorticotrophic hormone syndrome associated with poor prognosis in metastatic parotid acinic cell carcinoma. Otolaryngol Head Neck Surg 145: 878-879.
- Wade L, Kitching P, De Winton E (2020) Ectopic ACTH secretion secondary to metastatic acinic cell carcinoma of the parotid gland: A case report and review of current evidence for systemic therapy. J Investig Med High Impact Case Rep 8: 2324709620918080.
- Pires FR, Pringle GA, de Almeida OP, et al. (2007) Intra-Oral minor salivary gland tumors: A clinicopathological study of 546 cases. Oral Oncol 43: 463-470.
- Sepulveda I, Frelinghuysen M, Platin E, et al. (2015) Acinic cell carcinoma of the parotid gland: A case report and review of the literature. Case Rep Oncol 8: 1-8.
- Barnes L, Eveson JW, Reichert P, et al. (2005) WHO classification of tumors: Pathology and genetics of head and neck tumors. (3 Edn), World Health Organization, 9.
- Skalova A, Hyrcza MD, Leivo I (2022) Update from the 5th edition of the World Health Organization classification of head and neck tumors: Salivary glands. Head Neck Pathol 16: 40-53.
- Zhu S, Schuerch C, Hunt J (2015) Review and updates of immunohistochemistry in selected salivary gland and head and neck tumors. Arch Pathol Lab Med 139: 55-66.
- Ohtomo R, Mori T, Shibata S, et al. (2013) SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: A clue to the histogenesis for tumor diagnosis. Mod Pathol 26: 1041-1050.
- Chenevert J, Duvvuri U, Chiosea S, et al. (2021) DOG1: a novel marker of salivary acinar and intercalated duct differentiation. Mod Pathol 25: 919-929.
- Neskey DM, Klein JD, Hicks S, et al. (2013) Prognostic factors associated with decreased survival in patients with acinic cell carcinoma. JAMA Otolaryngol Head Neck Surg 139: 1195-1202.
- Zenga J, Parikh AS, Emerick KS, et al. (2018) Close margins and adjuvant radiotherapy in acinic cell carcinoma of the parotid gland. JAMA Otolaryngol Head Neck Surg 144: 1011-1016.
Corresponding Author
Jeff Zhang, BS, Jacobs School of Medicine and Biomedical Sciences, State University of New York at Buffalo, 955 Main Street; Buffalo, NY 14203, USA
Copyright
© 2022 Zhang JF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.