Hürthle Cell Carcinoma of the Thyroid - Well-Differentiated Thyroid Cancer or Something Else?
Summary
Hürthle cell carcinoma is a rare thyroid neoplasm with unpredictable development. Ever since its first description in 1907 by Langhans there have been a debate about their origin, clinical course, treatment and prognosis. Here, we describe a case of an 81-year-old male which after great diagnostic dilemma proved to be an aggressive Hürtle cell carcinoma. This case illustrates an aggressive Hürthle cell carcinoma with unpredictable slow progressive development and resistant to treatment options including surgery and radioactive iodine.
Introduction
Hürthle cell carcinoma is a rare thyroid neoplasm with unpredictable development [1]. It represents only 1 to 3% of all thyroid cancers. This type of tumors is well differentiated-solitary masses in the thyroid, comprised of Hürthle cell (at least 50%) and confined by a capsule. Ever since its first description there have been a debate about their origin, clinical course, treatment and prognosis. At the core of these debate stand the fact that these tumors are extremely rare, and no single institution can gain enough experience in their treatment [2]. Here, we describe a case of an 81-year-old male which after great diagnostic dilemma proved to be an aggressive Hürtle cell carcinoma.
Case History
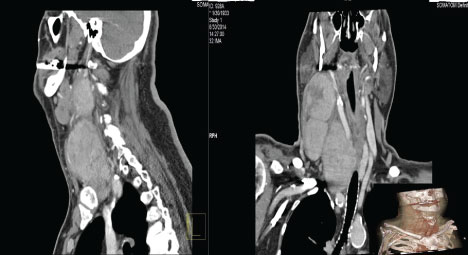
A 81-year-old man presented to the surgery clinic of our hospital with complaint of recurrent nodule in right lateral lymph node compartment (Level IIA). This nodule first appeared in May 2014. It was painless and gradually progressive in size. The patient was admitted in a ENT unit where they made surgical biopsy. During the procedure the ENT specialist in charge palpated another nodule. It was larger and located in levels III and VA. Histopathological examination showed benign oncocytoma. The patient was treated with antibiotics and then released home. Because of the intraoperative findings the patient wasdirected to conduct CT scan of the neck and chest. The CT scan showed a significantly enlarged right thyroid lobe 9/5 cm descending in the mediastinum. The right carotid artery and jugular vein were shifted medially and backwards paravertebrally along the periphery of the formation. In the right lateral lymph node compartment the CT scan showed two nodular formation-in level III 6 cm in diameter and in level Va 5 cm (Figure 1).
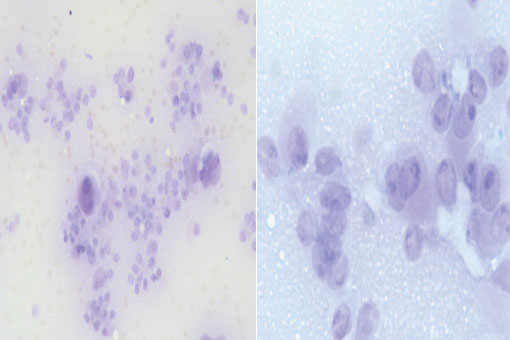
Because of this findings the patient was referred to our Endocrine surgery center for management. We made FNA biopsy of the right thyroid lobe. The result was inconclusive because of the limited material (Figure 2).
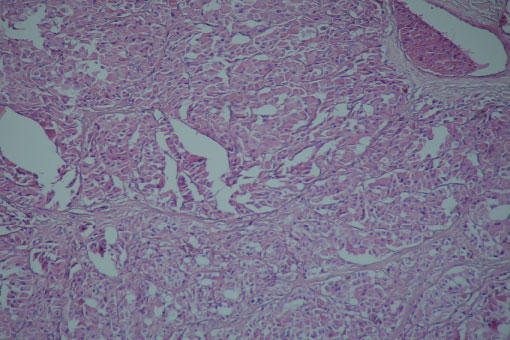
When we revised and compared the previous biopsy to ours we decided that the most likely diagnosis was Hürthle cell carcinoma. The patient underwent total thyroidectomy with right cervical lymphadenectomy of regions IIA, III, and VA. During the operation we used THUNDERBEAT® energy device to free the tumors of the surrounding structures. Unfortunately the right laryngeal nerve was covered on all sides by the tumor and it had to be excised. The histopathological examination showed Hürthle cell carcinoma invading the capsule and the surrounding soft tissues with nodal metastasis (Figure 3).
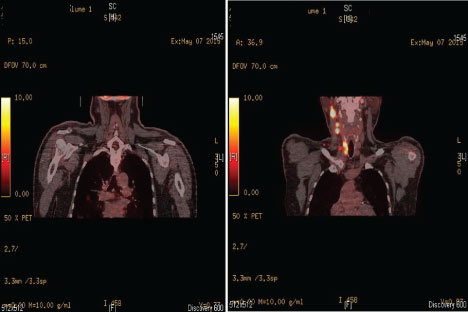
TNM classification after definite histopathological analysis was pT3N1aMx. He recovered but had unilateral RLN palsy. The patient began TSH suppressive therapy with 150 µg L thyroxin. The 131 I scintigraphy showed thyroid remnant tissue from the left thyroid lobe and two months later he underwent radioablation with 129 mCi. On the 6 months follow up the ultrasonography and the 131 I scintigraphy showed new lymph nodes in the right IIB and VB regions. The Tg levels were elevated-82.55 ng/ml. Ten months after the previous operation the patient was readmitted in our department and he underwent right cervical lymphadenectomy of regions II B and VB. The excised lymph nodes returned positive for a Hürthle cell carcinoma invasion. After the operation the Tg levels remained elevated and the patient was directed for a second radioablation with tsh recombinante (thyrogen) stimulation. He underwent radioablation with 127 mCi. Six months after the second radioablation the patient was instructed to perform a whole body PET scan. The result of the PET scan showed right lateral cervical and mediastinal lymph node engagement, as well as metabolically active lesions in parenchyma of the right and left lung (Figure 4).
Discussion
The German histologist Karl W Hurthle is actually not the first one to describe the Hürthle cell because what he described initially were the parafollicular or C cells. It was Askenazy who initially described these oncocytic cells [3]. In 1974, Thompson first described a series of 25 patients with Hürtle cell neoplasms [4]. The interesting fact was that 75% of those lesions were initially described as benign but unfortunately subsequently died of Hürtle cell carcinoma. Since then, however, several studies have shown that histologic criteria can reliably differentiate between Hürtle cell adenomas and carcinomas [5]. Nowadays the main criteria for differentiation between benign from malignant Hürtle cell neoplasm is full-thickness capsular and/or vascular invasion [6]. At present, the general agreement is that Hurthle cell neoplasms are a subset of all differentiated thyroid cancer, irrespective of the papillary or follicular growth pattern. But there are some The (WHO Committee) who considers this tumour theoxyphilic variant of follicular thyroid cancer [7]. They two admit that there are some differences - Hürtle cell carcinomas tend to be larger, with nodal metastases in approximately 30% of cases and occur in older patients [8]. In contrast follicular carcinomas of conventional type present as unifocal masses with nodal metastases in less than 5% of cases. However one must have in mind that, oncocytic tumors may also occur in major salivary glands and generally account for < 1% of all salivary tumors [9]. These facts makethe differentiation between benign and malignant lesion from a node biopsy near the parotid gland extremely difficult. Fine-needle aspiration can reliably detect Hürtle cell neoplasms [10]. FNA cannot accurately distinguish Hürtle cell adenomas from carcinomas. Molecular profiling for expression of specific markers (ras, p53 and others) have failed to show any correlation with Hürtle cell carcinomas [11].
Hürtle cell carcinomas are known to be able to secrete thyreoglobulin but generally fail to concentrate 131 I and therefore, in most patient, radioablation does not offer a therapeutic benefit. As a result, the only curative treatment is surgical resection. However many studies have showed that Total thyroidectomy and RAI ablation of thyroid remnant tissue can impact the survival of patients [12]. Also this offers the advantage of screening for recurrence by thyreoglobulin levels [13,14]. In our case the patient underwent radioablation two times with no significant benefit. After the second one we detected on PET scan distant lung metastasis. Although chemotherapy has proved ineffective in the past there are some initial reports that some new molecules might be an effective chemotherapy regimen for multi-metastatic of Hurthle cell carcinoma [15,16].
Hürthle cell carcinoma is a unique and unpredictable disease. This case illustrates an aggressive Hürthle cell carcinoma with unpredictable slow progressive development and resistant to treatment options including surgery and radioactive iodine. However there are some initial reports that new drug regiments might be effective in multi-metastatic Hurthle cell cancer.
Consent
Written informed consent was obtained from the patient for the publication of this report and any accompanying images.
Competing Interests
The authors declare that they have no competing interests.
References
- Barnabei A, Ferretti E, Baldelli R, et al. (2009) Hurthle cell tumours of the thyroid. Personal experience and review of the literature. Acta Otorhinolaryngol Ital 29: 305-311.
- Chindris AM, Casler JD, Bernet VJ, et al. (2015) Clinical and molecular features of Hurthle cell carcinoma of the thyroid. J Clin Endocrinol Metab 100: 55-62.
- Asa SL (2004) My approach to oncocytic tumours of the thyroid. J Clin Pathol 57: 225-232.
- Thompson NW, Dunn EL, Batsakis JG, et al. (1974) Hurthle cell lesions of the thyroid gland. Surg Gynecol Obstet 139: 555-560.
- Stojadinovic A, Hoos A, Ghossein RA, et al. (2002) Hurthle cell carcinoma: a 60-year experience. Ann Surg Oncol 9: 197-203.
- Hedinger C, Williams ED, Sobin LH (1989) The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer 63: 908-911
- Mai KT, Thomas J, Yazdi HM, et al. (2004) Pathologic study and clinical significance of Hurthle cell papillary thyroid carcinoma. Appl Immunohistochem Mol Morphol 12: 329-337.
- Maximo V, Soares P, Lima J, et al. (2002) Mitochondrial DNA somatic mutations (point mutations and large deletions) and mitochondrial DNA variants in human thyroid pathology: a study with emphasis on Hurthle cell tumors. Am J Pathol 160: 1857-1865.
- Brandwein MS, Huvos AG (1991) Oncocytic Tumors of Major Salivary Glands: A Study of 68 Cases With Follow-up of 44 Patients. Am J Surg Pathol 15: 514-528.
- Giorgadze T, Rossi ED, Fadda G, et al. (2004) Does the fine-needle aspiration diagnosis of "Hurthle-cell neoplasm/follicular neoplasm with oncocytic features" denote increased risk of malignancy? Diagn Cytopathol 31: 307-312.
- Lee KH, Shin JH, Ko ES, et al. (2013) Predictive factors of malignancy in patients with cytologically suspicious for Hurthle cell neoplasm of thyroid nodules. Int J Surg 11: 898-902.
- Petric R, Gazic B, Besic N (2014) Prognostic factors for disease-specific survival in 108 patients with Hurthle cell thyroid carcinoma: a single-institution experience. BMC Cancer 14: 777.
- Mazzaferri EL, Robbins RJ, Spencer CA, et al. (2003) A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma. J Clin Endocrinol Metab 88: 1433-1441.
- Pisanu A, Di Chiara B, Reccia I, et al. (2010) Oncocytic cell tumors of the thyroid: factors predicting malignancy and influencing prognosis, treatment decisions, and outcomes. World J Surg 34: 836-843.
- Dadu R, Waguespack SG, Sherman SI, et al. (2014) Efficacy and tolerability of different starting doses of sorafenib in patients with differentiated thyroid cancer. Oncologist 19: 477-482.
- Zhang H, Zeng L, Liang C, et al. (2012) Successful treatment of Hurthle cell thyroid carcinoma with lung and liver metastasis using docetaxel and cisplatin. Jpn J Clin Oncol 42: 1086-1090.
Corresponding Author
Dr. Kalin Nicolaev Vidinov, MD, PhD, Department of Endocrine Surgery, CCEG, USBALE "Akad. Iv. Penchev" Hospital, Medical University, Sofia, Bulgaria, Tel: +3592895-6066.
Copyright
© 2019 Vidinov K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.