Microscopic Omental Metastasis in Type II Endometrial Cancer Clinically Confined to the Uterus
Abstract
Background: Omental metastasis in endometrial cancer is associated with high-risk factors and portend a poor prognosis but have no impact on survival. There are no clear guidelines for performing omentectomy in Type II endometrial cancer.
Objective: To determine the incidence of microscopic omental metastasis in Type II endometrial cancer clinically confined to the uterus and to determine the effect of omentectomy on surgical outcome, staging accuracy, and survival.
Study design: A retrospective chart review of patients who underwent robot-assisted surgical staging for endometrial cancer between January 1st, 2012, and June 1st, 2020, at a single institution. Patients with grade-3 endometrioid adenocarcinoma, uterine serous cancer, clear cell carcinoma, and carcinosarcoma with no clinical evidence of disease outside the uterus were included.
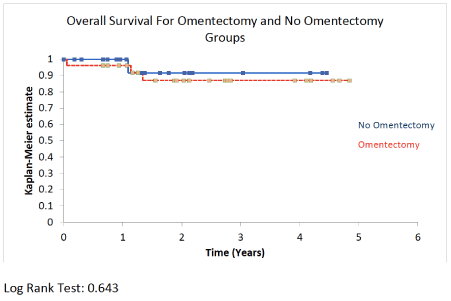
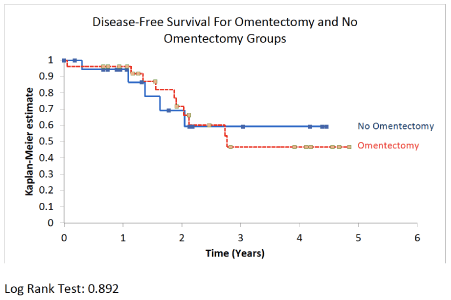
Results: A total of 56 patients met inclusion criteria. Twenty-seven patients underwent omentectomy and 21 patients did not and comprised the control group. Mean age was 70.6 years in the omentectomy group vs. 70.6 in the control group (P = 0.99). Mean BMI was 32.3 Kg/m2 in the omentectomy group vs. 31.2 in the control group (P = 0.63). On postoperative pathology, 26 (96.3%) patients in the omentectomy group had non-endometrioid histology vs. 15 (71.4%) in the control group (P = 0.03). Three (11.1%) patients in the omentectomy group had stage-4 disease vs. no patients in the control group (P = 0.44). Four (14.8%) patients in the omentectomy group had lymph node metastasis vs. 3 (14.3%) patients in the control group (P = 1.0), and 4 (14.8%) patients in the omentectomy group had adnexal metastasis vs. 2 (9.5%) in the control group (P = 0.68). Positive cytology was recorded in 4 (15.4%) patients in the omentectomy group vs. 10 (47.6%) in the control group (P = 0.025). The incidence of omental metastasis was 7.4%. The estimated blood loss was 126 cc in the omentectomy arm vs. 99 cc in the control arm (P = 0.06). There was no difference in the rate of complications (P = 1), readmission (P = 1), overall survival (P = 0.643), or recurrence-free survival (P = 0.892) between the two groups. Omental metastasis was associated with peritoneal cytology (P = 0.019) and elevated serum CA125 (P = 0.048).
Conclusion: The incidence of omental metastasis in Type II endometrial cancer clinically confined to the uterus is 7.4%. Omentectomy has a low contribution to staging, management and prognosis. It does not, however, add significant surgical risk and should be performed when judged to be prudent.
Keywords
Endometrial cancer, Omental metastasis, Omentectomy
Introduction
Endometrial cancer is the most common gynecologic cancer in high income countries [1]. It is divided into Type I and Type II from a histopathological as well as a molecular perspective. Type II endometrial cancer comprises some grade-3 endometrial cancers, undifferentiated endometrial cancer, uterine serous cancer, clear cell carcinoma, and carcinosarcoma. Although these tumors are less common than low grade endometrial cancer, they are associated with a higher stage at diagnosis and with most recurrences and deaths [2].
Endometrial cancer commonly spreads via the lymphatic pathway as well as trancoelomic where it involves the omentum. Although omentectomy has not been shown to influence survival [3,4] yet it is commonly performed during surgery for high grade endometrial cancer [5]. Furthermore, omentectomy is not formally included in all staging guidelines for Type II endometrial cancer. For example, ESMO-ESGO-ESTRO report on endometrial cancer recommends omentectomy be performed on uterine serous cancer only [6]. FIGO recommends omental palpation in endometrial cancer staging along with a total abdominal hysterectomy, bilateral salpingo-oopherectomy and lymph node sampling [7]. NCCN guidelines state that an omental biopsy is commonly performed in uterine serous cancer, clear cell carcinoma, and carcinosarcoma [8].
The main objective of this study is to determine the incidence of omental metastasis in Type II endometrial cancer clinically confined to the uterus. Our secondary objectives are to determine the effect of omentectomy on surgical outcome, staging accuracy, and survival. Additionally, we aim to identify risk factors associated with omental metastasis.
Materials and Methods
IRB approval was obtained for a retrospective chart review of patients who underwent robot-assisted surgical staging for endometrial cancer between January 1st, 2012, and June 1st, 2020, at a single institution. All data were abstracted form a single comprehensive electronic medical record (EMR).
We included patients 18-years and older with grade 3 endometrial cancer, uterine serous cancer, clear cell carcinoma or carcinosarcoma on postoperative pathology report. Excluded were grade 1 and 2 carcinoma, uterine sarcoma, and patients with disease spread outside the uterus either clinically, on CT scan at presentation, or during surgical exploration. Cases with incomplete records were also excluded.
Demographic and clinical data abstracted included age, body mass index (BMI), initial CT scan findings and serum CA125 levels. A CA125 level > 35 was considered elevated.
Pathological data abstracted included tumor size, depth of invasion, lymph-vascular space invasion, histopathology, grade, and stage according to the International Federation of Gynecology and Obstetrics (FIGO) 2009 classification [9]. Metastases to the omentum, lymph nodes, adnexa, and peritoneum were also recorded.
Operative data abstracted included the type of procedure, conversion to laparotomy, operative times, length of hospital stay, and 30-day complications or readmission. Only grade 3 or higher complications according to the Clavien-Dindo system [10] were recorded.
Tumor board conference recommendations, adjuvant treatment received, and surveillance data were also abstracted from the EMR. Progression-free survival and overall survival were estimated. The former was calculated from the time of surgery to recurrence, and the latter was calculated from the time of surgery to death or the time of last office or hospital visit.
Statistical analysis was conducted using RStudio 1.2.5033 program working on the Mac OS X 10.15.5 system. Continuous variables were summarized using mean. Categorical variables were summarized using frequency and percentage. Two-sided t-test was used to analyze means of continuous variables. Fisher's exact test was used to compare categorical patient characteristics, pathological data, operative data, and complications in patients that received an omentectomy and patients that did not receive an omentectomy. Risk factors for omental metastasis, including histology, stage, peritoneal cytology, lymph-vascular space invasion, tumor size, depth of invasion, lymph node metastasis, adnexal metastasis and CA-125 were also analyzed using Fisher's exact test. The Kaplan-Meier method was used to analyze disease-free survival and overall survival. Bonferroni's correction was used to calculate the level of significance for preoperative and postoperative histology (α < 0.01), preoperative and postoperative grade (α < 0.017), and stage (α < 0.0125). The level of significance calculated for stage was 0.0125. For all other variables, the level of significance used was α < 0.05.
Results
A total of 630 charts were reviewed, 56 met inclusion criteria. Twenty-seven patients underwent omentectomy and 21 patients did not and comprised the control group.
Mean age was 70.6 years in the omentectomy group vs. 70.6 in the control group (P = 0.99) (Table 1). The mean BMI was 32.3 Kg/m2 in the omentectomy group vs. 31.2 in the control group (P = 0.63). The mean length of stay was 2.07 days in the omentectomy group vs. 1.57 days in the control group (P = 0.51). Twenty-six (96.3%) patients in the omentectomy group had preoperative grade-3 histology vs. 20 (95.3%) in the control group (P = 1.00). Sixteen (59.3%) patients in the omentectomy group had a preoperative diagnosis of uterine serous cancer, while 19 (90.5%) cases in the control group had a preoperative diagnosis of endometrioid adenocarcinoma (P < 0.0001) (Table 1). All patients had a preoperative CT scan showing no metastatic disease. Five (23.8%) patients in the omentectomy group had an elevated (> 35 units/ml) serum CA125 level vs. 3 (18.8%) patients in the non-omentectomy group (P = 1.00).
On postoperative pathology, 26 (96.3%) patients in the omentectomy group had non-endometrioid histology vs. 15 (71.4%) in the control group (P = 0.03) (Table 2). Specifically, 18 (66.7%) patients in the omentectomy group had uterine serous cancer USC vs. 8 (38.1%) patients in the control group (P = 0.03) (Table 2), whereas 1 (3.7%) patient in the omentectomy group had an endometrioid histology vs. 6 (28.6%) patients in the control group (P = 0.03). There was no difference in stage distribution, lymph node metastasis, or adnexal metastasis between the two groups. Five (18.5%) patients in the omentectomy group had stage-3 disease vs. 5 (23.8%) patients in the control group (P = 0.44), while 3 (11.1%) patients in the omentectomy group had stage-4 disease vs. no patients in the control group. Four (14.8%) patients in the omentectomy group had lymph node metastasis vs. 3 (14.3%) patients in the control group (P = 1.00), and 4 (14.8%) patients in the omentectomy group had adnexal metastasis vs. 2 (9.5%) in the control group (P = 0.68). Positive cytology was recorded in 4 (15.4%) patients in the omentectomy group vs. [10] (47.6%) in the control group (P = 0.025). The mean length of the omental sample was 22.1 cm, mean breadth was 9.5 cm, and mean thickness was 1.3 cm. Omental metastasis was recorded in 2 (7.4%) patients in the omentectomy group (Table 2).
Operating room time was 284 minutes in the omentectomy arm vs. 258 minutes in the control arm (P = 0.19), surgery time was 240 minutes in the omentectomy arm vs. 210 minutes in the control arm (P = 0.12), and console time was 151 minutes in the omentectomy arm vs. 146 minutes in the control arm (P = 0.81). Patients in the omentectomy arm underwent laparoscopic infra-colic omentectomy prior to docking of the robot. Twenty-one (77.8%) patients in the omentectomy group had pelvic lymph node dissection vs. 17 (81%) patients in the control group (P = 1.00), while 18 (66.7%) patients in the omentectomy group underwent para-aortic lymph node dissection vs. 11 (52.4%) in the control group (P = 0.38) (Table 3).
The estimated blood loss (EBL) was 126 cc in the omentectomy arm vs. 99 cc in the control arm (P = 0.06). One (3.7%) patient (80-year-old, BMI 17) in the omentectomy group underwent a conversion to laparotomy for subcutaneous emphysema (Table 3). The same patient was readmitted with small bowel obstruction and pneumonia that were successfully treated with gastric drainage and antibiotics. Another patient in the control group was readmitted for postoperative fever. Her workup was negative, and she was discharged home after medical management (Table 3).
Table 4 lists clinic-pathologic risks factors associated with omental metastasis (7.4%) in the omentectomy group (N = 27) (Table 4). Only peritoneal cytology (P = 0.019) and CA-125 levels (P = 0.048) were found be statistically significant compared to cases without omental metastasis.
Finally, after a mean follow up time of 24 months, there was no difference in recurrence-free survival (P = 0.73) or overall survival (P = 0.62) between the omentectomy and the control group (Table 1) (Figure 1 and Figure 2).
Comment
In this study we found that the incidence of microscopic omental metastasis in Type II endometrial cancer clinically confined to the uterus was 7.4%. All surgeries were laparoscopic robot assisted. We excluded all patients with evidence of disease outside the uterus by preoperative CT scan and during laparoscopic survey of the abdomen, and all patients had an infracolic omentectomy.
Kaban, et al. reported a similar incidence of microscopic omental metastasis of 6.9% in a retrospective chart review of 218 patients with non-endometrioid cancer [11]. Gehrig, et al. reported an incidence of micrometastasis to the omentum of 4% in a study of 65 patients with uterine serous cancer of which 52 underwent either partial or total omentectomy [12]. In a study by Luz, et al. [3] the incidence of microscopic omental metastasis in 106 patients with uterine serous cancer who had negative CT scan and no palpable or visible omental nodules was 3%. Most patients underwent omental biopsy rather than omentectomy. The overall incidence of omental metastasis was 12% but 6 of the 8 patients with omental metastasis had visible lesions or palpable omental nodules, and the CT scan was suspicious in 3 of them. The authors reported a negative predictive value for CT scan of 92% and of the operative findings of 97% [3].
The incidence of omental metastasis in uterine carcinosarcoma was reported to be 16% by Ross, et al. Most patients in this study of 153 patients had an omentectomy with a 35% (6/17) incidence of microscopic omental metastasis [4].
The mean length of the omental sample in our study was 22.1 cm, mean breadth was 9.5 cm, and mean thickness was 1.3 cm. In their prospective report of 134 patients with clinical stage-1 endometrioid adenocarcinoma of the endometrium, Fujiwara, et al. [13] proposed the removal of at least 10 × 5 cm of omentum for adequate detection of metastasis or a complete omentectomy if gross metastasis was suspected [13]. Turan, et al. [14] studied 811 endometrial cancer patients and found that total omentectomy vs. infracolic omentectomy significantly increased the odds of detecting microscopic metastasis (11.3% vs. 2.1%, P < 0.001) [14].
In our study we found that CA-125 levels (P = 0.048) and peritoneal cytology (P = 0.019) significantly correlated with omental metastasis. Elevated CA125 serum levels are well documented in Type II endometrial cancer literature to be associated with advanced stage as well as extra-uterine metastasis including to the lymph nodes and omentum [15,16]. Kaban, et al. found that adnexal involvement significantly correlated with omental metastasis in non-endometrioid cancer (P = 0.003) [11]. In carcinosarcoma, residual disease (P = 0.004) and depth of myometrial invasion (P = 0.017) were found to be predictors of omental metastasis on multivariate analysis [4]. In 3 other studies including both endometrioid and non-endometrioid clinical stage I endometrial cancer patients who underwent total or infracolic omentectomy, risk factors for omental metastasis were grade, cytology, adnexal involvement, depth of myometrial invasion and lymph node involvement [17-19].
We did more omentectomy procedures in non-endometrioid histology than in grade-3 endometrioid cancer in this retrospective chart review. Omentectomy did not, however, impact staging, nor did it impact delivery of adjuvant treatment. All our patients received adjuvant treatment consistent with well-established standards of care and guidelines [20-23]. Ultimately, omentectomy in this clinical scenario had no impact on recurrence-free or overall survival.
Peled, et al. [24] reviewed 52 women with USC initially diagnosed as EAC and found that performing an omentectomy had no impact on disease-free interval (24.5 months with omentectomy vs. 30.5 months without, P = 0.29), or overall survival (33 months with omentectomy vs. 29 months without, P = 0.32), or on recurrence pattern [24]. In another study of 106 USC patients, there was no difference in overall or disease-free survival between the patients that did not have an omentectomy and those that had an omentectomy or an omental biopsy [3].
Finally, Ross, et al. found that omental sampling had no impact on median overall survival (P = 0.7432) in carcinosarcoma. However omental metastasis on multivariate analysis was associated with poor overall survival (11.4 months vs. 128.7 months, P < 0.001) and 35% of all omental metastasis (16%) was microscopic [4].
We found that performing a laparoscopic infracolic omentectomy did not contribute significant additional surgical time, blood loss or complications. Others reported on the safety of laparoscopic and robotic surgery for endometrial cancer including omentectomy [25-27].
The limitations of this study are its small sample size, due to our strict inclusion criteria, which may have obscured statistical differences in some areas or exaggerated it in others. The retrospective design may have contributed to selection bias whereby omentectomy was performed less frequently in grade-3 endometrioid cancer compared to non-endometrioid cancer. The strengths of this study, however, are the adherence to strict clinical, radiological, and pathological inclusion criteria, and the uniformity of surgical management as well as adjuvant treatment following well-established standards of care.
In conclusion laparoscopic infracolic omentectomy in Type II endometrial cancer clinically confined to the uterus has a low contribution to staging, management and prognosis. The incidence of omental metastasis in this scenario, however, is not negligible. Omentectomy does not add significant surgical time or risk and hence should be performed when judged to be prudent.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144: 1941-1953.
- Brinton LA, Felix AS, McMeekin DS, et al. (2013) Etiologic heterogeneity in endometrial cancer: Evidence from a Gynecologic Oncology Group trial. Gynecol Oncol 129: 277-284.
- Luz R, Macdonald N, Mould T (2016) Omental Biopsy for Surgical Staging of Uterine Serous Carcinoma. Int J Gynecol Cancer 26: 1448-1454.
- Ross MS, Elishaev E, Berger JL, et al. (2018) Prognostic significance of omental disease and the role of omental sampling in patients with uterine carcinosarcoma. Int J Gynecol Cancer 28: 254-259.
- Joo WD, Schwartz PE, Rutherford TJ, et al. (2015) Microscopic Omental Metastasis in Clinical Stage I Endometrial Cancer: A Meta-analysis. Ann Surg Oncol 22: 3695.
- Colombo N, Creutzberg C, Amant F, et al. (2016) ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Ann Oncol 27: 16-41.
- Lewin SN (2011) Revised FIGO staging system for endometrial cancer. Clin Obstet Gynecol 54: 215-218.
- Dursun P, Ayhan A (2017) Gynecologic oncologist perspective about ESMO-ESGO-ESTRO consensus conference on endometrial cancer. Int J Gynecol Cancer 27: 826-831.
- Amant F, Mirza MR, Koskas M, et al. (2018) Cancer of the corpus uteri. Int J Gynecol Obstet 143: 37-50.
- Dindo D, Demartines N, Clavien P-A (2004) Classification of Surgical Complications. Ann Surg 240: 205-213.
- Kaban A, Topuz S, Erdem B, et al. (2018) Is omentectomy necessary for non-endometrioid endometrial cancer. Gynecol Obstet Invest 83: 482-486.
- Gehrig PA, Van Le L, Fowler WC (2003) The role of omentectomy during the surgical staging of uterine serous carcinoma. Int J Gynecol Cancer 13: 212-215.
- Fujiwara H, Saga Y, Takahashi K, et al. (2008) Omental metastases in clinical stage I endometrioid adenocarcinoma. Int J Gynecol Cancer 18: 165-167.
- Turan T, Üreyen I, Karalök A, et al. (2014) What is the importance of omental metastasis in patients with endometrial cancer? J Turkish Ger Gynecol Assoc 8: 164-172.
- Olawaiye AB, Rauh-Hain JA, Withiam-Leitch M, et al. (2008) Utility of pre-operative serum CA-125 in the management of uterine papillary serous carcinoma. Gynecol Oncol 110: 293-298.
- Gupta D, Gunter MJ, Yang K, et al. (2011) Performance of serum CA125 as a prognostic biomarker in patients with uterine papillary serous carcinoma. Int J Gynecol Cancer 21: 529-534.
- Saygili U, Kavaz S, Altunyurt S, et al. (2001) Omentectomy, peritoneal biopsy and appendectomy in patients with clinical stage I endometrial carcinoma. Int J Gynecol Cancer 11: 471-474.
- Dilek S, Dilek U, Dede M, et al. (2006) The role of omentectomy and appendectomy during the surgical staging of clinical stage I endometrial cancer. Int J Gynecol Cancer 16: 795-798.
- Bayrak M, Yllmaz A, Yllmaz F, et al. (2019) Omental Micro metastasis in Endometrial Cancer. Oncol Res Treat 42: 466-469.
- Randall ME, Filiaci V, McMeekin DS, et al. (2019) Phase III trial: Adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early-stage endometrial cancer. J Clin Oncol 37: 1810-1818.
- Matei D, Filiaci V, Randall ME, et al. (2019) Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. N Engl J Med 380: 2317-2326.
- de Boer SM, Powell ME, Mileshkin L, et al. (2018) Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 19: 295-309.
- Koh WJ, Abu-Rustum NR, Bean S, et al. (2018) Uterine Neoplasms, Version 1.2018: Clinical practice guidelines in oncology. J Natl Compr Canc Netw 16: 170-199.
- Peled Y, Aviram A, Krissi H, et al. (2015) Uterine papillary serous carcinoma pre-operatively diagnosed as endometrioid carcinoma: Is omentectomy necessary? Aust New Zeal J Obstet Gynaecol 55: 498-502.
- Praiss AM, Chen L, St Clair CM, et al. (2019) Safety of same-day discharge for minimally invasive hysterectomy for endometrial cancer. Am J Obstet Gynecol 221: 239e1-239e11.
- Melamed A, Katz Eriksen JL, Hinchcliff EM, et al. (2016) Same-Day Discharge After Laparoscopic Hysterectomy for Endometrial Cancer. Ann Surg Oncol 23: 178-185.
- Lee J, Aphinyanaphongs Y, Curtin JP, et al. (2016) The safety of same-day discharge after laparoscopic hysterectomy for endometrial cancer. Gynecol Oncol 142: 508-513.
Corresponding Author
Dr. Karim ElSahwi, MD, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Hackensack Meridian Health, Jersey Shore University Medical Center, 19 Davis Ave, HOPE Tower E7129, Neptune, NJ 07753, USA.
Copyright
© 2021 Boak K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.