NeoEssentialism, Species, and Molecular Phylogenetics Regarding Hominin Evolution
Abstract
A number of recent articles have appeared on the hominin Denisova fossil remains. Many of them focus on attempts to produce DNA sequences from the extracted samples. Often these project mtDNA sequences from the fossil remains of a number of Neandertal fossils and the Denisovans in an attempt to understand the evolution of Mid Pleistocene human ancestors. There are two problems with these papers, one concerns the degradation of the ancient DNA and its interpretation as authentic genetic information and the other concerns the idea of "species" versus that of "population" and the use of these ideas in the building of evolutionary diagrams to indicate ancestry and extinction. Since I have dealt with the issue of degradation elsewhere [1]. I will limit this paper to ideas of species and population. A central issue is what does human variation mean, how much population variation has there been in the past and how does this variation distinguish hominid speciation or simply a process of anagenesis. Some businesses today claim to be able to use DNA analysis to discover past ethnic identities and a new niche in restaurants is producing DNA menus. Perhaps some caution is in order.
Keywords
Ancient DNA, Denisova, Neandertals, Populations, Speciation, Haplotypes, Anagenesis
Introduction
As a young undergraduate I was exposed to the lectures and seminars given by Alan Wilson and Vincent Sarich at UC Berkeley. The excitement of the introduction of biomolecular analysis of vertebrate proteins and DNA affected most all of the anthropology students I knew. The idea that the fossil record might be filled in where there were gaps by the analysis of existing patterns of DNA and proteins in living Primates and could be used to verify or correct errors in interpretation was a powerful theme at the time. The promise of this work has been largely fulfilled in the near five decades that have passed since those days [2].
The central debate I recall that engaged those who had built their careers on fossil analysis versus the new methods, was that the assumptions that DNA and protein substitutions could be used as clocks to construct phylogenetics was a shore too far to accept. That mutations could accumulate at steady rates unaffected by selection or back mutation seemed simply a metaphysical dream. Most of those who were skeptical at the time were staunch Darwinists and the idea of neutral theory seemed heresy. My own feeling at the time was that all tools have uses and that methods may be flawed but that they can be perfected.
In recent years claims have been made and controversy produced by the application of DNA analysis in the production of evolutionary trees. And while general comprehensive analyses have been produced regarding molecular phylogenies [3] others have been more specific especially regarding how or if horizontal gene transfer (HGT) has corrupted our interpretation of the evolution and organization of great groups of living organisms like the archaeal and bacterial phyla [4]. A number of sequences have been identified as being key to the evolution of the brain without clearly delineating which ones where actually "key" or just involved [5]. The search for unique sequences produced claims of the discovery of "intelligence" genes and sequences that could be used to produce genealogies for contemporary people reaching into the mists of antiquity and beyond. These problems, regarding how sequences are to be considered to have significance, relates to attempts to create phylogenetic trees of hominin evolution. In the recent past conflicts over the specific importance of anatomical features found on different fossils created "bushy" trees, especially with Homo erectus/Homo ergaster and the Archaic Homo finds [6,7], but more so in identifying specific "modern" traits in hominins after 200,000 B.P. [8]. Battles between splitters (those giving species designation to every fossil) and lumpers (those given to proposing variation equals population diversity) have produced a lively intellectual milieu, as when Tattersal [7] referred to the category, Homo habilis as a "wastebasket taxon", but also confused the public. It seems the molecular evidence, as it has accumulated supports a more direct scheme, one defined as anagenesis.
The Brain, Intelligence and Ethnicity
The big brain became a central focus for paleoanthropologists as it had biologists and anatomists for centuries. Krantz [9] and Tobias [10] claimed that the size of the brain in children at the end of the first year of life (approximately 750 cc) should be the meridian when a brain size at this point indicates the arrival of symbolic behavior [11]. This is entirely based on the assumption that the human child today creates symbolic language at this time and with this amount of brain. Yet humans do produce speech with smaller brains (e.g. nanocephalic dwarves) and the language of children at 12 months can hardly be said to represent a fully achieved human consciousness. The child gains brain size and neuronal numbers up to about 90% of adult size by 2 to 3 years of age, age [12-14]. Yet organizationally and functionally it is quite unlike an adult brain due to incomplete segmental development, as in the fact that grey matter in the frontal lobe undergoes protracted structural development to reach its maximal volume at 11-12 years, while that of the temporal lobe does not achieve its full volume until 16-17 years and most cortical regions under a cycle of thickening and then thinning reflecting synaptic "pruning" and cell death [15]. Still, the capacity of a child can at this age hardly be said to be fully human. By ages 6 to 8 this level of performance usually does arrive, given proper exposure to human society and nutrition. In this regard, Holloway [11] von Bonin [16] and Deacon [17] all placed more emphasis on organization of the brain and connectivity, than simply size.
This problem has surfaced in the debate over the status of the fossils from the island of Flores, the idea that a new small species could be described from the remains led to discussions of potential dwarfism, pathology and later the process of island dwarfism after the discovery of the more recent fossils of small individuals discovered by Berger on Palau [18-20]. Thus these cases represent likely pathology in the Flores example or insular dwarfism, in the Palau example as they generally fall within the range of certain local groups of Andaman Islanders (Onge). But the effects of mummification and different conditions of preservation should also be considered as in cases like those of the Alaskan and Aleutian mummies [21] in the case of Palau and the Rising Star Cave finds or "Homo naledi" [22].
In fact, brain size variation in modern humans is considerable, yet performance as a human is unclear as related to brain size, weight, regions' size, etc. [23,24]. Holloway [11] also pointed out the arbitrary nature of the size of the brain associated with species designation, especially regarding Neandertals. As he notes, it is difficult to understand why hominid brains evolved after Homo habilis and I take on this problem in a book out this fall [25]. He points out that the slow increase in brain size over the past 2 million years also undermines Dunbar's assertion concerning brain size, social grooming and grooming.
Gene Sequences, Populations and Species
Access to DNA sequences for study, especially ancient DNA is limited, the best resource is GENBANK, the Cambridge DNA Concordance appears to be no longer available. Other databases with population comparison capability like the International Genome Sample Resource (IGSR) lacks comprehensive data and similar sites have been strapped to expand due to costs and lack of funding. The final 1000 IGSR data set contains some 2,500 present-day individuals from 26 populations with a low coverage of 2-6x whole-genome sequencing (WGS) data, mainly exome sequence data available for all individuals and only high coverage for 24 [26,27]. Low-coverage Whole Genome Sequencing (WGS) is a sampling strategy that overcomes some of the deficiencies seen in fixed content SNP array studies. Linkage-disequilibrium (LD) aware variant callers, such as the program Thunder, may provide a calling rate and accuracy that makes a low-coverage sequencing strategy viable. This is due to the costs of sequencing whole genomes. Tests of accuracy, as by Bizon, et al. [28] indicate acceptable results.
However, making assumptions from this small sample of contemporary humans given a population of over 6 billion may skew the actual penetration and retention of Neandertal genes and other archaic populations (e.g. Denisovans). Sankararaman, et al. [29] used this data base to make a number of propositions concerning modern human ancestry, derived also from assumptions regarding both Neandertal demography and the evolution of population expansions and selection over the past 30,000 years. Their methodology of inferring Neandertal haplotypes due to the impossibility of reconstructing the short ancient Neandertal DNA fragments is interesting but cannot be relied on to be more than suggestive. Other assumptions are equally curious as to propose that reduced genes in Neandertal ancestry that are highly expressed in testes than other tissue, or a discovered 5 fold reduction of Neandertal ancestry X chromosome genes are the result of selection against them. Projecting such conclusions over 30,000 years from partial knowledge of the Neandertal populations and from a lack of complete sequences and a small contemporary sample seems premature at best. More comprehensive sampling, deeper coverage and more precise methods of producing ancient DNA sequences from preserved samples will probably change this picture.
To some extent much of this thinking attempts to show direct relationships between adaptation and changes in genes and rather borders on Lamarkianism. This is particularly strong in efforts to explain the evolution of the "big brain" in hominins and the appearance of language. Deacon [17] refers to the work of James Mark Baldwin [30] whose work was limited to human psychology but argued that "biases" coupled with learning and behavioral flexibility could modify inheritance of future kin. Little in Baldwin's work differs from the theory of inheritance of Lamark [31] but Lamark's work was bolstered in a wide knowledge of the animal and plant world, embryology and development. He created a coherent theory of evolution and laid the framework for today's transgenerational epigenetics [32-34]. Though flawed, and given the knowledge and instrumentation of his day, Lamark certainly provides a more coherent view of epigenetics than Baldwin. In fact, few can even discuss or explain the "Baldwin effect" without reference to Lamark [35]. Even Waddington's [36] "genetic assimilation" which he tried to distinguish from the "Baldwin effect" was called "Lamarkian" by Simpson, though Waddington [37] had the benefit of extensive experimentation with cells and the problem of agents of development and genetics. Where Baldwin's idea appears metaphysical in its connections, Waddington's is based in his experience in embryology and cytochemistry. One might argue that Baldwin's concept is an elaboration of Darwin's [38] conjecture on Baboon "metaphysics" drawn out recently in Cheney and Seyfarth's [39] and the evolution of the "mind". But the adaptive "arms race" in nature, as between Bordetella bacteria and bacteriophage [40] is a process of communication. The bacteria is able to change its outside cell surface almost at any time, yet phage has evolved diversity-generating retroelements (DGRs) that achieve "targeted" genomic adaptation. Such successful response is a process of "information processing" by the phage, no matter at all if we call it Baldwinian or Lamarkian.
Graph theory, nodes and neighbor joining for discovering paleospecies
How charts are composed using traits depends on concepts of affinity and difference. As John von Newmann [41] noted, this starts with a conception of the complexity of the organism and how they can be subdivided into parts, seen as elementary units. This stands for the actual biological process that has arrived at the complexity we address, but allows us to apply logic or math to the complexity, though that complexity has built up over time and represents systems that are difficult to isolate in any organism or populations of organisms over time. It leads to the consideration of similarities and differences and differences among individuals in populations and fossils which can be set into categories of primitive and derived in relative emphasis and from this are erected cladistics stepping stones isolated from reality yet posing as representations of transitions as Le Gros Clark [42,43] carefully described. This is especially true regarding the distribution of traits in populations and assigning specific associations as derived. An example of this is the generally shorter tibia of Neandertals [44] when at least one contemporary population, the Maori, have tibia in the same range [45-47]. At the base of this process is the principle of parsimony (the fewest steps to a conclusion) in the reconstruction of ancestral character states [48] or that of maximum likelihood (using "local" or "global methods") based on models of how evolution proceeds.
Math has been a major tool in this process, in the need to interpret relations of traits and the categories formed from observed differences. Bootstrapping and graph theory are also often used methods. The use of graph theory in phylogenetics has a long history. The idea stems from the use of a field of points carrying certain values that are joined together as pair wise relations into nodes, these may be connected by arcs, edges or most often by lines. The means of associating specific fossils to unique points depends on the interpretation of the qualities of the fossils to some scientifically established model of a species. The creation of "rooted" and "unrooted" trees, in the former case producing a most recent common ancestor (MRCA) depends on the determination of genetic distance, in either case both are based on ideas of divergence rates, including the molecular clock theory. Bootstrapping was a product of Bradley Efron's efforts to estimate variances in a population from simple or limited data. It involves the statistical mechanics of "resampling" from the available data to project logical aspects of the true population and missing data [49].
How closely any specific fossil can be so associated depends on how measurements are made and fossils described given the model. Efforts by Hess [50] and Brothwell [51] to increase confidence in measurements of variants led to considerations of multiple measurements [52-55]. Penrose's approach has been found more useful in some cases than M-statistic methods [56] but for large data sets M-statistic methods have produced more useful results, especially in genetic and medical meta-studies [57]. However, some researchers, especially in cranial studies, favored concentration on a "single measure of divergence" as developed by Smith [58] for convenience as modified by Grewal [59]. The validity of this approach has been discussed by Berry [60] Berry, Evans and Sennitt [61], Howe and Parsons [62] and Berry, Berry and Ucko, [63]. This involved averaging measures of divergence and applying principal component analysis (PCA) to the data as shown by Howells [64], and sensitive to the relative scaling of the original variables. PCA was developed by Pearson [65] as a means of finding "…ines and planes of closest fit to systems of points in space", basically making approximations between points of measurement [66].
This procedure attempts to deal with variation of variables and transform them into a set of values often using additional computations to adjust the set of values, as in eigenfunction decomposition where the "noise" of the measurement system will have undue influence and could hamper solutions (detection).
To attempt this with paleospecies requires a means of describing the population of the fossil model and its hypothetical variation over time, including gender variation, age characteristics, population size over time and potential hybridization events. In this way the existence of Australopithecus afarensis can be described as existing for some million years and probably produced over that time some 3 or 4 million individuals considering a low crude birth rate (CBR), survival given predation and disease beginning with some 100 fertile individuals. We assume stability in morphology to come to this conclusion given our sample over this million year period. But do these fossil samples represent the real population diversity of the species we call Australopithecus afarensis? The same problem was addressed by Rightmire for Homo erectus and Tobias [10] and Wood [67] for Homo habilis.
Data produced by a variety of individual scientists measuring and interpreting fossil remains and reconstruction produce the same problem Howells [64,68] faced in addressing variability in human crania. This resulted in his attempt to eliminate operator error by making new computations himself and comparing these other exisiting ones. Later studies [69-71] argued from their own computations, that they could associate cranial architecture with genetic constraints and there were limited environmental variability in samples studied. Later studies contradicted this finding, with Williams, Belcher, and Armelagos [72] arguing, that misclassification of a high percentage of ancient skulls into modern reference samples, produced "the possibility that skeletal material could be accurately sorted by geographic origin, at any other level than geographic extremes, is quite small". Hubble and Neves [73] criticized the software they used in their analysis and agreed with Relethford [70] and Roseman [71].
The same problem attends producing phylogenetic charts from DNA or ancient DNA (aDNA), establishing notes based on agreement of samples is complicated by the number of different transcripts produced during amplification and resulting from either degradation or contamination and by sequence variations between samples that are separated in time by thousands if not tens of thousands of years [74]. How much variability can be smoothed in the computing process and still have a relationship that accounts for population diversity and microevolution? The production of phylogenetic trees based on neighbor joining requires measurement that have been collected and inputted as data points that are manipulated by an algorithm to discover relationships between individuals in groups that are considered [75]. The main problem is how many variations create groups, how distinct are the aDNA sequences and how authentic? The use of DNA barcodes and thin-film biosensor chips have improved species identification in living species, limitations remain [76].
As Caldararo argued in a recent paper [1] definitions of what is a species are necessary to understand differences in DNA sequences in descendent populations. How many differences makes a species? We have many differences that can be demonstrated in contemporary populations, different ear shape, hair color, hair type, cranial shape, skin color, blood types, etc. Yet no one would today propose that such differences identified those individuals possessing such differences or combination of differences as different species. As Gould [77] argued, we then to be driven by extremes or by means and miss the nature of population diversity in either case. We base our general determination on the reliable process of fertility and the production of fertile young, known as the Biological Species Concept [78]. However, we cannot apply this rule to fossil species and some "species" we have acknowledged in the past can produce viable young in matings, as in Papio anubis and Papio hamadryas [79,80].
It is interesting that the time line of discoveries of major fossil entities has taken place in almost reverse order to their age (Table 1). Sahelanthropus being the most ancient at about 7 million years. Yet one must also recall that the Piltdown Hoax took place between #s 2 and 3 in the table above and distorted our understanding of hominin evolution for about half a century. Fashion in ideas and precedent have had their effects, as Weidenreich [81] notes, first people tried to divide humanity by skin color, the by constitution and vapors, then head shape and ideas of civilization, all of which failed to pass the test of fact. Today we face a similar problem with the use of DNA.
There are at least a dozen concepts concerning designating animal groups as different species, some of the more used include the Phylogenetic Species Concept (PSC), the Species Recognition Concept (SRC) and the Ecological Species Concept (EPC). Each has problems and refining definitions requires agreement. Obviously the SRC, like the BSC would be impossible to apply to fossil paleospecies. With the PSC of Eldredge and Caracraft [82] the focus is on morphology to determine a diagnostic cluster of traits from individuals from which a parental pattern can be discerned. However, what is the necessary number of traits and individuals required to establish the diagnosis? Numerous methods have been used, Rightmire [83] used detailed measurements and computation to arrived a diagrams to value trends in trait appearance. But how reliable are these methods in terms of what we are expecting from them. Meirmans [84] points out a similar problem of expectations with genetic distance analysis residing concerning factors like Isolation by Distance (IBD) and the use of programs like a Mantel test, or data checks using AMOVA. He also tests SAM (Special Analysis Software for detecting candidate loci for selection) and FDIST (as a measure of differentiation or diversity), he found both identified an excess of loci with p ≤ 0.05 due to program assumptions. This is also confounded where taxonomists have found groups of organisms that are morphologically indistinguishable from each other to belong to different evolutionary lineages by genetic analysis [85]. We are not concerned here with back mutations, reversals [86] or pseudo-atavism (e.g. Hypertrichosis) or pathological conditions that mimic these ideas, as in Uner Tan Syndrome where affected individuals must walk in quadrupedal posture [87,88].
Similarly differences in coding of characters have been suggested as being the source of conflicting phylogenetic conclusions in termites [89]. As Maynard Keynes [90] once cautioned, one should not mistake probability for reality. How much gene flow and population variation undermines the veracity of such an analysis and how can this be applied reliably to paleospecies? An approach to the problem was published by O'Hara [91,92] who focuses attention on the nature of the generalizations that lead to representations of phylogenetic relationships. The same caution was expressed by Sean Eddy (one of the authors of the book, Biological Sequence Analysis) [93], who is abused by the way people added to "sequence weighing" in an ad hoc fashion to where he considered there was so little statistical basis for many models he called their work, "sequence weighting crap" [94]. Lior Pachter, author of the program SLAM criticized the claims of program creators who compare programs and argue theirs is always better but then everyone cannot be right. On a more troubling note, Shen, Hittinger and Rokas [95] argue that contentious relationships in phylogenomic studies can be the result of small subsets of genes affecting large data matrices on specific nodes of data. Elimination of often single gene inclusion can diminish support for branching. This expands on earlier work by Rokas, Williams and Carroll [96].
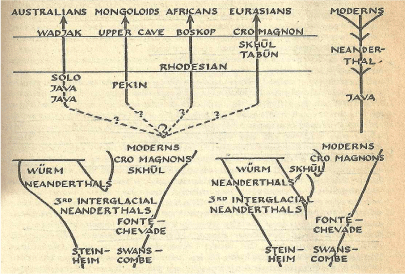
For hominids we have attempted to use physiological differences in the surviving fossil material to construct logical species groupings. That is not to say there have not been successes, certainly advances in gene therapy (for example in Car-T research and treatment) indicate greater precision in identifying sequences and interpreting variations for biological ends, even though there have been terrible setbacks (for example see review in Crow, et al. [97]). Unfortunately, there has been a long history of disagreement over what significance to place on differences. In Figure 1 we have reproduced a drawing from Howells' 1959 book [98]. It contrasts the idea of a straight line evolution of hominines or anagenesis, to a bushy "hat rack" idea. How do we determine the differences or similarities that are possible to group or separate fossil evidence into such charts?
Much is being made concerning certain groups of DNA, called haplotypes and designations of ethnicity, race and evolution of populations [99]. Trees have been constructed detailing evolutionary changes, associations with locations and historic events, as in migrations. Templeton [100] defines a haplotype as, "A haplotype is a multisite haploid genotype at two or more polymorphic sites on the same chromosome in a defined DNA region". He argues that, "Haplotype trees can be used to reconstruct past human gene-flow patterns and historical events, but any single tree captures only a small portion of evolutionary history, and is subject to error". Therefore, as with discussions of species, a caution on the discussion of haplotypes is in order: Claims that certain sequences have evolutionary significance should be considered carefully.
We saw in the 1990s studies of the genomes of various organisms produced general ideas of horizontal gene transfer (HGT) from Archaea and Bacteria to vertebrates without any evolutionary intermediates. Subsequent research seemed to show these findings were in error [101]. Other, later work, clarified some evidence of (HGT) but the issue is still clouded [4]. Crisp, et al. 2015 [102], demonstrates that there does appear to be more support for the idea of HGT in vertebrates and humans. This example shows how tenuous are our findings and the problems that interpretation can bring. HGT reeked havoc with ideas of species and trees. But new information and technology will cause us to reexamine our assumptions, the same should be the case with haplotype analysis.
Haplotypes, despite a lack of evidence or understanding of a selective advantage, are often credited with substantial significance in evolution, as in the case of FAM72 and SRGAP2 [103,104]. FAM72 has been identified as a neuronal progenitor cell self-renewal protein with tumourgenic effects [105]. Haplotypes are not eternal and arguments that they are associated with nations, peoples or specific geographic areas are at best wrong-headed [106]. This is most evident in the way haplotypes have been associated with ethnic groups and localities. For example, in the Wainscoat, et al. 1989 [107] discussion of the frequency of the Taq I y-globin polymorphism, they argue that it is found in 0.47% of the Nigerian population and at 0.36% of the South African Black, and that this is not found outside of Africa and thus indicates a major division of human population. The fact that sample sizes were small (132 to 11 individuals) and that we do not know what Nigerians this included or what South African "Blacks" lends a rather small indication for a "major" division. What about those Nigerians, the majority who did not have the haplotype or the Blacks can 0.47% and 9.36% represent all Africans? It is obvious selective pressures change and affect distribution of such traits as in the variation between highland and lowland populations in y-globin gene promoter polymorphisms [108].
Haplotype pattern of inheritance differs from that of genes common to descendent groups or species from a common ancestor [109], as illustrated in Figure 2. They change over time as does their frequency among local groups due to selection and mutation, appearing and disappearing and thus statements that specific haplotypes can be used to identify ancient migration patterns or "homelands" are ill-advised and most likely entirely false. Ideas of such essentialism of haplotypes in specific people or peoples creates the same problems as did ideas that certain cranial types were associated with specific peoples in the 19th century, and represented certain behavioral qualities or abilities much to the disgrace of the anatomists who championed those theories [110].
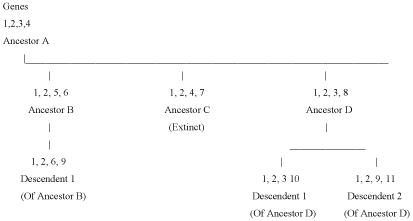
Here we have an ancestor species A from which there are 3 species derived: B, C & D.
Genes 1, 2, 3, and 4 are the ancestral genes. Genes 1 and 2 are retained as conservative and appear in each descendent species. Where they appear in descendent species they are inherited directly from the ancestor. Genes 5, 6, 7, 8, 9, 10, and 11 are mutations appearing in the individual histories of the separate species. In the Vernot, et al. 2016 [111] chart reproduced here as Figure 3 the lines from Neandertals and Denisovans indicate "introcession" of mutations newly produced in these hominids into populations of early Homo sapiens sapiens at some time in the past. This difference, inherited genes from common ancestors is a distinctly different process from genes transmitted via sexual matings of hominids that are interfertile as Vernot, et al. 2016 [111] assume.
Certainly sequences have been gained and lost by individuals in populations and by some populations but the history of gene flow, transmission and loss is yet to be fully understood. Relethford [70], reviewing Alan Fix's [112] book on the use of molecular biology to study human migrations puts this elegantly describing the danger in trying to abstract a single pattern of human migration, particularly when the goal is to make inferences about ancient human populations. Fix concludes "perhaps the real take-home message from these comparisons is that there is not one 'real' human population that typified human populations throughout the long span of our evolution".
In some cases the DNA sequences [113] identified as causative agents, as with megacephaly and autism, are so variable and diffuse in association with clinical expression as disease, that causation seems doubtful at best [114,115]. The assumptions surrounding "association", "correlation", and statistical validity have also been often, if not false, distorting [116]. The finding that dyslexia varies by language, as in Chinese children vs. English children indicates that dyslexia is not the same in every culture or have a universal biological cause [117]. The same research suggests Japanese is intermediate between Chinese and a language like English [118]. What is most interesting is the fact that the English-speaking children and Chinese-speaking children develop dyslexia pathologies of the brain in different areas. The affects of culture on brain development would seem possible, given our long history of studies on brain development and experience [119], but epigenetic studies in promoter and enhancer activity is clearly an area of future work in human corticogenesis [120] and may define a number of exogenous factors.
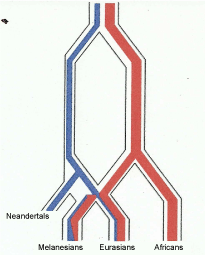
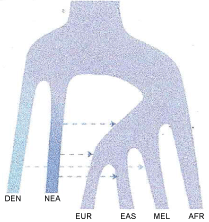
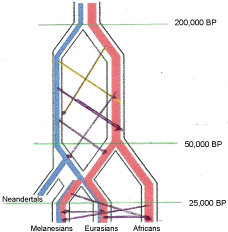
The Vernot, et al. 2016 [111] chart is unfortunate as it does not clearly indicate the idea of genetic exchange by living co-mingling populations of the same species. It is easily read as indicating separate species and an undefined transmission of genes, perhaps by hybrids. A chart by Mendez, et al. 2014 [121], their Figure 4 (reproduced here as Figure 3), suffers a similar distortion as Vernot, et al. 2016 [111] and both give the impression of speciation between Melanesians, Eurasians and African populations. A more representative means of illustrating the transmission of the haplotype at STAT2 between these groups is shown in Figure 5, where I have modified their chart to indicate interfertile populations of the same species, and clarified the time frame of the past 200,000 years and indicating by the arrows continued gene flow between populations of hominids. This is a form of anagenesis, or the idea that the human species of Homo sapiens probably should include Neandertals, Denisovans and other mid Pleistocene hominids if we assume inter fertility which is the basis for the Biological Species Concept. This seems all the more reasonable as in recent years the extreme views of Neandertals, both in terms of physiological differences of the Archaic Homo group in general [8], as well as the behavioral differences assumed in the past have been undermined [122]. The Mendez, et al. 2014 [121], chart does not appear in the published paper, but only in the supplemental materials. And the new find at Jebel Irhoud [123] supports my view of a more inclusive transition of Archaic Homo to modern human.
As I have mentioned in an earlier paper [1] we are limited in our understanding of hybridization in paleospecies, but some, as in the case of Clifford Jolly, have discussed hybridization in other contexts in primates, as with Papio [124]. Sankararaman, et al. [29] have made a number of suggestions regarding Neandertal and Modern Human sterility based on the current aDNA recovery and sequencing of the X chromosome. I think just as the discovery of Neandertal DNA in Modern Human populations today [125] disproved earlier arguments of the lack of such transmission [126], it is likely the Sankaraman, et al. [29] speculations will be unsupported in the future.
The problem with behavior differences has been significant in determining the transition to sapiens status. Emphasis on tool making and especially the theories of speciation associated with "advanced" blade industries lose their force when early Australian tool kits are examined [127]. The very fact that the simple tools used by the first Australians had served them (and their ancestors) well in crossing not only the vast territories and varied environments from Africa to South Asia, but had been sufficient to cross the sea barrier to Australia itself. It was their ability and not the tools they carried that made the difference. This points out the fallacy of using technology to define species.
Association and Not Causation
In the same fashion as discussed above with haplotypes and disease or origin, genetic studies of ability have confused association of SNPs with causation of ability. Some of these efforts to parse out the factors of IQ have turned to Meta studies, looking at large groups of data derived from specific surveys of both adults and children. They search the studies for potential markers of biologically determined ability. Others collect responses from populations they create from available sources and manipulate the populations to fit certain criteria of their study, for example, availability of genetic data. The study by Benyamin, et al. [128] is one of these, they focused on the FNBP1L gene and used data available from a number of pre-existing sources from which genetic data was available.
Their sample contained 17,989 children of European ancestry and was purged of outliers due to "missingness" heterozygosity, relatedness, population and ethnic outliers and other undefined cohort-specific quality control steps. Most of the studies drawn from also were not random samples, for example, The Avon Longitudinal Study of Parents and Children, was collected from mothers having multiple pregnancies or 2 births between 1991 and 1992, thus it is a select population and not a random sample. The authors state that they found that, "There was no SNP that reached nominal significance after a Bonferroni adjustment". This is a statistical test to ensure that when multiple comparisons or multiple hypotheses are tested the chance of a rare event increases, and therefore, the likelihood of incorrectly rejecting a null hypothesis (a Type One error) increases. This method is named after the Italian mathematician, Carlo Emilio Bonferroni, but it was standardized by the work of Olive Jean Dunn. The authors then meta-analysed their data from the cohorts and found there were no SNPs that reached a genome-wide significant threshold. But plotting the estimated regression coefficients from the top 100 SNPs between their samples they found a positive correlation and identified FNBPIL (Formin Binding Protein I-Like) as associated with childhood intelligence.
The two main problems with studies like this is the idea that one factor can be the causative agent and the idea that association is causation. If we ask the question of what does FNBPIL do, we find a rather disappointing answer. This is a protein of the BAR Domain Super family of proteins. These are involved in endocytosis and cell migration. This class of proteins are evolutionarily conserved from yeast to human. The amino acid sequence of the F-BAR domain of Toca-1/formin binding protein 1-Like (FNBPIL) is almost identical to those of CIP4 and FBPI7. These latter two are involved in the diameter of tubles, correspond to the curvature of the initial stages of clathrin-coated pits and CIP4 regulates insulin signaling, the F-BAR and SH3 domains of FBP17 are essential for the formation of podosome and phagocytic cups in macrophages. Toca-1/FNBPIL is essential for autophagy of the intracellular pathogen Salmonella enterica serovar Typhimurium [129] and we can go on. In other words, this protein which they have attempted to associate with intelligence is involved in so many biochemical interactions in the body that it is rather surprising they would isolate it as a causative agent in a capacity like intelligence without defining a role specific to the quality. But this tendency is characteristic of other such studies of intelligence using SNPs, as those by Davies, et al. [130]. Such mistaken ideas of single gene expression fail to understand the nature of transcriptional activity of alleles, as in the case of mono or biallelic expression [131].
This brings up another consideration that of Linkage Disequilibrium (LD), a recent study by Koch, et al. [132] demonstrated an excess of associations between pairs of distant sites on all of the 22 autosomes. It is clear that "detecting" LD does not ensure linkage or a lack of equilibrium. LD has generally been defined as an association between pairs of sites or loci, yet Koch, et al. [132] argues that there seems to be associations between pairs of chromosome blocks separated by large intervening chromosome regions, referred to as Long-range linkage disequilibria (LRLD). Several types of confounding data problems exist including, miscalled SNPs and phasing errors.
Assumptions alone are not the only problem with associations, in the initial work done by Stoneking and Cann [133] they produce a Figure 1 where they claim "the average sequence divergence that has accumulated since the common mtDNA ancestor is 0.57%" however, when one looks at the data we find that it represents groups with numbers of individuals in the groups ranging from 134 to 21, they have weighted all these groups equally though they vary in sequence divergence from 0.00 in the Venezuela sample to 0.59 among the San. The sample with the largest number of individuals, the Sardinians at 134, has one of the lowest rates or 0.29. The median rate of all the individuals given would be 0.32 which would substantially change their date of separation. Also, it is unclear if the estimations of mtDNA mutation rates are clearly understood, Parsons, et al. [134] found higher rates than are generally recognized and most rates are based on the idea that paternal mtDNA never enters the ovum and never contributes to the fertilized cell, yet there has been clear evidence this is false given demonstration published by Aitken [135] and Ankel-Simons and Cummins [136]. Further evidence of this "paternal leakage" is found in sheep [137] and humans [138,139]. No comprehensive study of such transmission across populations has yet been done. However, Pyle, et al. [140] question reports of paternal mtDNA inheritance. Assumptions that mtDNA variations are neutral are also at odds with the biology of mtDNA in vertebrates [141]. To reduce problems in control region studies, Ingman, et al. [142] produced a study of the entire mtDNA genome of a larger sample that most.
Conclusions: Over Extension and Futures
While the technology of ancient DNA extraction, processing and preservation has increased dramatically in the past 40 years, our ability to interpret the results is still in the developmental stage. Models and simulations of how specific sequences might have been transmitted across the globe and promoted survival or been selected against have produced contradictory results. Perhaps this is what we should have expected, not just because the writing of an algorithm must be based on a certain understanding of prior information on a subject, and on past histories of patterns of events, but because, in the case of hominin evolution, selection has changed over time. Selection pressures 2 million years ago were obviously different that those today, traits adaptive then lost their benefits for survival, not just due to migrations into new territories (less solar radiation, less need for melanin in the skin the loss of which can promote bone growth but also be a factor associated with autoimmune disease like hypervitaminosis and MS, [74]. Trade-offs in mutations, silencing of genes, polygenic effects and duplications have created new complex interaction with the environment and plants and animals. New ecological systems are created and humans become substrates for new and old pathogens and symbionts. An algorithm developed to account for factors of environment and selection today can hardly be expected to pattern selective pressures of the distant past.
On the other hand, our attempts to use math to construct computer systems (algorithms) to model trends over time can be compared with the human genome's attempt to produce genetic responses to improve the survival of its gene-machine (in Dawkin's sense here), thus haplotypes are really nature's algorithms based on past responses in the biosphere for the hominins of the time, for example, the immune/pathogen co-evolutionary history [143]. Eventually, our techniques will bring us closer to an understanding of the relationships of paleospecies representing human evolution.
We find the idea of the DNA code continues to stimulate new frontiers of research and commerce, some concerning as in the identification of people in data bases uncovering potential risks for corporate health care or life insurance. Other concepts are simply as odd as revealing ancient past ethnicities, as in DNA menus provided by Life genetics (http://lifegenetics.net/1-click-dna-menu-planning/) or DNA Pizza (http://www.dnapizza.com/menu.html).
References
- Caldararo Niccolo (2016) Denisovans, melanesians, europeans, and neandertals: The confusion of DNA assumptions and the biological species concept. J Mol Evol 83: 78-87.
- Lowenstein JM (1999) Evolutionary information from fossil molecules. In: Shirley C Strum, Donald G Lindburg, David Hamburg, The new physical anthropology: Science, humanism and critical reflection. 174-180.
- Suarez-Diaz Edna, Anaya-Munoz Victor H (2008) History, objectivity, and the construction of molecular phylogenies. Stud Hist Philos Biol Biomed Sci 39: 451-468.
- Puigbo Pere, Wolf Yuri I, Koonin Eugene V (2009) Search for a 'Tree of Life' in the thicket of the phlylogenetic forest. J Biol 8: 59.
- Lu Zhi-xiang, Peng Jia, Su Bing (2007) A human-specific mutation leads to the origin of a novel splice form of Neuropsin (KLK8), a gene involved in learning and memory. Hum Mutat 28: 978-984.
- Tattersall Ian (1986) Species recognition in human paleontology. Journal of Human Evolution 15: 165-175.
- Tatersall Ian (1992) The many faces of Homo habilis. Evolutionary Anthropology 1: 33-35.
- Pearson OM (2000) Postcranial remains and the origin of modern humans. Evolutionary Anthropology 9: 229-247.
- Krantz GS (1961) Pithecanthropine brain size and its cultural consequences. Man 11: 85-87.
- Tobias Phillip (1971) The Brain in hominid evolution. Columbia University Press, New York.
- Holloway Ralph (1996) Evolution of the human brain. In: A Lock, C Peters, Handbook of human symbolic evolution. Oxford University Press, New York, 74-116.
- Blinkov SM, Glezer II (1969) The human brain in figures and tables: A quantitative handbook.
- DeSilva Jeremy M, Julie J Lesnik (2008) Brain size at birth throughout human evolution: A new method for estimating neonatal brain size in hominins. J Hum Evol 55: 1064-1074.
- Herculano-Houzel Suzana (2016) The human advantage. MIT Press, Cambridge.
- Semple Bridgette D, Blomgren Klas, Grimlin Kayleen, et al. (2013) Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 106-107: 1-16.
- Bonin von G (1963) The Evolution of the human brain. University of Chicago Press, Chicago, USA.
- Deacon Terrence W (1997) The Symbolic species. W.W. Norton, New York.
- Berger Lee R, Church Steven E, De Klerk Bonita, et al. (2008) Small-bodied humans from Palau, Micronesia. PLoS One 3: e1780.
- Wilford John Noble (2008) Debate over 'Little People' intensifies after recent island discovery. New York Times.
- Henneberg Maciej, Eckhardt Robert C, Sakdapong Chavanaves, et al. (2014) Evolved developmental homeostasis disturbed in LB1 from Flores, Indonesia, denotes Down syndrome and not diagnostic traits of the invalid species Homo floresiensis. Proc Natl Acad Sci U S A 111: 11967-11972.
- Zimmerman MR (1998) Alaskan and Aleutian mummies. In: Aidan Cockburn, Eve Cockburn, Theodore A, Reyman, Mummies, Disease and Ancient Cultures. (2nd edn), Cambridge, Cambridge University Press, 138-153.
- Berger LR, Hawks J, de Ruiter DJ, et al. (2015) Homo naledi, a new species of the genus Homo from the Dinaledi Chamber, South Africa. Elife 10: 4.
- Holloway RL (1980) Within-species brain-body weight variability: A re-examination of the Danish data and other primate species. Am J Phys Anthropol 53: 109-121
- Dekaban AS (1978) Changes in brain weights during span of human life: Relation of brain weights to body heights and body weights. Ann Neurol 4: 345-356.
- Caldararo Niccolo (2017) Big brains and the human super organism: Why special brains appear in humans and other social animals. Lexington Press, Lanham.
- Sims D, Sudbery I, Ilott NE, et al. (2014) Sequencing depth and coverage: Key considerations in genomic analyses. Nat Rev Genet 15: 121-132.
- Sudmant PH, Rausch T, Gardner EJ, et al. (2015) An integrated map of structural variation in 2,504 human genomes. Nature 526: 75-81.
- Bizon C, Spiegel M, Chasse SA, et al. (2014) Variant calling in low-coverage whole genome sequencing of a Native American population sample. BMC Genomics 15: 85.
- Sankararaman Sriram, Mallick Swapan, Dannemann Michael, et al. (2014) The genomic landscape of Neandertal ancestry in present-day humans. Nature 507: 354-357.
- Baldwin James Mark (1902) Development and Evolution. Macmillan, New York.
- Lamark Jean-Baptiste (1830) Philosophie zoologique. Bailliere, Paris.
- Cameron Nicole M (2011) Maternal programming of reproductive function and behavior in the female rat. Front Evol Neurosci 3: 10.
- Haig David (2007) Weisman Rules! Ok? Epigenetics and the Lamarkian temptation. Biol Philos 22: 415-428.
- Weaver IC, Cervoni N, Champagne FA, et al. (2004) Epigenetic programing by maternal behavior. Nat Neurosci 7: 847-854.
- Burman JT (2013) Updating the baldwin effect: The biological levels behind Piaget's new theory. New Ideas in Psychology 31: 363-373.
- Waddington CH (1942) Canalization of development and the inheritance of acquired characters. Nature 150: 563-565.
- Waddington CH (1956) Principles of embryology. George Allen & Unwin, London.
- Darwin Charles (1989) Charles Darwin's notebooks, 1836-1844: Geology, transmutation of species, metaphysical enquiries. Cornell University Press, Ithaca, New York.
- Cheney Dorothy L, Seyfarth Richard M (2007) Baboon metaphysics. University of Chicago Press, Chicago, USA.
- Heyman Karen (2005) Retroelements guide adaptation. The Scientist.
- Neumann Von, John (1951) The general and Logical theory of automata. In: Lloyd A Jeffress, Cerebral Mechanisms in Behavior. The Hixon Symposium. John Wiley & Sons, New York, 1-31.
- Clark Le, Gros WE (1934) Early Forerunners of Man: A morphological study of the evolutionary origins of the primates. Oxford University Press, Oxford.
- Clark Le Gros WE (1964) The fossil evidence for human evolution. Revised Edition.
- Lovejoy C Owen, Trinkaus Erik (1980) Strength and robusticity of the Neandertal tibia. Amer J Phys Anth 53: 465-470.
- Davidson Janet (1984) The Prehistory of New Zealand. Longman Paul, Auckland, 270.
- Houghton Philip (1980) The First New Zealanders. Hodder and Stoughton, Auckland.
- Schofield G (1959) The metric and morphological features of the femur of the New Zealand Maori. J Royal Anth Inst 89: 10.
- Pagel Mark (1999) The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Systematic Biology 48: 612-622.
- Efron B (1979) Bootstrapping methods: another look at the jackknife. The Annals of Statistics 7: 1-26.
- Hess L (1945) The metopic suture and the metopic syndrome. Human Biology 17: 107-136.
- Brothwell DE (1961) The biology of earlier human populations. In: DR Brothwell, E Higgs, Thames and Hudson, Science in Archaeology. London, 330-341.
- Rao CR (1948) The utilization of multiple measurements in problems of biological classification. J R Statist Soc 10: 159-203.
- Laughton WS, Jorgensen JB (1956) Isolate variation in Greelandic Eskimo crania. Acta Genet Stat Med 6: 3-12.
- Brothewell DE (1958) The use of non-metrical characters of the skull in differentiating populations. Dt Ges Anthrop 6: 103-109.
- Penrose LS (1954) Distance, size and shape. Annals of Human Genetics 17: 337-343
- Groeneveld HT, Kieser JA (1987) An evaluation of the M-statistic in human odontomorphometric distance analyses. International Journal of Anthropology 2: 29-36.
- Magosi Lerato E, Goel Anuj, Hopewell Jemma, et al. (2017) Identifying systematic heterogeneity patterns in genetic association meta-analysis studies. PLoS Genet 13: e1006755.
- Smith G Elliot (1911) The ancient Egyptians and their influence upon the civilization of Europe. Harper, London.
- Grewal MS (1962) The rate of genetic divergence in the C57BL strain of mice. Genetics Research 3: 226-237.
- Berry RJ (1964) The evolution of an island population of the house mouse. Evolution 18: 468-483.
- Berry RJ, Evans IM, Sennitt BFC (1967) The relationships and ecology of Apodemus sylvaticus from the Small Isles of the Inner Hebrides, Scotland. Journal of Zoology 132: 333-346.
- Howe WL, Parsons PA (1967) Genotype and environment in the determination of minor skeletal variants and body weight in mice. J Embryol 17: 283-292.
- Berry A Caroline, Berry RJ, Ucko Peter J (1967) Genetical change in ancient Egypt. Man New Series 2: 551-568.
- Howells WW (1973) Cranial variation in man: A study by multivariate analysis of patterns of differences among recent human populations. Papers of the Peabody Museum of Archeology and Ethnology, Harvard University, Cambridge, 67: 259.
- Pearson K (1901) On lines and planes of closest fit to systems of points in space. Philosophical Magazine 2: 559-572.
- Wold Svante, Esbenson Kim, Geladi Paul (1987) Principal component analysis. Chemometrics and Intelligent Laboratory Systems 2: 7-52.
- Bernard W (1991) The many faces of homo habilis. Koobi Fora research project, Hominid cranial remains. Oxford: Clarendon Press 1: 33-37.
- Howells WW (1989) Skull shapes and the map: Craniometric analyses in the dispersion of modern homo. Papers of the Peabody Museum of Archaeology and Ethnology, Harvard University, Cambridge, 79: 189
- Relethford John (1994) Craniometric variation among modern human populations. Am J Phys Anthropol 95: 53-62.
- Relethford John H (2001) Migration and colonization in human microevolution. American Journal of Human Biology 13: 280-281.
- Roseman Charles C (2004) Detecting interregionally diversifying natural selection on modern human cranial form by using matched molecular and morphometric data. Proceedings of the National Academy of Sciences U S A 101: 12824-12829.
- Williams Frank L'Engle, Robert L Belcher, George J Armelagos (2005) Forensic misclassification of ancient Nubian crania: Implications for assumptions about human variation. Current Anthropology 46: 340-346.
- Hubbe Mark, Neves Walter A (2007) On the misclassification of human crania: Are there any implications for assumptions about human variation? Current Anthropology 48: 285-288.
- Caldararo Niccolo, Gabow Steven (2000) Mitochondrial DNA and the place of neandertals in homo. Ancient Biomolecules 3: 135-158.
- Saitou N, Nei M (1987) The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4: 406-425.
- Pecnikar Fiser, Buzan EV (2014) 20 years since the introduction of DNA barcoding: From theory to application. J Appl Genet 55: 43-52.
- Gould SJ (1996) Full house: The spread of excellence plato to darwin. Harmony Press, New York.
- Mayr Ernst (1982) The growth of biological thought. Belknap Press, Cambridge.
- Lewin R (1989) Species questions in Modern Human origins. Science 243: 1666-1667.
- Bergman TJ, Beehner JC (2004) Social system of a hybrid baboon group (Papio anubis x P. hamadryas). International Journal of Primatology 25: 1313-1330.
- Weidenreich, Franz (1946) Apes, Giants and Man. University of Chicago Press, Chicago, USA.
- Eldredge N, Cracraft J (1980) Phylogenetic patterns and the evolutionary process. Method and Theory in Comparative Biology. Columbia University Press, New York.
- Philip Rightmire G (1990) The evolution of homo erectus. Cambridge University Press, Cambridge.
- Meirmans Patrick (2012) Trouble with isolation by distance. Mol Ecol 21: 2839-2846.
- Saez AG, Lozano E (2005) Body doubles. Nature 433: 111.
- Domes Katia, Norton Roy A, Maraun, et al. (2007) Reevolution of sexuality breaks Dollo's law. Proc Natl Acad Sci U S A 104: 7139-7144.
- Humphrey N, Skoyles JR, Keynes R (2005) Human hand-walkers: Five siblings who never stood up. LSE Research Online, Discussion Paper.
- Tan Üner (2006) A new syndrome with quadrupedal gait, primitive speech, and severe mental retardation as a live model for human evolution. Int J Neurosci 116: 361-369.
- Roisin, Yves, Korb, et al. (2011) Social organization and the status of workers in termites. In: David Edward Bignell, Yves Roisin, Nathan Lo, Biology of Termites A Modern Synthesis. Springer, Dordrecht, 133-164.
- Keynes John Maynard (1962) A treatise on probability. Harper Torch books.
- Robert JOH (1993) Systematic generalization, historical fate, and the species problem. Systematic Biology 42: 231-246.
- Robert JOH (1994) Evolutionary history and the species problem. American Zoologist 34: 12-22.
- Durbin Richard, Eddy Sean R (1998) Biological Sequence Analysis: Probabilistic Models of Proteins and Nucleic Acids. Cambridge University Press, Cambridge.
- Heyman K (2003) Gene finding with hidden Markov models. The Scientist 28.
- Shen XX, Chris TH, Antonis R (2017) Contentious relationships in phylogenomic studies can be driven by a handful of genes. Nature Ecology and Evolution 1.
- Rokas A, Williams BL, King N, et al. (2003) Genome-scale approaches to resolving incongruence in molecular phylogenies. Nature 425: 798-804.
- Crow, David (2017) Vein-to-Vein solutions. The Financial Times.
- Howells, William W (1959) Mankind in the making. Garden City, Doubleday.
- Kimchi-Sarfaty C, Marple AH, Shinar S, et al. (2007) Ethnicity-related polymorphisms and haplotypes in the human ABCB1 gene. Pharmacogenomics 8: 29-39.
- Templeton AR (2005) Haplotype tress and modern human origins. Am J Phys Anthropol 48: 33-59.
- Stanhope MJ, Lupas A, Italia MJ, et al. (2001) Phylogenetic analyses do not support horizontal gene transfers from bacteria to vertebrates. Nature 411: 940-944.
- Crisp A, Boschetti C, Perry M, et al. (2015) Expression of multiple horizontally acquired genes is a hallmark of both vertebrate and invertebrate genomes. Genome Biol 16: 50.
- Charrier C, Joshi K, Coutinho-Budd J, et al. (2012) Inhibition of SRGAP2 function by its human-specific paralogs induces neoteny during spine maturation. Cell 149: 923-935.
- Dennis MY, Nuttle X, Sudmant PH, et al. (2012) Evolution of human-specific neural SRGAP2 genes by incomplete segmental duplication. Cell 149: 912-922.
- Kutzner A, Pramanik S, Kim PS, et al. (2015) All-or-(N)One - an epistemological characterization of the human tumorigenic neuronal paralogous FAM72 gene loci. Genomics 106: 278-285.
- Hay Maciamo (2017) Origin, spread and ethnic association of European haplotypes.
- Wainscoat JS, Hill AVS, Thein SL, et al. (1989) Geographic distribution of Alpha and Beta-globin gene cluster polymorphisms. In: Paul Mellars, Chris Stringer, The Human Revolution. Princeton University Press, Princeton, 31-38.
- Inken R, Francisco R, Manuela D (2010) Native highland and lowland populations differ in γ-globin gene promoter polymorphisms related to altered fetal hemoglobin levels and delayed fetal to adult globin switch after birth. Anthropological Science 118: 41-48.
- Carroll SB (1995) Homeotic genes and the evolution of arthropods and chordates. Nature 376: 479-485.
- Firmin Antenor (2002) The equality of the human races. Champaign, University of Illinois Press, (originally published 1885).
- Vernot B, Tucci S, Kelso J, et al. (2016) Excavating neandertal and denisovan DNA from the genomes of melanesian individuals. Science 352: 235-239.
- Alan GF (1999) Migration and colonization in human microevolution. Cambridge University Press, New York.
- Dumas LJ, O'Bleness MS, Davis JM, et al. (2012) DUF1220-Domain copy number implicated in human brain-size pathology and evolution. Am J Hum Genet 91: 444-454.
- Brunetti-Pierri N, Berg JS, Scaglia F, et al. (2008) Recurrent reciprocal 1q21.1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nat Genetic 40: 1466-1471.
- Perry GH, Ben-Dor A, Tsalenko A, et al. (2008) The fine-scale and complex architecture of human copy-number variation. Am J Hum Genet 82: 685-695.
- Nuzzo Regina (2014) Statistical errors. Nature 506: 150-152.
- Siok WT, Perfetti CA, Jin Z, et al. (2004) Biological abnormality of impaired reading is constrained by culture. Nature 431: 71-76.
- Helen P (2004) Chinese dyslexics have problems of their own. Nature.
- Marian CD (1988) Enriching heredity. The Free Press, New York.
- Reilly SK, Yin J, Ayoub AE, et al. (2015) Evolutionary changes in promoter and enhancer activity during human corticogenesis. Science 347: 1155-1159.
- Mendez FL, Watkins JC, Hammer MF (2012) Haplotype STAT2 introgressed from neandertals and serves as a candidate of positive selection in Papua New Guinea. Am J Hum Genet 91: 265-274.
- Francesco DE (2003) The invisible frontier. A multiple species model for the origin of behavioral modernity. Evolutionary Anthropology 12: 188-202.
- Jean JH, Abdelouahed BN, Shara EB, et al. (2017) New fossils from Jebel Irhoud, Morocco and the pan-African origin of Homo sapiens. Nature 546: 289-292.
- Clifford JJ, Tamsin WB, Shimelis B, et al. (1997) Intergeneric hybrid baboons. International Journal of Primatology 18: 597-627.
- Vernot B, Akey JM (2014) Resurrecting surviving Neandertal lineages from modern human genomes. Science 343: 1017-1021.
- Krings M, Geisert H, Schmitz R, et al. (1999) DNA sequence of the mitochondrial hypervariable region II from the neandertal type specimen. Proc Natl Acad Sci U S A 96: 5581-5585.
- Mellars Paul, Stringer Chris (1989) The Human Revolution. Princeton, Princeton University Press, 1-16.
- Benyamin B, Pourcain BSt, Davis OS, et al. (2014) Childhood intelligence is heritable, highly polygenic and associated with FNBP1L. Molecular Psychiatry 19: 253-258.
- Safari F, Suetsugu S (2012) The BAR Domain superfamily proteins from subcellular structures to human diseases. Membranes 2: 91-117.
- Davies G, Tenesa A, Payton A, et al. (2011) Genome-wide association studies establish that human intelligence is highly heritable and polygenic. Mol Psychiatry 16: 996-1005.
- Gimelbrant A, Hutchinson JN, Thompson BR, et al. (2007) Widespread monoallelic expression on human autosomes. Science 318: 1136-1140.
- Evan K, Mickey R, Mark K (2013) Long range linkage disequilibrium across the human genome. PLoS One 8: e80754.
- Stoneking Mark, Cann Rebecca L (1989) African origin of human mitochondrial DNA. In: Paul Mellars, Chris Stringer, The Human Revolution. Princeton University Press, Princeton, 17-30.
- Parsons TJ, Muniec DS, Sullivan K, et al. (1997) A high observed substitution rate in the human mitochondrial DNA control region. Nat Genet 15: 363-368.
- Aitken RJ (1995) The complexities of conception. Science 269: 39-40.
- Ankel-Simons F, Cummins JM (1996) Misconceptions about mitochondria and mammalian fertilization: Implications for theories on human evolution. Proc Natl Acad Sci U S A 93: 13859-13863.
- Zhao X, Li N, Guo W, et al. (2004) Further evidence for paternal inheritance of mitochondrial DNA in the sheep (Ovis aries). Heredity 93: 399-403.
- Schwartz M, Vissing J (2002) Paternal inheritance of mitochondrial DNA. N Engl J Med 347: 576-580.
- Guo Y, Li CL, Sheng Q, et al. (2013) Very low-level heteroplasmy mtDNA variations are inherited in humans. J Genet Genomics 40: 607-615.
- Pyle A, Hudson G, Wilson IJ, et al. (2015) Extreme-Depth re-sequencing of mitochondrial DNA finds no evidence of paternal transmission in humans. PLoS Genet 11: e1005040.
- Niccolo C, Guthrie M (1998) Mitochondrial DNA, the Y Chromosome and the origins of modern humans. HOMO 49: 225-240.
- Max I, Henrik K, S vante P, et al. (2000) Mitochondrial genome variation and the origin of modern humans. Nature 408: 708-713.
- Niccolo C (1996) The HIV/AIDS epidemic: Its evolutionary implications for human ecology with special reference to the immune system. The Science of the Total Environment 191: 245-269.
Corresponding Author
Niccolo Caldararo, Ph.D, Department of Anthropology, San Francisco State University, 1600 Holloway Ave, San Francisco, CA 94132, USA.
Copyright
© 2018 Caldararo N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.