Evaluation of Patients Suspected Gastric Cancer with Multidedector Computed Tomography
Introduction
Gastric cancer is one of the major causes of deaths related to cancer worldwide [1]. Its prevalence shows a great variation according to geographic regions. It is especially frequent in countries such as Japan, Korea and China [2]. Early detection of gastric carcinoma program has yielded successful results in Japan with current screenings. In the Western World, 80% of patients have gastric carcinoma at advanced stages at diagnosis and have a poor diagnosis [3]. The sole proven curative treatment of gastric carcinoma consists of total gastric resection and resection of adjacent lymph nodes [4]. On the other hand, 5-year survival rates of resectable gastric carcinomas is between 10- 30% [5]. Treatment modalities such as chemotherapy and radiation therapy decrease the stage of disease in inoperable advanced stage gastric carcinomas and increase survival rates [6-8]. Thus, determination of depth of gastric wall invasion, adjacent organ invasion, nodal involvement and distant organ metastasis possess vital importance.
Radiological examination with double contrast and endoscopic examination are effective in detection of small mucosal gastric lesions at early stages. But they are inadequate in the evaluation of whole gastric wall, extra-serosal dissemination, and lymph node and distant organ metastasis [9]. Similarly, endoscopic ultrasonography (EUS) cannot be used in routine examination due to its limitations and dependence on the operator [10].
Although multidedector computerized tomography (MDCT) has limitations in the detection of lymph node metastasis, peritoneal dissemination and detection of small hematogenous metastasis, it is the modality of choice in the preoperative evaluation of gastric cancer, due to its current developments such as thin slice and multiplanar imaging. We aimed to investigate the role of MDCT in cases in whom endoscopic examination was performed due to suspicion of gastric cancer and a pathology was found.
Material and Methods
Endoscopic biopsy results and abdominal CT findings of patients admitted at the endoscopy unit of Gastroenterology Department between September 2008 and December 2014 with a working diagnosis of gastric carcinoma were retrospectively compared. Patients who had gastric surgery before, those in whom gastric distention was not at an optimal level and those without appropriate investigations were excluded in this study. Of the 151 patients included in this study, 103 (63%) were males and 48 (37%) were females. Their mean age was 59.8 ± 14.9 years, with an age range between 20-89 years. Our study was a retrospective archive study, and the protocol was approved by the local ethics committee.
Abdominal CT examinations of the patients included in the investigation group were done with multislice Philips Brillance V2.6.1 (2007) device with 64 detectors and Toshiba Activion V3.00 (2010) device with 16 detectors. The images were evaluated with two radiologists specialized on abdominal CT at the work station (Philips Extented Brilliance Workspace Philips Medical systems, Best The Netherlands). The images were examined in axial sections. After that, 3 dimensional reformatted images were created and sagittal and coronal sections were also used when required. In routine abdominal CT examination, imaging was started from the xiphoid process and continued until the symphysis pubica, including the inguinal canal orifices, while the patient held his/her breath. Optimal distention of the stomach was obtained by making the patients drink 1.5 L water containing 10 cc ionic 76% contrast material or only water after fasting for 6 hours. The patients were examined after oral liquid intake and 1-1.5 cc IV contrast material was administered at a velocity of 3 ml/second. The images were obtained at the portal phase. All patients were included in gastric wall thickness, which was measured at the thickest point. The thickest area of the mass was measured in patients in whom there was a mass. The short axis of the lymph node was measured, if lymph nodes were present at the perigastric area. Lymph node metastasis and CT findings were compared in patients who had surgical intervention at the Surgery Department and lymph node dissection was administered. Presence of distant metastasis and mesenteric peritoneal increase in density were searched. Also, the distribution of the tumor according to its location in the stomach was calculated. Endoscopic reports and histopathological examination results of all patients were examined and recorded. Pathologic examination results were considered as the gold standard.
Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables as absolute values and percentages. The sensitivity, specificity values of detecting the primary tumor by MDCT was calculated. The highest sensitivity and specificity values for gastric wall thickness were measured, considering the ROC curve. SPSS (Statistical Package for the Social Sciences, ver.15.0, Chicago, Illinois-USA, 2006) software was used in the statistical analysis.
Results
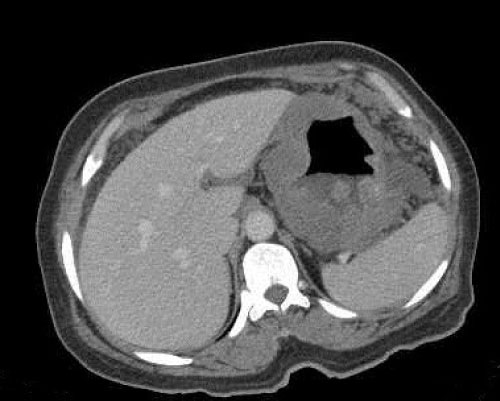
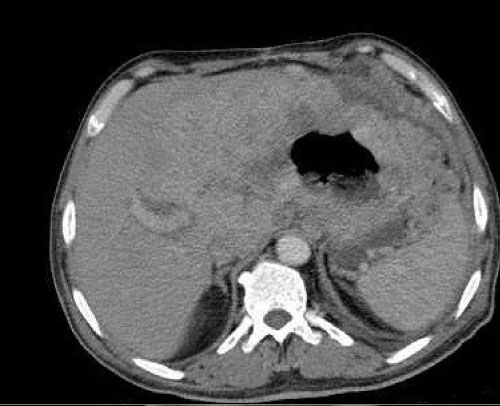
According to endoscopic biopsy results of patients included in this study, 129 patients (85%) had malignant and 22 patients (15%) had benign findings (Table 1). Of the malignant patients, 115 (90%) had adenocarcinoma, 8 (6%) had lymphoma, 3 (2%) had squamous cell carcinoma and 3 (2%) had neuroendocrine cell tumor as diagnosis (Figure 1). In patients in whom pathologic results were benign, the findings were considered as gastropathic changes (Figure 2).
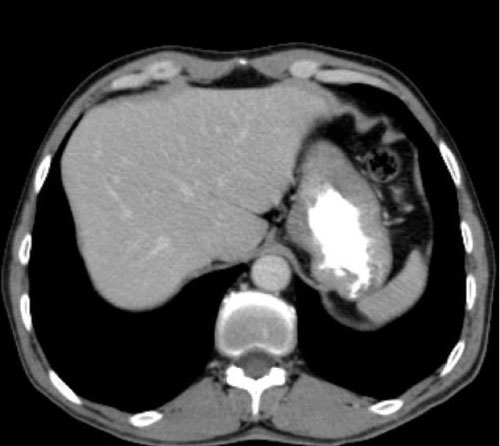
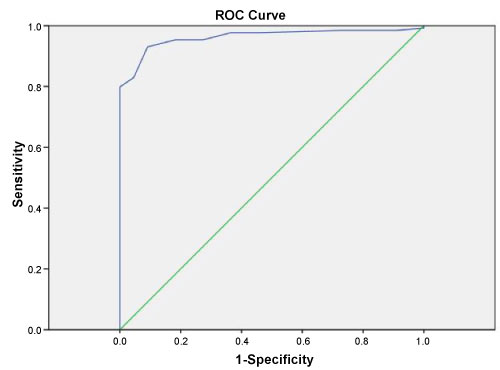
In this study the sensitivity and specificity of CT in detection of gastric cancer was 96.9% and 31.8%, respectively. When the value of gastric wall thickness was taken as 9.5 mm in consideration with ROC curve, the sensitivity of MDCT in detecting gastric cancer was calculated as 95.3%, and specificity was calculated as 81.8% (Figure 3). Perigastric region lymph node metastasis was present in 22 of 28 patients (78%) who had undergone surgical intervention. In MDCTs of patients with lymph node metastasis, the short axis of the largest lymphadenopathy at the perigastric region was measured between 6 mm and 22 mm. In the MDCTs of 6 patients (22%) in whom lymph node metastasis was not detected, short axis of perigastric region lymph nodes were between 6 mm and 10 mm. There was peritoneal spread in 77 of patients with a malignant diagnosis (60%), while 35 (27%) had distant metastasis. There were both peritoneal spread and distant organ metastasis in 83 patients (63%) (Figure 4). The malignant mass was located at antropyloric region in 65 patients (50%), at the gastric corpus in 33 patients (26%), diffuse in 17 patients (14%) and located at the cardia in 14 patients (10%).
Discussion
We found our precision as 96% when pathologic examination result was considered as the gold standard in patients who had admitted with a working diagnosis of gastric cancer in this study. Sohn, et al. and Kleinhaus and Militianu had found precision values of 86% and 81% in detection of gastric tumors, respectively in their studies [11,12]. Our precision value is higher than the values found by other investigators. We feel that the CT technique that we have used and our patient profile may have played a role in this difference. As there are no screening programs for gastric cancer in our country, patients seek medical attention at late phases and this makes detection of gastric cancer with MDCT easier.
Lee, et al. have reported an increase in diagnostic rates of early gastric cancer from 64.5% to 93% when 3 dimensional images were used with 'surface-shaded display' technique. In the study by Chen CY, et al. which was supported by virtual gastroscopy, this rate was found to be 96% [13,14]. None of these methods were used in our study. In the study by Pickhardt, et al. on 153 patients on the normal gastric wall thickness, the mean gastric wall thickness was found to be 5 ± 1.9 mm. On the other hand, the gastric wall thickness range of the normal patients examined by these investigators was between 1.8 mm and 12 mm, which is rather wide [15]. In the study by Erik, et al. where benign and malignant lesions of 36 patients were evaluated with MDCT, the mean gastric wall thickness was 1.5 cm. The range of gastric wall thickness of these 36 patients was between 0.7 cm and 7.5 cm. In patients with malignant lesions (n = 8) among the patients that were examined, the sensitivity of MDCT was 100% but specificity was 42% in those in whom wall thickness was over 1 cm [16].
In the study by Tongdee, et al. on gastric wall thickness on 154 patients, when cut-off value of gastric wall thickness was taken as 10 mm, the sensitivity, specificity, negative predictive value, positive predictive value and power of MDCT in detecting gastric cancer were 81.8%, 97.7%, 97%, 85.7% and 95.5%, respectively [17]. In our study, when the cut-off value was taken as 9.5 mm according to ROC analysis, sensitivity, specificity, negative predictive value, positive predictive value and power of MDCT in detecting gastric cancer were 95.3%, 81.8%, 5%, 89% and 87%, respectively. When our study is compared with studies by Erik, et al. and Tongdee, et al. we found a correlation between sensitivity and positive predictive values, but that specificity and negative predictive values were different. The main reason of this difference is presence of relatively fewer patients with malignant tumors in comparison to our study in the other studies among all patients that were examined. Another important factor for the prognosis of patients is N staging. Lymph nodes may enlarge due to reactive hyperplasia, inflammatory processes or tumoral spread, and lymph nodes having a normal size may contain micrometastasis. Thus, discrimination of enlargement of lymph nodes may not always be possible with CT in lymph nodes with micrometastasis.
In 1253 lymph nodes of 31 patients who had undergone surgical intervention and examined by Mönig, et al. the mean dimensions of lymph nodes in which there was no metastasis was 4.1 mm, and the mean diameter of lymph nodes in which metastasis was found was 6 mm. In 80% of lymph nodes in which there was no metastasis, the diameter was smaller than 5 mm. In 55% of lymph nodes in which metastasis was found, the diameter was under 5 mm. For this reason, these investigators have concluded that measurement of lymph nodes was not indicated in detection of lymph node metastasis. In our study, perigastric lymph node metastasis was found in 22 of 28 patients (78%) who had undergone surgical intervention. In patients who had lymph node metastasis, the shortest diameter of the largest LAP measured by MSCT at the perigastric region was between 6 mm and 22 mm. In MDCT of 6 patients (22%) in whom lymph node metastasis was not found, the diameter of perigastric lymph nodes was between 6 mm and 10 mm. As it was found by Mönig, et al. we also concluded that dimensions of lymph nodes were not useful in the detection of metastasis [18].
Detection of preoperative liver and distant organ metastasis and peritoneal spread are important in determination of inoperability status of a patient. Screening with MDCT is useful in the detection of liver metastasis larger than a diameter of 5 mm. But MDCT is not reliable in the detection of solitary pulmonary or hepatic metastasis smaller than 5 mm [19]. Hepatic metastasis seems hypodense in the liver parenchyma. Contrast enhancement is observed in the lesion after IV injection of contrast material. Precision of new-generation CTs in the detection of metastasis larger than 1 cm is about 90% [12]. In peritoneal involvement, sometimes small nodular implants may not be seen in CT or peritoneal density increases due to inflammation may be mistaken as peritoneal involvement. In our study, we found distant organ or hepatic metastasis and peritoneal spread in 83 (63%) of the patients with malignant diagnosis and commented this in favor of inoperability. As we could not verify these findings with histopathological findings, sensitivity and specificity of MDCT for this could not be determined. Our study is limited in this respect. Another limitation of our study is the presence of fewer benign lesions in comparison to malignant lesions.
While the sensitivity of MDCT in detection of gastric cancer is high, it is not equally successful in detecting lymph node metastasis. It may provide guidance in the treatment of patients due to its sensitivity in detecting distant organ metastasis. The role of MDCT in clinical practice is increasing every day, with its high detection rates in gastric cancers.
References
- Parkin DM (2004) International variation. Oncogene 23: 6329-6340.
- Coleman MP, Estève J, Damiecki P, et al. (1993) Trends in cancer incidence and mortality. IARC Sci Pub 1-806.
- Roukos DH (2000) Current status and future perspectives in gastric cancer management. Cancer Treat Rev 26: 243-255.
- Kim JP (1999) Surgical results in gastric cancer. Semin Surg Oncol 17: 132-138.
- Green D, Ponce de Leon S, Leon-Rodriguez E, et al. (2002) Adenocarcinoma of the stomach: Univariate and multivariate analysis of factors associated with survival. Am J Oncol 25: 84-89.
- Ott K, Sendler A, Becker K, et al. (2003) Neoadjuvant chemotherapy with cisplatin,12-FU, and leucovorin (PLF) in locally advanced gastric cancer: A prospective phase II study. Gastric Cancer 6: 159-167.
- Bando H, Yamada Y, Tanabe S, et al. (2016) Efficacy and safety of S-1 and oxaliplatin combination therapy in elderly patients with advanced gastric cancer. Gastric Cancer 19: 919-926.
- Yoshikawa T, Sasako M, Yamamoto S, et al. (2009) Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg 96: 1015-1022.
- Levine MS (1995) Role of the double-contrast upper gastrointestinal series in the 1990s. Gastroenterol Clin North Am 24: 289-308.
- Kwee RM, Kwee TC (2007) Imaging in local staging of gastric cancer: A systematic review. J Clin Oncol 25: 2107-2116.
- Sohn KM, Lee JM, Lee SY, et al. (2000) Comparing MR imaging and CT in the staging of gastric carcinoma. AJR Am J Roentgenol 174: 1551-1557.
- Kleinhaus U, Militianu D (1998) Computed tomography in te preoperative evaluation of gastric carcinoma. Gastrointest Radiol 13: 97-101.
- Chen CY, Hsu JS, Wu DC, et al. (2007) Gastric cancer: Preoperative local staging with 3D multi-detector row CT--correlation with surgical and histopathologic results. Radiology 242: 472-482.
- Lee JH, Jeong YK, Kim DH, et al. (2000) Two-phase helical CT for detection of early gastric carcinoma: Importance of the mucosal phase for analysis of the abnormal mucosal layer. J Comput Assist Tomogr 24: 777-782.
- Pickhardt PJ, Asher DB (2003) Wall thickening of the gastric antrum as a normal finding multidedector CT with cadaveric comparison. Am J Roentgenol 181: 973-979.
- Insko EK, Levine MS, Birnbaum BA, et al. (2003) Benign and malignant lesions of the stomach: Evaluation of CT criteria for differentiation. Radiology 228: 166-171.
- Tongdee R , Kongkaw L, Tongdee T (2012) A study of wall thickness of gastric antrum: Comparison among normal , benign and malignant gastric condition MDCT scan. J Med Assoc Thai 95: 1441-1448.
- Mönig SP, Zirbes TK, Schröder W, et al. (1999) Staging of gastric cancer: Correlation of lymph node size and metastatic infiltration. AJR Am J Roentgenol 173: 365-367.
- Layke JC, Lopez PP (2004) Gastric cancer: Diagnosis and treatment. Am Fam Physician 69: 1133-1140.
Corresponding Author
Cihad Hamidi, Department of Radiology, Medical School, Dicle University, Sur 21280, Diyarbakır, Turkey.
Copyright
© 2019 Hamidi C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.