5,10-Dihydro-7,8-Dimethyl Alloxazine as an Anticancer Agent from Lumichrome of Riboflavin
Abstract
Scientists are continuing their relentless endeavor to find a remedy for cancer or therapeutic agents which will minimize the growing burden of this seemingly incurable disease to a great extent. From literature review, we got the insight that a photoproduct of riboflavin named lumichrome has anticancer effect against lung cancer and this demands working on this compound and evaluation of anticancer activity. The present study represents the potential anticancer property of lumichrome and its synthesized derivative 5,10-dihydro-7,8-dimethyl alloxazine against liver, breast and colorectal cancer. Cytotoxic activity was investigated by in vitro sulforhodamine B (SRB) assay against HepG2 (Hepatocellular carcinoma), MCF-7 (Breast Adenocarcinoma), Colo-205 (Colorectal cancer) cell lines. IC50 values were between 7.7 to 23.9 μg/ml. HepG2 cell line was most sensitive to lumichrome as well as its synthesized derivative with IC50 values of 7.7 and 17.2 μg/ml respectively. The mechanisms of actions of these compounds are thought to involve induction of apoptosis and interference with the transcription process. Future studies will be required to confirm their mechanism of actions (Graphical Abstract).
Keywords
Anticancer, Lumichrome, 5,10-dihydro-7,8-dimethyl alloxazine, Breast cancer, Liver cancer, Colorectal cancer
Introduction
Cancer is still considered as one of the most dreaded diseases in the world. In 2020, an estimated 19.3 million new cancer patients were reported by the International Agency for Research on Cancer (IARC). It is predicted that this number of cancer patients will rise up to 28.4 million in the next two decades, mostly from developing countries [1]. Breast cancer, liver cancer and colorectal cancer are some commonly occurring cancers. Of almost 9.9 million cancer deaths, lung cancer was the leading cause of demise followed by colorectal and liver cancer [1]. But the number of females diagnosed with breast cancer (2.3 million) has exceeded the number of lung cancer patients, unprecedentedly [2]. This increased mortality, growing resistance of cancer cells to anticancer drugs and intolerable side effects of most of the agents have always compelled the scientists to give attention and relentless endeavor to find new chemotherapeutic agents with better safety and efficacy.
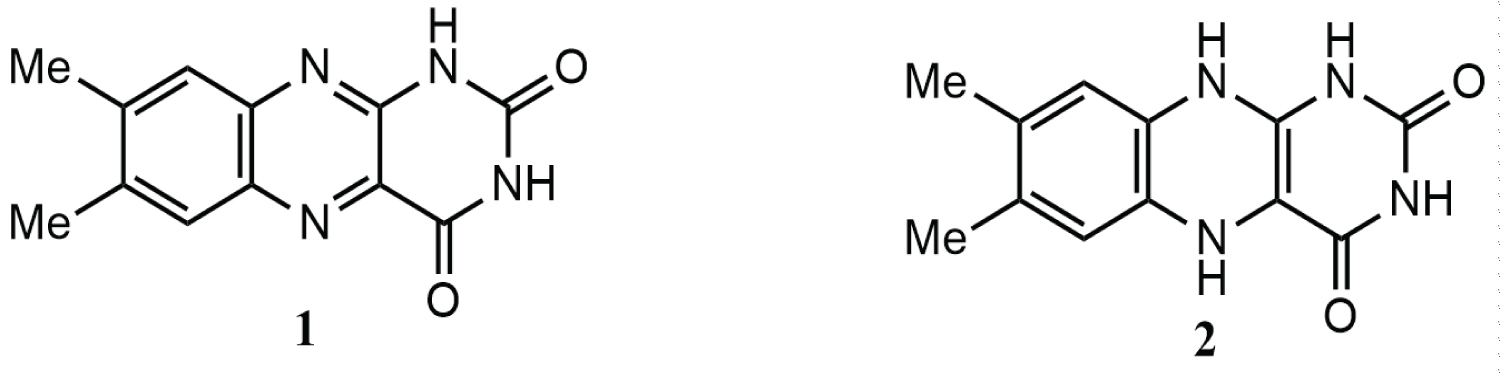
Lumichrome 1(7,8-dimethyl alloxazine) (Figure 1), one of the major derivatives of riboflavin, is produced by irradiation of riboflavin in neutral or weakly acidic solutions [3]. The structure of lumichrome is thought provoking as 5,10-dihydro-7,8-dimethyl alloxazine 2 (Figure 1) can be designed from it. The lumichrome 1, on reduction with sodium borohydride in THF gives the compound 2 [4]. The structure of 5,10-dihydro-7,8-dimethyl alloxazine 2 seems to be interesting. The ring at the right side of its structure is exactly similar to uracil, the pyrimidine base present in RNA (Figure 1). Due to this similarity, the Molecular docking study suggested that 5,10-dihydro-7,8-dimethyl alloxazine 2 might be incorporated in place of uracil in RNA during transcription forming non-functional analog of RNA which, in turn, might cause the inhibition of protein synthesis and eventually cell growth [5].
Lumichrome 1 is also reported for its effect against lung cancer [6]. To compare the anticancer activity of 5,10-dihydro-7,8-dimethyl alloxazine 2 with that of lumichrome 1, its anticancer activity was also investigated further. In this article both the compounds’ activities against three different cancer cell lines: HepG2 (Hepatocellular carcinoma), MCF-7 (Breast Adenocarcinoma), Colo-205 (Colorectal cancer) are reported.
Materials and Methods
Chemistry
Lumichrome was purchased from a commercial source named Sigma Aldrich. The compound, 5,10-dihydro-7,8-dimethyl alloxazine 2 (Figure 1) was prepared from lumichrome (1) by its reduction with sodium borohydride in THF [4]. Doxorubicin for cytotoxicity test, was purchased from Beacon Pharmaceuticals Ltd., Dhaka, Bangladesh.
Cell culture
There are HepG2 (Hepatocellular carcinoma), MCF-7 (Breast Adenocarcinoma), and Colo-205 (Colorectal cancer) cell lines in Nawah Scientific Inc., (Mokattam, Cairo, Egypt). Culture cells were preserved there in RPMI media supplemented with 100 mg/mL of streptomycin, 100 units/mL of penicillin and 10% of heat-inactivated fetal bovine serum in humidified, 5% (v/v) CO2 atmosphere at 37 °C.
Cytotoxicity test
The sulforhodamine B (SRB) assay is now more commonly used than the formazan-based assays because of its more simplicity, sensitivity and resolution [7,8]. The assay is based on the interaction of the anionic pink aminoxanthine dye sulforhodamine B (SRB) with basic amino acids of the viable cells. The amount of dye taken up depends on the number of cells and more intense color as well as greater absorbance is produced when the cells are lysed after fixation [9]. Therefore cytotoxic effects of the test compounds on HepG2 (Hepatocellular carcinoma), MCF-7 (Breast Adenocarcinoma), Colo-205 (Colorectal cancer) cell lines were determined using the SRB assay.
1. 100 μL cell suspension (5 × 10^3 cells) of each type of culture (HepG2, MCF-7, Colo-205) was taken in 96-well plates and incubated for 24 h in complete media.
2. Another aliquot of 100 μL media containing the test compound at various concentrations ranging from 0.01 μg/ml to 100 μg/ml were added in cells.
3. Fixation of cells was carried out by changing media with 150 μL of 10% TCA solution after 72h of exposure of the test compound and incubated for 1 h at 4 °C.
4. Cells were then washed 5 times with distilled water to remove TCA solution and incubated for 10 min. in a dark place by adding 70 μL SRB solution (0.4% w/v) at room temperature.
5. Plates were set to air-dry overnight after 3 times washing with 1% acetic acid and finally, protein-bound SRB stain was dissolved by adding 150 μL of TRIS (10 mM).
6. BMG LABTECH®- FLUOstar Omega microplate reader (Ortenberg, Germany) was utilized to measure the absorbance at 540 nm.
All experiments were carried out in triplicate independently. Data were presented as mean± SEM. Sigma Plot software, version 12.0 (System Software, San Jose, CA, USA) for statistical analysis and an E-max model equation were used to determine IC50 values from concentration-response curves:
Where "D" is the concentration of test compound used, "F" is the residual unaffected fraction (the resistance fraction), "Kd" is the concentration of the test compound that causes a 50% reduction of the maximum inhibition rate and "m" is a Hill-type coefficient. IC50 was defined as the concentration of compound required to reduce absorbance to 50% of that of the control [10].
Results and Discussion
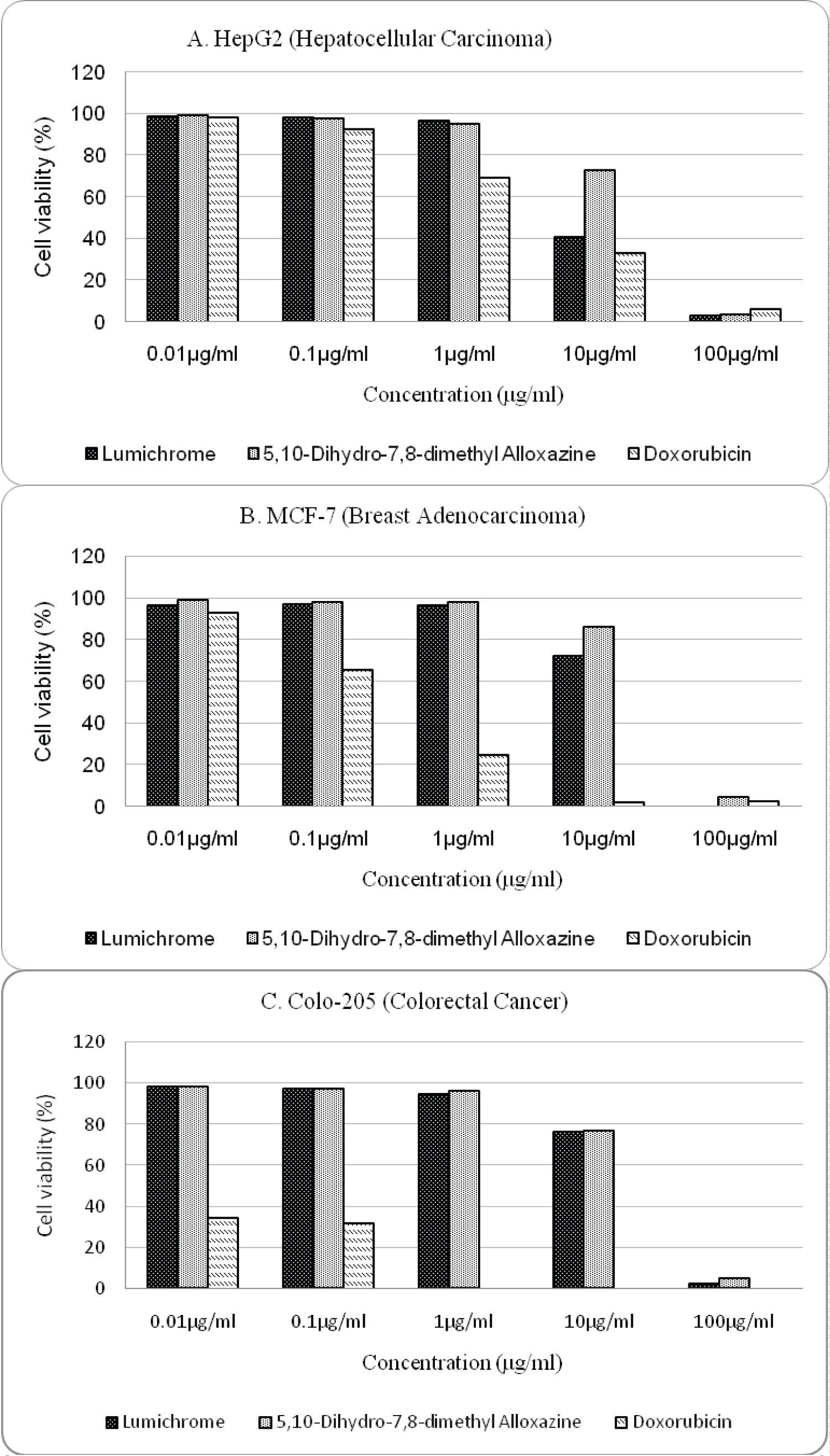
Through the SRB Assay, cytotoxic effects of the test compounds against HepG2, MCF-7, Colo-205 cancer cell lines were determined while taking doxorubicin as a standard. It is clear that both lumichrome and 5,10-dihydro-7,8-dimethyl alloxazine have cytotoxic effects against all three cell lines. At 100 μg/ml, test compounds significantly reduced the percentages of the cell viability, accounting for less than 6% at every cell line. Compared to MCF-7 and Colo-205 cell lines, the viability of HepG2 cell lines decreased gradually with the increase of concentration of lumichrome and 5,10-dihydro-7,8-dimethyl alloxazine (Figure 2A). However lumichrome had better cytotoxic profile than 5,10-dihydro-7,8-dimethyl alloxazine and lowered the viability of HepG2, MCF-7 and Colo-205 cell lines more in accordance with the rise of concentration (Figure 2B and Figure 2C).
The cell inhibition (IC50) results are shown in Table 1. IC50 values determined from cytotoxic profile of lumichrome against HepG2, MCF-7, Colo-205 cancer cell lines were 7.7 μg/ml, 15.3 μg/ml, 17.6 μg/ml and it was 17.2 μg/ml, 23.9 μg/ml and 19.7 μg/ml in case of 5,10-dihydro-7,8-dimethyl alloxazine respectively. Although IC50 values of test compounds against HepG2 cell lines were close to the value of standard doxorubicin but against MCF-7 and Colo-205 values were more than 100 times higher than standard.
From the cytotoxic property profile (Figure 2) of lumichrome and 5,10-dihydro-7,8-dimethyl alloxazine against HepG2, MCF-7, Colo-205 cancer cell lines it was observed that at highest concentration the cytotoxic effects of both lumichrome and 5,10-dihydro-7,8-dimethyl alloxazine were comparable to doxorubicin. After 10 μg/ml, the inhibitory effect of lumichrome against all three cancer cell lines rapidly increased (Figure 2). The most potent cytotoxic activity was expressed by lumichrome against liver cancer (HepG2 cell line) with the IC50 value of 7.7 μg/ml compared to standard (IC50 = 3.4 μg/ml). On the other hand, 5,10-dihydro-7,8-dimethyl alloxazine also had its most cytotoxic activity against liver cancer with the IC50 value of 17.2 μg/ml. Therefore, as expected 5,10-dihydro-7,8-dimethyl alloxazine possessed anticancer potential but lower than its precursor lumichrome. It has already been speculated that lumichrome provides its cytotoxic effect against lung cancer through induction of apoptosis by activation of tumor suppressor p53 protein as well as attenuation of cancer stem cells properties by suppression of protein kinase B (AKT) function and β-catenin level [6]. On the other hand, 5,10-dihydro-7,8-dimethyl alloxazine (2) may give its anticancer effect through formation of a non-functional analog of RNA during transcriptionand targeting multiple oncogenic proteins [5]. Further wet lab testing is required to understand their mechanism of actions.
Conclusion
Novel treatments are needed to improve survival in cancer patients as the burden of cancer worldwide is no way declining. Although most promising result was given by lumichrome against hepatocellular carcinoma, this study also provides evidence that both the compounds lumichrome and 5,10-dihydro-7,8-dimethyl alloxazine have significant cytotoxic effects against three major cancers: breast, liver and colorectal. So, further research on lumichrome and 5,10-dihydro-7,8-dimethyl alloxazine, for example, synthesis of more analogs and investigation of their anticancer activity might be undertaken to obtain doxorubicin-like more potent anticancer compounds.
Conflict of Interest
The author(s) declare that this article has no any type of conflict of interest.
Acknowledgement
The anticancer activity tests were performed in Nawah Scientific Inc., Mokattam, Cairo, Egypt.
References
- Sung H, Ferlay J, Siegel RL, et al. (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA: A Cancer J Clin 71: 209-249.
- GLOBOCAN (2020) New Global Cancer Data.
- Kino K, Kobayashi T, Arima E, et al. (2009) Photoirradiation products of flavin derivatives, and the effects of photooxidation on guanine. Bioorg Med Chem Lett 19: 2070-2074.
- Qais N, Etu SF (2020) Antioxidant activity of lumichrome and its reduced form, 5,10-dihydro- 7,8-dimethyl Alloxazine. IOSR J Pharm Biol Sci 15: 50-53.
- Etu SF, Hossain MA, Rouf ASS, et al. (2021) Molecular docking and anticancer activitydetermination of 5,10-dihydro-7,8-dimethyl alloxazine derived from lumichrome of riboflavin. Main Group Chem 20: 81-88.
- Chantarawong W, Kuncharoen N, Tanasupawat S, et al. (2019) Lumichrome Inhibits human lung cancer cell growth and Induces apoptosis via a p53- dependent mechanism. Nutr Cancer 71: 1390-1402.
- Fricker SP, Buckley RG (1996) Comparison of two colorimetric assays as cytotoxicity end points for an in vitro screen for antitumor agents. Anticancer Res 16: 3755-3760.
- Keepers YP, Pizao PE, Peters GJ, et al. (1991) Comparison of the sulforhodamine B protein and tetrazolium (MTT) assays for in vitro chemosensitivity testing. Eur J Cancer 27: 897-900.
- Skehan P, Storeng R, Scudiero D, et al. (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82: 1107-1112.
- Allam RM, Al-Abd AM, Khedr A, et al. (2018) Fingolimod interrupts the cross talk between estrogen metabolism and sphingolipid metabolism within prostate cancer cells. Toxicol Lett 291: 77-85.
Corresponding Author
Nazmul Qais, Department of Clinical Pharmacy and Pharmacology, Faculty of Pharmacy, University of Dhaka, Dhaka, Bangladesh, Tel: +880-1714417750
Copyright
© 2021 Etu SF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.