Frontal EEG Asymmetry Differs between High and Low Trait Anxious Individuals as a Function of Lateralized Vibrotactile Stress
Abstract
Anxiety is an aversive emotional state marked by hyperarousal and cognitive impairments. Studies using Quantitative Electroencephalography (QEEG) have shown asymmetric right frontal and parietotemporal activity at resting baseline in trait anxious subjects. However, there has been a lack of research investigating changes in QEEG asymmetries in trait anxious subjects following stress. The current study aimed to assess changes in QEEG asymmetries following lateralized vibrotactile and affective stress in trait anxious subjects. Following a two-minute baseline, subjects underwent two 2-minute stress periods, during which they were presented with auditory and counterbalanced unilateral vibrotactile stressors, each followed by a two-minute recording period. Subjects were classified as high or low trait anxious based on their scores on the Spielberger Trait Anxiety Inventory. A repeated measures ANOVA revealed significant asymmetry score differences between the high and low anxious groups in the F3/F4 (F(1,32) = 5.30, p < 0.03), and F7/F8 (F(1,32) = 4.20, p < 0.05) electrode pairs as a function of lateralized vibrotactile stress. No significant differences were found between groups in the Fp1/Fp2, C3/C4, P3/P4, and P7/P8 electrode pairs or between trials in any of the electrode pairs for either group. These results replicate previous findings demonstrating differences in EEG frontal asymmetries between subjects scoring high or low in trait anxiety with but do not support the use of changes in asymmetry scores as a marker of acute stress. More work needs to be done regarding EEG frontal asymmetry and acute stress.
Keywords
Trait anxiety, Anxiety, Electroencephalogram, EEG, Brain asymmetry, Stress, Emotion, Brain laterality, Neuropsychology
Introduction
Anxiety is a debilitating condition marked by chronic hyperarousal, a preponderance of negatively-valenced emotions, and cognitive impairments [1]. For the purposes of this paper, anxiety was defined as a prolonged state of fear induced by the anticipation of an unpredictable contextual threat [2]. While typically associated with a negative connotation, anxiety is an adaptive mechanism [3] that increases an individual's ability to detect and respond to threats in the environment. However, when anxiety is invoked too frequently or in response to innocuous stimuli, it becomes maladaptive.
Previous studies using quantitative EEG have found right frontal activity to be associated with negative, withdrawal states while left frontal activation is associated with positive, approach oriented states [4,5]. With regard to anxiety, Keller, et al. proposed a model in which asymmetric right frontal activation is associated with the negatively-valenced emotional component of anxiety [6], while asymmetric right parietotemporal activation is associated with the arousal component of anxiety. However, while some studies have been supportive of right sided parietotemporal EEG asymmetry as an indicator of anxious arousal [6-8], there have been a number of studies that have shown otherwise [7,9,10]. In contrast, converging evidence has been provided in support of EEG frontal asymmetry differences in anxiety [4].
It has been suggested that frontal asymmetry is sensitive to changes in emotional state. Davidson, et al. found right frontal EEG asymmetry increased in individuals with social phobia during anticipation of an impromptu speech [7]. In a separate study, a previous or current diagnosis of Major Depressive Disorder was found to correlate with decreased left frontal activity during resting baseline and when the patients were told to make approach and withdrawal facial expressions [10]. Additionally, the correlation was stronger during the emotional challenge condition than for resting baseline suggesting stress induced changes in EEG frontal asymmetry. Right frontal EEG activation has also been found using a within-subjects double-blind design, following acute cortisol administration in healthy male subjects [9], providing evidence of asymmetry in the frontal regulatory control over sympathetic drive underlying emotional states [11].
In addition to studies finding a shift toward right frontal asymmetry following stress, there have been studies [12] finding a shift toward left frontal asymmetry following application of Cognitive Behavioral Therapy (CBT). Moscovitch, et al. found a shift from right frontal EEG asymmetry to left frontal EEG asymmetry following completion of a 12-week CBT program [12]. Therefore a shift toward left frontal asymmetry may be a desirable outcome for symptom improvement in affective disorders. Meditation has also been shown to reduce stress level and to alter frontal theta activity in subjects scoring high and low in trait anxiety [13], whereas these authors did not assess for cerebral laterality effects.
We have found differences in frontal asymmetry mechanisms in the regulation of emotion across multiple projects with addition evidence provided from the laboratory that these potentially dynamic states reflect the "capacity" for regulatory control under stress and that the regulatory control is modifiable [14,15]. The current study aimed to replicate previous findings linking right frontal EEG asymmetry to anxious populations and to assess state changes in EEG frontal asymmetry following lateralized vibrotactile stress and meditation. A dual concurrent processing demand approach was used to induce a state of anxious arousal. Specifically, angry infant vocalizations (an aversive auditory stressor) and left hand vibrotactile stimulation, which both asymmetrically depend on right hemisphere cerebral systems, were utilized as stressors in a similar manner to Holland, et al. and Foster, et al. [16,17].
We hypothesized that subjects scoring high in trait anxiety would have less right frontal relative to left frontal and parietotemporal alpha power, an inverse marker of activation [18], following presentation of the stressors compared to subjects scoring low in trait anxiety at resting baseline. We also hypothesized that both groups would experience a shift in EEG asymmetry toward right hemisphere activation following the stressors with the high anxious group showing a greater shift and that both groups would experience a shift toward left hemisphere activation during meditation.
Materials and Methods
Thirty-four participants (20 females and 14 males) were recruited from the undergraduate subject pool at Virginia Polytechnic Institute. Participants were right handed with no existing medical conditions or current use of any anti anxiety, antidepressant, or antipsychotic medications. The trait section of the Spielberger State-Trait Anxiety Inventory was used to separate participants into high and low groups [19]. A Hitachi Magic Wand vibrator with a frequency setting of 60 Hz was used as the vibrotactile stimulus [17]. A recording of a baby crying sound effect extracted from YouTube was used as the auditory stressor [16]. The same two minutes of the recording were used for both stress sessions. The recordings were presented using over-the-ear headphones.
EEG was recorded and analyzed using the James Long Company EEG analysis system and routine acquisition and sampling procedures [20,21]. Data were sampled at 256 Hz. Data were re-referenced offline from the central vertex electrode to linked earlobes. A band pass filter with frequency cutoffs of 0.1 Hz and 100 Hz was used prior to data analyses. Epochs containing sharp rises and falls exceeding 75 micro volts (indicative of eye blinks) in the Fp1 and Fp2 electrodes were automatically rejected. Proper rejection of artifacts and retention of artifact free data were confirmed visually. Spectral power in the alpha bandwidth (7.5-12.5 Hz) was calculated using a Discrete Fourier Transformation with overlapping Hamming windows of 1.5 seconds in length.
Upon arriving at the laboratory and completion of a written informed consent form, participants completed the Spielberger Trait Anxiety Inventory. The EEG cap was then prepared for recording. A 24 electrode Electro-cap International EEG cap following the International 10-20 electrode placement system was used for recording. Electrode sites were prepared by lightly abrading the scalp with a wooden cue tip and injecting conductive saline solution into each electrode. Impedance levels were kept below 5 Kohms at all electrode sites. Following preparation of the cap, subjects underwent a 2-minute baseline EEG recording.
Subjects then completed a 2-minute period under four Conditions during which the auditory and vibrotactile stressors were presented concurrently. Immediately following presentation of the stressors, another two-minute EEG recording was taken. This process was repeated a second time with the vibrotactile stress at the other hand (counterbalanced order). Participants then completed a 5-minute period of meditation while EEG was recorded. Meditation was used to promote a relaxed state or baseline for comparative purposes. These procedures included having the subjects close their eyes for each recording in order to minimize ocular artifact.
Following scoring of the STAI, participants were categorized as high or low trait anxious using a median split which occurred at a score of 34 (17 high anxious participants and 17 low anxious participants). A repeated measures ANOVA was performed to assess the differences between asymmetry scores (Ln(Rα)-Ln(Lα)) at the Fp1/Fp2, F3/F4, F7/F8, C3/C4, P3/P4, and P7/P8 electrode pairs between groups across each condition. Positive asymmetry scores were indicative of greater left than right hemisphere activity and negative asymmetry scores were indicative of greater right than left hemisphere activity [4].
Results
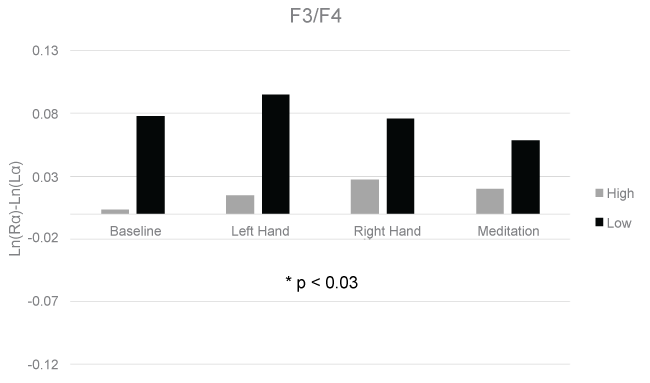
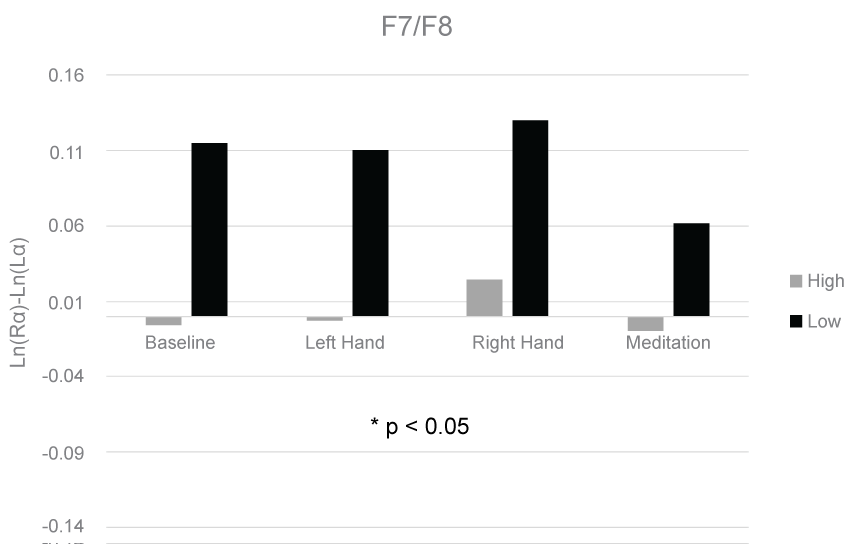
The means and standard deviations for each condition at each electrode pair are shown in Table 1. A 3-way GLM ANOVA with fixed measures of group (high/low) and sex (male/female) and a repeated measure of Condition (lateralized vibrotactile stress) revealed a significant difference in asymmetry scores between the high and low anxious groups in the F3/F4 (F(1,32) = 5.30, p < 0.03), and F7/F8 (F(1,32) = 4.20, p < 0.05) electrode pairs. The group effects for the F3/F4 and F7/F8 electrode pairs are displayed in Figure 1 and Figure 2, respectively. No significant differences were found between groups in the Fp1/Fp2, C3/C4, P3/P4, and P7/P8 electrode pairs. No significant differences were found between trials or sex in any of the electrode pairs.
Discussion
Our results support previous findings that frontal EEG asymmetry differs significantly between anxious populations and healthy controls at resting baseline [4]. However, our results do not support previous findings of differences in EEG parietotemporal asymmetries between anxious populations and healthy controls [1,5-8,11]. Additionally, our results do not support previous studies finding changes in EEG frontal asymmetry following stress [7,9,10].
Our null findings, with regard to state changes in frontal asymmetry following stress, may have been due to methodological limitations and especially the use of a median-split for anxiety scores. Recordings were taken immediately following each stress condition, rather than during them, due to concerns over mechanical artifact produced by the vibrator. Therefore, we may have been recording a post-stress calming down phase rather than a stress phase. Additionally, the stressors may not have been unpleasant enough. Regardless, left handed vibrotactile stimulation and recorded sounds of a baby crying were found to differentially affect High and Low Anxious Groups using asymmetry scores. However, it is reasonable that the stressors were not used in a way that properly modeled anxiety. There was no contextual element of unpredictability in the presentation of the stressors [2]. Another potential confound could have occurred due to the fact that our participants had their eyes closed for the entire recording period. Given that alpha power is increased globally when the eyes are closed, it is possible that this may have reduced the difference in alpha power between electrode sites [22].
It is important to note that while a significant group effect was found in two frontal electrode pair comparisons, this effect was not driven by right frontal asymmetry in the high anxiety group. Rather it was driven by left frontal asymmetry in the low anxious group. This could reflect an adaptive mechanism present in the low anxious group that is absent from the high anxious group. The low anxious group may have held more positive attributions toward the research environment while the high anxious group may have been more tentative. Perhaps a paradigm shift should occur regarding differences between anxious and non-anxious populations. While previous research has focused on positive symptoms of anxiety, such as increased sympathetic activation and cognitive impairments [6,2,23], less research has highlighted adaptive mechanisms that non-anxious individuals may utilize that anxious individuals may lack. Integrating this perspective with the dominant perspective regarding the maladaptive symptoms of anxiety may prove useful in increasing the understanding of anxiety disorders and help improve current treatments of those disorders.
References
- Harrison DW (2015) Positive and negative emotion. In: Brain Asymmetry and Neural Systems: Foundations in Clinical Neuroscience and Neuropsychology. Springer International Publishing, Switzerland.
- Grillon C (2002) Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biol Psychiatry 52: 958-975.
- Spielberger CD (2010) State‐Trait anxiety inventory. John Wiley & Sons Inc, NJ, USA.
- Coan JA, Allen JJ (2004) Frontal EEG asymmetry as a moderator and mediator of emotion. Biol Psychol 67: 7-49.
- Harrison DW (2015) Social Approach and Social Avoidance. In: Brain Asymmetry and Neural Systems Springer International Publishing, 383-387.
- Keller J, Nitschke JB, Bhargava T, et al. (2000) Neuropsychological differentiation of depression and anxiety. J Abnorm Psychol 109: 3-10.
- Davidson RJ, Marshall JR, Tomarken AJ, et al. (2000) While a phobic waits: regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biol Psychiatry 47: 85-95.
- Metzger LJ, Paige SR, Carson MA, et al. (2004) PTSD arousal and depression symptoms associated with increased right-sided parietal EEG asymmetry. J Abnorm Psychol 113: 324-329.
- Tops M, Wijers AA, van Staveren AS, et al. (2005) Acute cortisol administration modulates EEG alpha asymmetry in volunteers: relevance to depression. Biol Psychol 69: 181-193.
- Stewart JL, Coan JA, Towers DN, et al. (2014) Resting and task‐elicited prefrontal EEG alpha asymmetry in depression: support for the capability model. Psychophysiology 51: 446-455.
- Harrison DW (2015) Parasympathetic and Sympathetic Tone. In: Brain Asymmetry and Neural Systems Springer International Publishing, 441-453.
- Moscovitch DA, Santesso DL, Miskovic V, et al. (2011) Frontal EEG asymmetry and symptom response to cognitive behavioral therapy in patients with social anxiety disorder. Biol Psychol 87: 379-385.
- Murata T, Takahashi T, Hamada T, et al. (2004) Individual trait anxiety levels characterizing the properties of Zen meditation. Neuropsychobiology 50: 189-194.
- Carmona JE, Holland AK, Harrison DW, et al. (2009) Extending the functional cerebral systems theory of emotion to the vestibular modality: a systematic and integrative approach. Psychol Bull 135: 286-302.
- Comer CS, Harrison PK, Harrison DW, et al. (2015) The dynamic opponent relativity model: an integration and extension of capacity theory and existing theoretical perspectives on the neuropsychology of arousal and emotion. SpringerPlus 4: 345.
- Holland AK, Newton SE, Hinson DW, et al. (2014) Physiological and behavioral indices of hostility: an extension of the capacity model to include exposure to affective stress and right lateralized motor stress. Laterality 19: 560-584.
- Foster PS, Hubbard T, Yung RC, et al. (2013) Cerebral asymmetry in the control of cardiovascular functioning: evidence from lateral vibrotactile stimulation. Laterality 18: 108-119.
- Bazanova OM, Vernon D (2014) Interpreting EEG alpha activity. Neurosci Biobehav Rev 44: 94-110.
- Spielberger CD (1983) Manual for the State-Trait Anxiety Inventory STAI (form Y) (" self-evaluation questionnaire").
- Foster PS, Williamson JB, Harrison DW, et al. (2005) The Ruff Figural Fluency Test: heightened right frontal lobe delta activity as a function of performance. Arch Clin Neuropsychol 20: 427-434.
- Foster PS, Harrison DW (2004) The covariation of cortical electrical activity and cardiovascular responding. Int J Psychophysiol 52: 239-255.
- Barry RJ, Clarke AR, Johnstone SJ, et al. (2007) EEG differences between eyes-closed and eyes-open resting conditions. Clin Neurophysiol 118: 2765-2773.
- Mathersul D, Williams LM, Hopkinson PJ, et al. (2008) Investigating models of affect: relationships among EEG alpha asymmetry, depression, and anxiety. Emotion 8: 560-572.
Corresponding Author
David W Harrison, Director, Behavioral Neuroscience Laboratory, Department of Psychology, College of Science, Virginia Polytechnic Institute, Blacksburg, Virginia 24061-0436, USA, Tel: +540-231-4422.
Copyright
© 2017 Woisard K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.