RBT-1, A Pro-Oxidant Preconditioning Agent, Mitigates Syndecan-1 Shedding in Patients Undergoing Cardiac Surgery and Following Experimental AKI
Abstract
Background: During systemic stress, e.g., sepsis or major surgeries, syndecan-1 (SDC-1) shedding into plasma results, imply endothelial glycocalyx damage. A recent phase 2 clinical trial indicated that RBT-1 induced ‘oxidant preconditioning’ mitigated post-operative complications following cardiac surgeries. The present study assessed whether RBT-1 preconditioning attenuated SDC-1 shedding in these patients, implying a vascular protective effect.
Methods: Patients (n, 112) were randomized to receive low-dose RBT-1, high-dose RBT-1, or placebo 24-48 hrs prior to surgery. Plasma samples were obtained before and 2 days post-surgery and assayed for SDC-1 (ELISA). The results were compared between the groups. To gain further insights, CD-1 mice were subjected to diverse forms of acute renal injuries (maleate, glycerol, endotoxemic AKI; acute heart failure). RBT-1’s impact on resulting plasma SDC-1 increases, vascular/aortic stress (NGAL/IL-6 gene induction), and the expression of two vascular cytoprotective pathways (Nrf2; ferritin) were assessed.
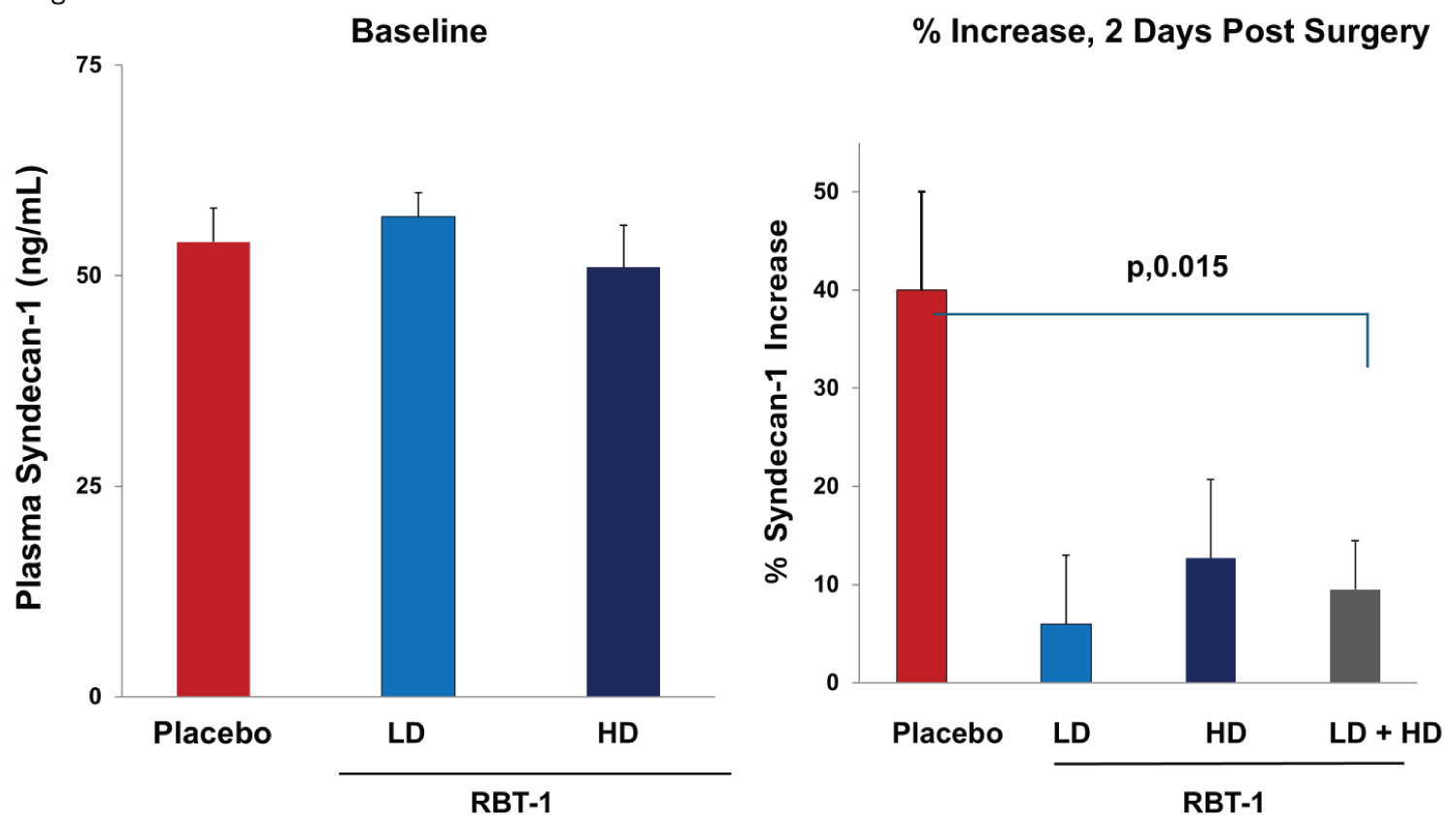
Results: Baseline plasma SDC-1 levels did not differ between the three patient groups. The placebo group developed a 40 ± 11% post-surgical plasma SDC-1 increase (p, 0.03). Conversely, no significant SDC-1 increases (< 10%) were observed in the RBT-1 treatment groups (< RBT-1 vs. placebos, p, 0.015). Each form of experimental injury markedly increased plasma SDC concentrations. This result was attenuated by RBT-1 preconditioning. RBT-1 also: i) Mitigated injury-induced aortic NGAL/IL-6 mRNA increases; ii) Activated aortic Nrf2; and iii) Increased ferritin expression in aorta and select renal vessels.
Conclusion: RBT-1 induced ‘oxidant preconditioning’ confers a vaso-protective effect, potentially by activating Nrf2 and up-regulating ferritin. Whether this can decrease post-cardiac surgery complications requires further study.
Keywords
Syndecan-1, Glycocalyx, Preconditioning, Nrf2, Acute kidney injury, Endotoxemia, Isoproterenol
Introduction
Transient bouts of tissue injury, e.g., as induced by ischemia, can trigger adaptive responses that can protect tissues against subsequent insults. This phenomenon, commonly referred to as ‘ischemic preconditioning’, was first demonstrated in kidney [1], and subsequently it has been documented in virtually every organ [2-4]. These observations have stimulated efforts to identify safe methods to mimic ‘ischemic preconditioning’ in humans. If a safe and effective method were identified, it could be used to protect patients who are about to undergo major cardiovascular surgeries or other ‘high risk’ medical interventions.
One approach towards achieving this goal has been ‘remote preconditioning’ (RP) in which repetitive bouts of limb ischemia, induced by inflating blood pressure cuffs, are performed shortly before major cardiovascular procedures [5]. However, inconclusive organ protection has been observed [6-10]. A major factor that potentially limits efficacy is the short time window between RP and the start of surgery. Hence, there is virtually no time prior to surgery to permit the production of cytoprotective anti-inflammatory and antioxidant proteins in target tissues.
We have sought an alternative strategy to safely induce a potent preconditioning response. To this end, RBT-1, a combination product composed of proprietary stannic protoporfin (SnPP) and iron sucrose (FeS) has been studied [11-16]. RBT-1 induces a transient pro-oxidant signal which frees Nrf2 from its inhibitory binding protein; KEAP-1 (e.g., ref. [14]). This permits Nrf2 translocation from the cytosol to the nucleus where it activates gene transcription [12-14]. Within 12-24 hrs of RBT-1 infusion, a host of Nrf2-dependent antioxidant and anti-inflammatory proteins are up-regulated in multiple organs (kidney, liver, heart, lung; ref. [11-16]; unpublished data). In addition, the FeS component of RBT-1 markedly increases tissue heavy chain (antioxidant) ferritin translation. As a result of these combined actions, synergistic protection against multiple experimental models of acute tissue damage has been observed [e.g., renal ischemia; nephrotoxin injuries; hepatic ischemia [11-16]; oleate-induced acute lung damage (unpublished data)].
An unresolved question is whether the intravenous infusion of RBT-1 might also up-regulate cytoprotective pathways directly within the vasculature, and if so, would vascular protection result? The endothelial glycocalyx, and its constituent protein, syndecan-1 (SDC-1), line the luminal side of blood vessels and modulate several key vascular functions, including the control of vascular permeability, inflammation, thrombosis, and cytokine signalling [17-20]. During critical illnesses, trauma, and major surgeries (e.g., ‘on pump’ open heart procedures), vascular SDC-1 shedding into plasma results [21-31]. Hence, plasma SDC-1 has become a widely accepted biomarker of endothelial glycocalyx damage, as well as a predictor of subsequent adverse clinical outcomes [20,22,25-31].
Given these considerations, we questioned whether RBT-1 preconditioning might mitigate glycocalyx injury, and thus, blunt plasma SDC-1 increases following ‘on pump’ cardiac surgeries. To address this question, we measured plasma SDC-1 levels in patients who participated in a recent double-blind, placebo-controlled phase 2 study which was conducted in subjects who underwent ‘on pump’ cardiac surgery with and without RBT-1 preconditioning [32]. In complementary mouse studies, we tested whether RBT-1 preconditioning might mitigate SDC-1 release following endotoxemia or nephrotoxic tissue damage. We also assessed whether RBT-1 would activate Nrf2 and up-regulate ferritin expression directly within vascular (aortic) tissues, potentially conferring cytoprotective effects. These combined clinical and experimental studies form the basis of this report.
Methods
Plasma SDC-1 levels before and after cardiac surgery: Impact of RBT-1 preconditioning
A recent IRB approved multicenter, randomized, double-blind, placebo-controlled phase 2 clinical trial (NCT04564833; START) evaluated whether RBT-1 preconditioning could decrease complications following ‘on pump’ cardiac surgery [32]. In that study, 135 patients were randomized (1:1:1) to receive either placebo, low dose RBT-1 (45 mg SnPP/240 mg FeS), or high dose RBT-1 (90 mg SnPP/240 mg FeS) 24-48 hrs prior to surgery. The results of that study implied significant clinical benefits, based on a post hoc hierarchical analysis of multiple clinical endpoints [33,34].
To assess whether this previously noted protection was associated with diminished SDC-1 release, in the current study, paired plasma samples that had been collected before surgery (baseline) and 2 days after surgery were assayed for SDC-1 by ELISA (ab46506; Abcam, Waltham, MA). Absolute SDC-1 concentrations (ng/mL), and the % increases in plasma SDC-1 levels (from baseline to 2 days post-surgery) for each of the groups were assessed. Paired samples were available for assay from 39, 36, and 37 subjects in the low dose RBT-1, high dose RBT-1, and placebo groups, respectively.
Ethics
The above study received Institutional review board approval from Advarra Inc (Columbia MD), and/or from the enrolling clinical sites. Informed written consent was obtained from each patient before enrollment. The protocol was approved by the relevant health authorities and institutional review boards. The trial was registered on ClinicalTrials.gov (NCT04564833) prior to study enrollment [35].
Mouse studies
Male CD-1 mice (30-40 grams; Charles River, Wilmington, MA) were used for all described experiments. The mice were housed under standard vivarium conditions with free food and water access. Deep anaesthesia was induced with sodium barbiturate (40-50 mg/Kg, administered IP). All experiments were performed at Bloodwork’s NW, Seattle, WA, with IACUC approval in accordance with NIH guidelines [36].
Mouse plasma SDC-1 levels following five diverse injury models
To assess whether a broad spectrum of injuries can release SDC-1 into the circulation, mice were subjected to one of three different AKI models (maleate, glycerol, cisplatin nephrotoxicity); isoproterenol-induced cardiomyopathy/acute heart failure [37]; or acute endotoxemia (see below). Collected plasma samples were assayed for mouse SDC-1 by ELISA (ab273165; Abcam, Waltham, Boston). Additionally, blood urea nitrogen (BUN) concentrations were measured (DIUR-500; Bioassay Systems, San Francisco, CA) as an index of renal function.
1) Glycerol model of AKI: Four mice were lightly anesthetized with isoflurane and the glycerol model of rhabdomyolysis AKI was induced (8 mL/Kg 50% glycerol; administered in two equally divided IM injections into the hind limbs) [11]. Eighteen hrs later, the mice were deeply anesthetized, the abdominal cavities were opened, and terminal vena cava blood samples were obtained. Sacrifice was performed by aortic transaction. Four saline injected mice served as controls.
2) Cisplatin (CP) model of AKI: Five mice received an intraperitoneal injection of CP (15 mg/kg; Sigma; stock solution, 1 mg/ml saline). Three days later, blood samples were obtained from the abdominal vena cava and the mice were then sacrificed, as noted above. Blood samples from 4 normal mice served as controls.
3) Maleate: Four mice were injected with Na maleate, a proximal tubule specific nephrotoxin (600 mg/Kg IP in ~500 μL PBS; ref. [36]). Approximately 18 hrs post maleate injection, terminal blood samples were obtained from the abdominal vena cava, followed by sacrifice as noted above. The plasma SDC-1 values were compared to those observed in 4 mice which did not receive prior RBT-1 preconditioning.
4) Isoproterenol (Iso) model of cardiac Injury: Iso administration is widely used as an experimental model of acute heart failure, resulting from extreme tachycardia, hypotension, and myocyte apoptosis and/or necrosis [37]. Five mice received a subcutaneous injection of Iso (50 mg/Kg dissolved in PBS; from Sigma; St. Louis, MO). Eighteen hours post injection, terminal blood samples were obtained, followed by sacrifice. Blood samples from 4 normal mice served as controls.
5) E. coli endotoxin (LPS): Six mice were injected with E. coli endotoxin (2 mg/Kg; 0111:B4; L-2630; Sigma Chemicals, St. Louis, MO; 150 µL via tail vein). Eighteen hrs post LPS injection, terminal blood samples were obtained for SDC-1 assay, followed by sacrifice, as noted above. The results were compared to those observed in 4 normal controls.
RBT-1 Preconditioning Effect on Experimental Injury-Induced SDC-1 Release
The following experiments were undertaken to determine whether RBT-1 preconditioning can mitigate SDC-1 release following two of the above injury models: Maleate-induced AKI or endotoxemia (LPS). Ten mice were injected with RBT-1 (1 mg FeS + 1 μ mole SnPP; ref. [11]; from Reni bus Therapeutics, Southlake, TX). Approximately 18 hrs later, they received either maleate or endotoxin injection (n, 5 for each). Plasma samples were obtained from these mice and mice that had been injected with maleate or end toxin without prior RBT-1 preconditioning (n, 5 each). The impact of RBT-1 preconditioning on LPS- or maleate-induced plasma SDC-1 increases was assessed. To assess whether RBT-1 independently alters plasma SDC-1 concentrations, the latter was measured in 3 normal mice and 3 mice 18 hrs post RBT-1 administration in the absence of any additional interventions.
Assessment of whether RBT-1 preconditioning mitigates aortic injury during AKI
The following experiment was designed to seek further evidence that RBT-1 preconditioning can decrease vascular (aortic) injury. Fifteen mice were divided into three groups: i) Control mice; ii) Maleate injected mice; and iii) Maleate injection, administered 18 hrs after RBT-1 administrations (n, 5 per group). Approximately 18 hrs post maleate injection, the abdominal aorta was rapidly excised, iced, RNA was extracted and assayed for NGAL and IL-6 mRNAs by RT-PCR, as previously described [11,13]. The results were factored by simultaneously obtained GAPDH product.
Potential RBT-1 effects on Nrf2 pathway activation and vascular ferritin expression
Nrf2 assessments: Ten mice received a tail vein injection of RBT-1, as noted above. Either 4 or 18 hrs later (n = 5 at each time point), mice were anesthetized, the abdominal cavity was opened, and the abdominal vasculature exposed. The perivascular adipose tissue was gently removed, and then the full-length abdominal aorta was carefully respected, immediately cooled to 4 °C, and total RNA was extracted (RN easy Kit; Qiagen, Germantown, MD). Nrf2 activation was assessed by measuring the mRNAs of two Nrf2 activated genes [heme oxygenase 1 (HO-1); NAD(P)H quinone dehydrogenase 1(NQO1)]. Aortic RNA samples from 5 normal mice served as controls. Samples were assayed for HO-1 and NQO1 mRNAs by RT-PCR, as previously described [11,13]. The values were factored by simultaneously determined GAPDH product. [Note, these (and the above) assessments were performed on aorta, given that it can be obtained free of potentially contaminating organ parenchyma which could obfuscate vascular results].
Vascular heavy chain ferritin assessments: Previous studies have indicated that RBT-1 up-regulates tissue ferritin expression primarily via increased translation, not gene transcription. Hence, to assess whether RBT-1 increased vascular heavy chain ferritin expression, it was probed in aortic and renal vascular tissues by immune histochemistry (IHC). Three mice were injected with RBT-1 and 18 hrs later, aorta and left kidneys were removed and fixed in 10% formalin. The tissues were then probed for heavy chain ferritin as described at the end of this Methods section.
SDC-1 expression, as assessed by IHC
The goal of this experiment was to confirm that SDC-1 shedding into plasma corresponds with loss of SDC-1 from endothelial cells. To this end, 3 mice were lightly anesthetized with isoflurane and then subjected to the glycerol AKI model, as noted above. Three sham injected mice served as controls. Approximately 18 hrs later, the aortas and kidneys were respected and fixed in 10% formalin. The tissues were then probed for SDC-1 by IHC (see below). Terminal plasma samples were also assayed for SDC-1, as noted above.
SDC-1 and heavy chain ferritin: IHC methodologies
IHC studies were performed by the Fred Hutchinson Cancer Centre Experimental Histopathology; an NIH supported shared resource (P30 CA015704). Paraffin sections were cut at 4 microns, air dried at room temperature overnight, and baked at 60 °C for 1 hr. Slides were stained on a Leica BOND Rx autotimer (Leica, Buffalo Grove, IL) using Leica Bond reagents. Epitope retrieval was performed at 100 °C for 20 minutes with Epitope Retrieval Solution 1 (Leica AR9640). Endogenous anperoxidase was blocked with 3% H 2 O 2 for 5 min followed by protein blocking with TCT buffer (0.05M Tris, 0.15M NaCl, 0.25% Casein, 0.1% Tween 20), and 0.05% Proclin 300 at pH 7.6 +/- 0.1. Rabbit anti-heavy chain ferritin, clone EPR18878 (Abcam ab183781) at a dilution of 1:160 was incubated for one hr and Refine Rabbit polymer HRP was applied for 12 minutes, followed by Mixed Refine DAB (Leica DS9800) for 10 min and counterstained with Refine Haematoxylin (Leica DS9800) for 4 min after which slides were dehydrated, cleared and cover-slipped with permanent mounting media. The same protocol was followed for SDC-1 immunohistochemistry with the exception that rat anti-syndecan-1, clone 281-2 (BD Biosciences 553715) at a dilution of 1:100 was incubated for one hour and Refine Rabbit polymer HRP was applied for 12 minutes, followed by Mixed Refine DAB (Leica DS9800) for 10 min and counterstained with Refine Haematoxylin (Leica DS9800) for 4 min, followed by slide dehydration, cleared, and cover-slipped with permanent mounting media, as above. Scanning was performed on the Aperio AT turbo scanner.
Statistics
All values are given as means ± 1 SEM. The clinical SDC-1 plasma values are presented as ng/mL. Baseline vs. 2-day post-surgery plasma SDC-1 values within each individual group were compared by paired Student’s t test. The % changes from base line values to the 2-day post-surgery values for each of the three groups were also calculated. The % plasma SDC-1 changes from baseline to 2 days post-surgery for the low dose and high dose RBT-1 groups were combined and compared vs. the placebo group unpaired t test). The mouse mRNA data were compared by ANOVA with Turkey’s HSD, or unpaired Student’s t test (for two set data comparisons). The NGAL mRNA and IL-6 mRNA data were converted to log base 10 prior to statistical analysis. Significance was judged by a p value of < 0.05.
Results
Human SDC-1 plasma concentrations
As shown in Figure 1, left panel, baseline plasma SDC-1 concentrations were highly comparable for the three treatment groups. The placebo group manifested a marked post-surgical plasma SDC-1 increase over baseline values (from 48 ± 3 to 73.4 ± 11.9 ng/mL (overall average, 52% increase; p, 0.03). In contrast, neither the low dose (LD) nor the high dose (HD) RBT-1 group developed significant post-operative SDC-1 elevations (LD dose RBT-1: From 53.7 ± 3.9 to 57.2 ± 5.2 ng/mL; overall average 6.4% increase; p,0.47); HD RBT-1: from 50.5 ± 5.7 to 56.9 ± 6.8 ng/mL, or 12.7%; p, 0.28). To assess the overall impact of RBT-1 on the % plasma SDC-1 increase, the LD and HD groups were combined and compared to the placebo group. This demonstrated a significantly lower % SDC-1 increase with RBT-1 preconditioning vs. that seen in the placebo group (p, 0.015; see Figure 1, right panel).
Mouse studies
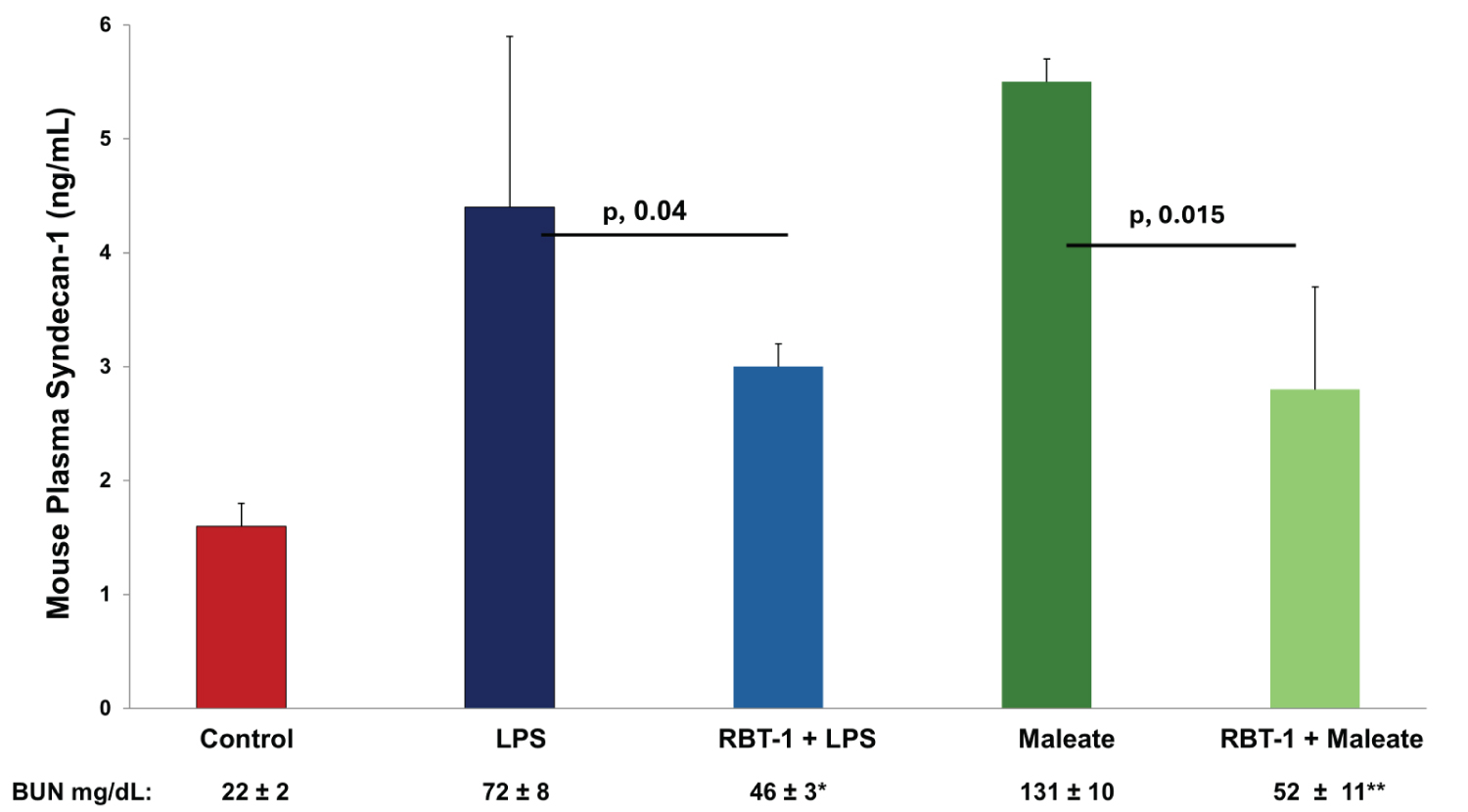
Plasma SDC-1 responses to glycerol, cisplatin, and Iso-induced cardiac stress: Glycerol, cisplatin, and Iso each caused significant increases in plasma SDC-1 concentrations, compared to their controls (glycerol, 5.1 ± 0.2; cisplatin, 3.8 ± 0.2; Iso, 5.4 ± 1.2; composite controls, 1.6 ± 0.2 ng/mL; each p < 0.05 vs. individual set of controls). Hence, these findings (as well as those from the maleate and endotoxin experiments presented immediately below), confirm that diverse forms of injury can evoke marked SDC-1 release in mice. As expected, each injury model caused significant increases in BUN concentrations (glycerol, 89 ± 7; Iso, 86 ± 5; cisplatin, 86 ± 8; composite controls, 22 ± 2 mg/dL; all p < 0.05 vs. individual controls).
RBT-1 preconditioning effect on LPS- and maleate-mediated SDC-1 release: Consistent with the above results, LPS and maleate injections also caused marked plasma SDC-1 increases (Figure 2). RBT-1 preconditioning significantly mitigated these SDC-1 increases by approximately 50% (Figure 2), mirroring the above noted clinical results (i.e., suppression of post-surgical SDC-1 elevations). RBT-1 also significantly decreased the degree of LPS- and maleate-induced BUN elevations (Figure 2), consistent with RBT-1’s previously documented renal cytoprotective effects (e.g., ref. [11]). Of note, RBT-1 did not independently alter SDC-1 concentrations (controls, 1.6 ± 0.2; 18 hrs post RBT1. 1.5 ± 0.1 ng/mL).
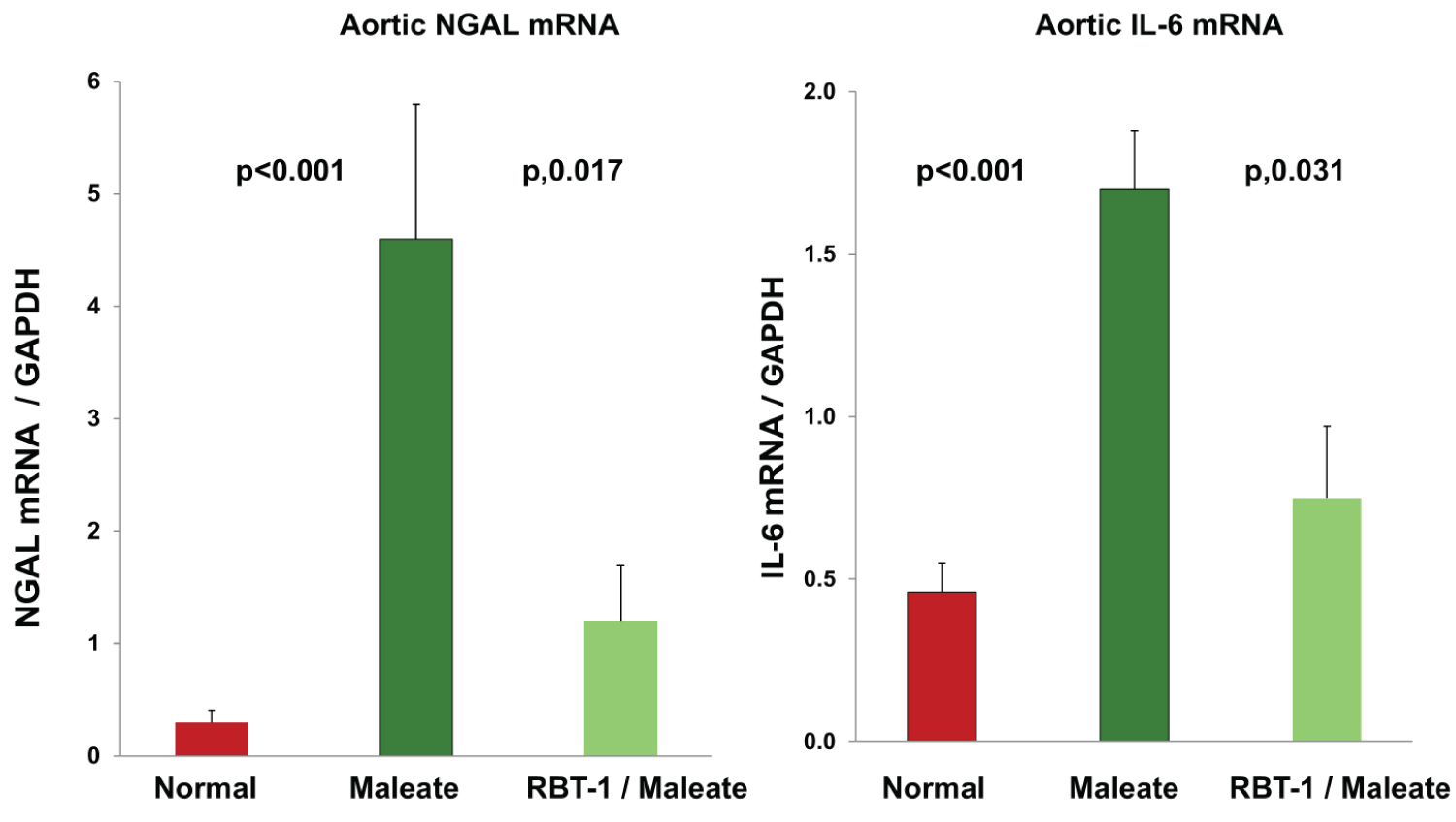
RBT-1 preconditioning protects the aorta from maleate-induced injury, as assessed by NGAL/IL-6 mRNAs: Maleate dramatically increased aortic NGAL mRNA and IL-6 mRNA at 18 hrs post injection, indicating aortic injury/stress (Figure 3). RBT-1 preconditioning significantly decreased the extent of these NGAL mRNA and IL-6 mRNA elevations, indicating a vasculature protective effect.
Aortic Nrf2 and ferritin responses to RBT-1 preconditioning
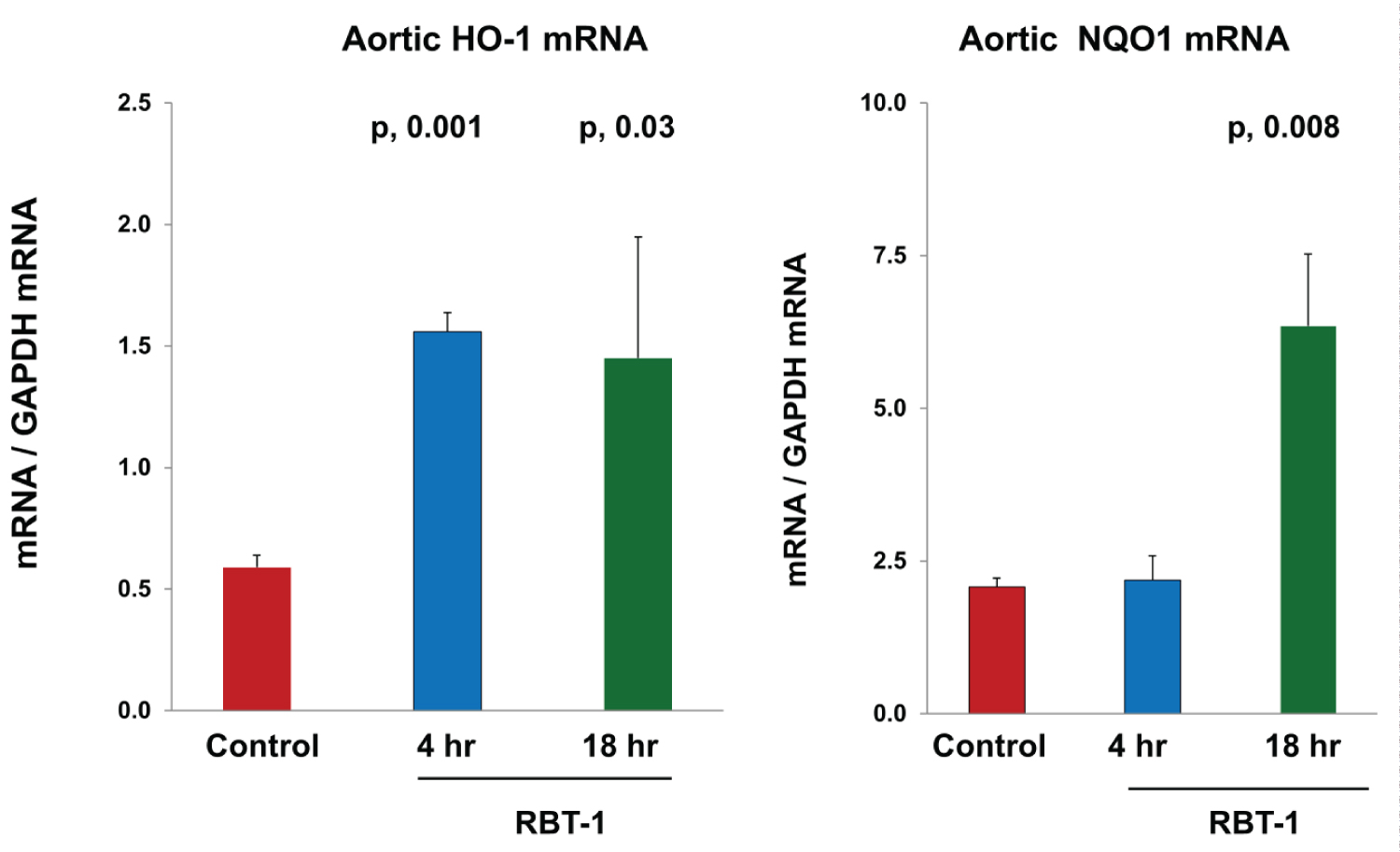
Nrf2: Within 4 hrs of RBT-1 infusion, a 3-fold HO-1 mRNA increase was observed and persisted for at least 18 hrs (p, 0.004) (Figure 4). A 3-fold NQO1 mRNA elevation was evident at the 18 hr (p, 0.018), but not at the 4 hr, time point. The latter is consistent with prior findings that NQO1 reacts more slowly to RBT-1 vs. other Nrf2 sensitive genes.
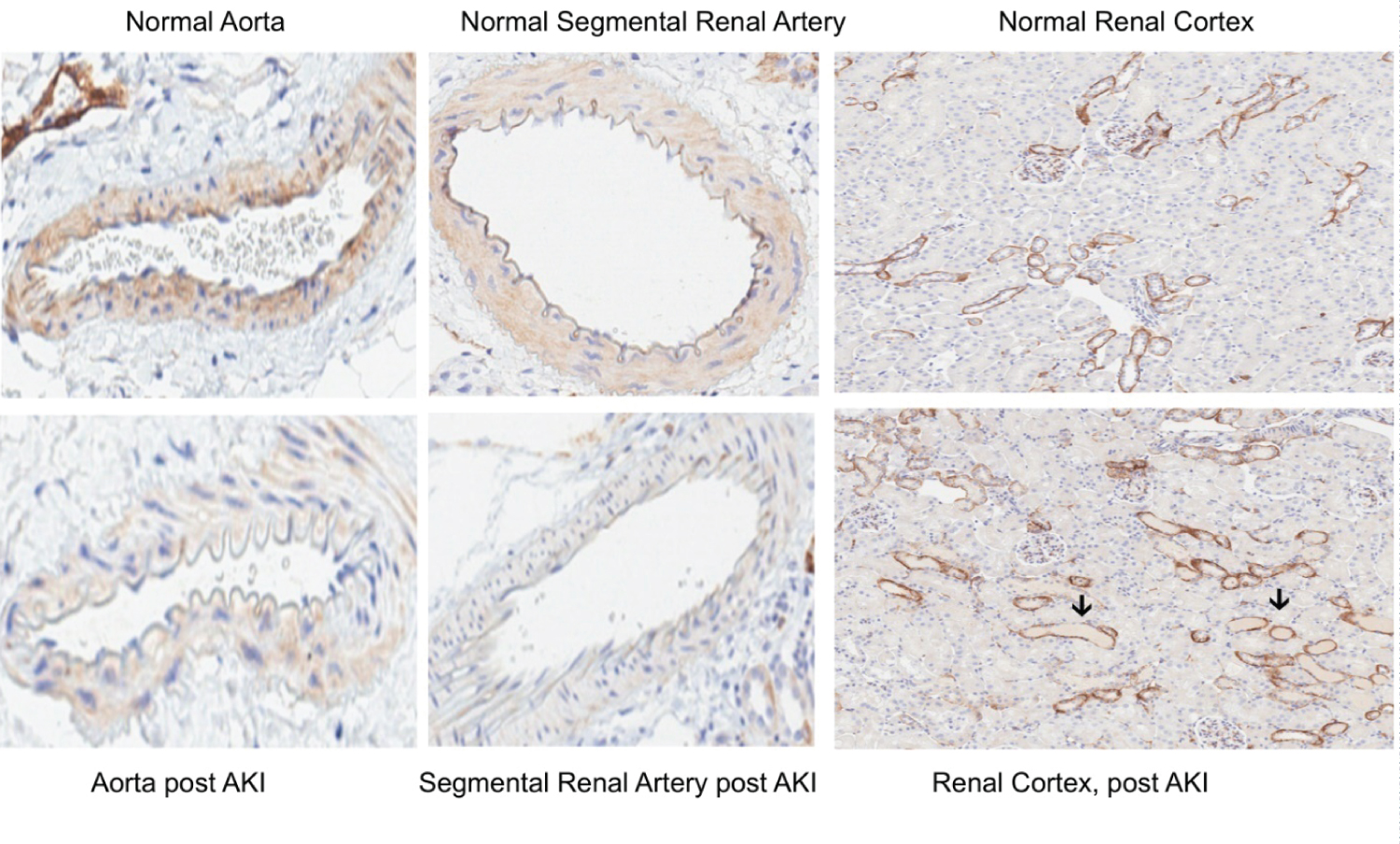
Ferritin: As shown in Figure 5, there was no apparent ferritin staining in normal aortas or in segmental renal arteries (panels A and B, respectively). However endothelial cell ferritin staining was observed in both vessels following RBT-1 injection (panels D and E). Whereas control renal tissues manifested foci of ferritin staining (panel C), essentially all proximal tubules demonstrated striking ferritin expression at 18 hrs post RBT-1 injection (panel F).
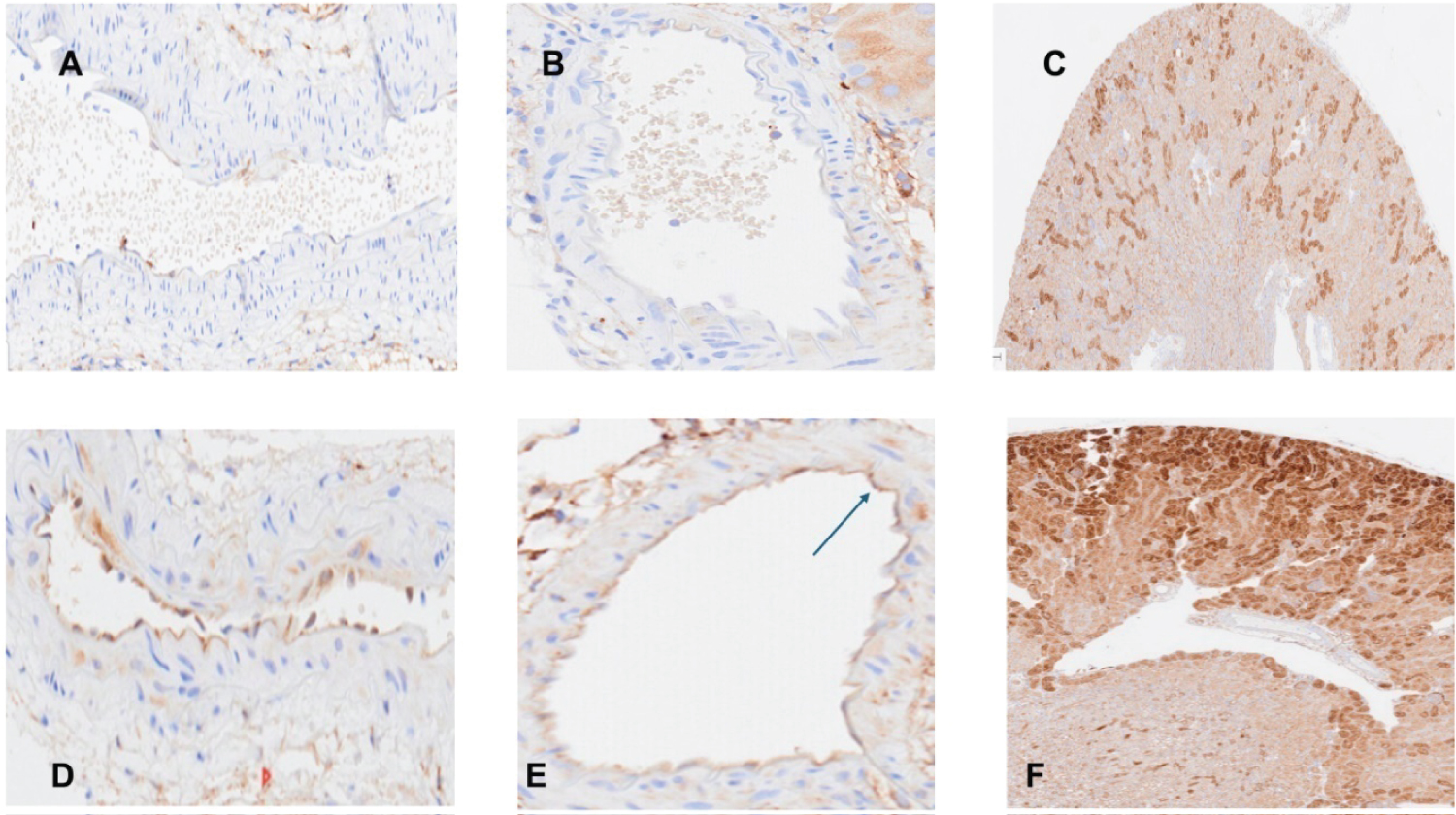
Vascular loss of SDC-1, as assessed by IHC
Post glycerol-induced AKI, marked decreases in aortic and renal vascular SDC-1 staining were observed, compared to normal samples (Figure 6). In contrast, no observable difference in renal tubular SDC-1 staining was observed between the normal and post AKI renal tissues. The loss of vascular SDC-1 post glycerol AKI corresponded with an approximate 4-fold increase in SDC-1 plasma concentrations (glycerol, 5.1 ± 0.2 vs. 1.6 ± 0.2; p < 0.05).
Discussion
In a recent phase 2 trial of RBT-1 preconditioning conducted in patients undergoing ‘on-pump’ cardiac surgery, several clinical benefits were suggested, including shorter post-operative ventilator times, shorter ICU stays, a decreased incidence of atrial fibrillation, less hypervolemia, less blood product use, and a reduction in 30-day hospital readmission rates [32]. We have previously documented that RBT-1 activates cytoprotective pathways in multiple organs, including mouse kidney [11-15], liver [16], heart [11], and lung (unpublished data). As a result, multi-organ protection against diverse forms of experimental tissue injury has been observed [11-16]. However, the impact of RBT-1 on the vasculature, and most notably the endothelial glycocalyx, has not previously been explored. It is noteworthy that SDC-1 and the glycocalyx are critical determinants of vascular function, potentially impacting multi-organ perfusion, and hence, potential clinical outcomes [20,22,25-31]. Thus, we sought to determine whether RBT-1 preconditioning can reduce SDC-1 shedding in post-operative cardiac surgery patients, suggesting a vasoprotective effect.
Consistent with the literature [20-22], we observed an approximate 40-50% increase in plasma SDC-1 levels at 2 days post cardiac surgery in the placebo group (p, 0.03). Conversely, only small and non-significant plasma SDC-1 increases were observed in RBT-1 preconditioned subjects. To confirm that diverse forms of injury can, indeed, evoke tissue SDC-1 release into plasma, multiple experimental renal and extra renal injury models were studied, and in each case marked plasma SDC-1 increases were observed. RBT-1 preconditioning decreased SDC-1 shedding into plasma by ~50% in each of two experimental injury models (endotoxemia, maleate-induced AKI), consistent with the above clinical results. Given that SDC-1 is a widely accepted biomarker of glycocalyx injury, these clinical and experimental data support the concept that RBT-1 preconditioning can confer a vascular protective effect.
To further test whether RBT-1 might decrease endothelial/vascular injury, NGAL mRNA and IL-6 mRNA were measured in mouse aorta following induction of maleate-induced AKI. In both instances, RBT-1 significantly blunted the AKI-induced mRNA increases. These data are congruent with the RBT-1-induced reductions in SDC-1 release, implying a vascular protective effect. Of note, although NGAL, and its mRNA, are generally considered markers of tubular cell injury, vascular damage can also increase their expression [38,39]. This provided the rationale for undertaking the aortic NGAL mRNA assessments.
We have previously documented that RBT-1 activates the Nrf2 pathway in multiple mouse organs and plays a key role in the RBT-1 preconditioning response [11-16]. Hence, the question arose as to whether the above noted vascular protection could have resulted from RBT-1-induced Nrf2 activation directly within vascular tissues. To address this issue, Nrf2-regulated HO-1 mRNA and NQO1 mRNA levels were measured in mouse aorta, and in both instances, RBT-1-mediated HO-1/NQO1 gene induction was observed. It is notable that Nrf2 activation up-regulates a host of antioxidant and anti-inflammatory genes [11,40]. Furthermore, oxidant stress and inflammation are potent inducers of endothelial injury and glycocalyx SDC-1 shedding [21-31]. Thus, it seems plausible that vascular Nrf2 activation could mediate, or contribute to, RBT-1’s vascular protective effect.
Heavy chain ferritin up-regulation, which occurs primarily via increased mRNA translation, as opposed to gene transcription, also contributes to RBT-1’s protective action. This is primarily driven by RBT-1’s Fe sucrose content. Hence, we assessed whether increased aortic, as well as renal vascular, heavy chain ferritin production might result from RBT-1 administration. This appears to be the case, given that increased heavy chain ferritin expression was observed in aorta and segmental renal arteries by immunohistochemistry. Given that cellular ferritin exerts a potent antioxidant effect via catalytic iron scavenging, it, along with Nrf2 activation, could confer a vasoprotective action.
It is important to point out several potentially significant limitations of this study. First, although SDC-1 shedding is widely accepted as a biomarker of endothelial glycocalyx injury, it is notable that SDC-1 is also expressed in, and can be shed from, epithelial cells [27,41-43]. Hence, the relative contributions of endothelial vs. epithelial cell SDC-1 shedding to the observed plasma SDC-1 increases remain unknown. However, that the glycerol AKI model depleted aortic and renal segmental artery SDC-1 staining without any discernable impact on renal tubule SDC-1 expression implies that vascular SDC-1 release, at a minimum, contributed to the observed plasma SDC-1 increases. Second, although RBT-1 activated Nrf2 and up-regulated ferritin in mouse aorta, we cannot conclude that these changes occurred throughout the vascular system. Indeed, it is small, rather than large, vessels that control tissue perfusion. Thus, a more thorough assessment of RBT-1 effects on Nrf2/ferritin expression throughout the vasculature remains an important area for study; Third, although Nrf2 activation and ferritin are known to exert cytoprotective actions on the endothelium [44], we do not have direct evidence that Nrf2 and ferritin either caused, or contributed to, the RBT-1-induced suppression of glycocalyx injury and SDC-1 release. Additional studies to test the mechanistic relevance of these pathways are clearly required. Finally, the ultimate utility of RBT-1 preconditioning as a method to decrease post-surgical complications remains to be defined. The results of a recently initiated phase 3, randomized, double blind, placebo-controlled international trial of RBT-1 preconditioning in 400 ‘on pump’ cardiac surgery patients (NCT 06021457) will provide critical information in this regard.
Acknowledgments
The authors wish to thank the participating study site investigators and staff for their conduct of the completed phase 2 study (NCT04564833; “Start” ref. [32], which was registered with Clinicaltrials.gov. This work was supported by research funds from Reni bus Therapeutics, Southlake, TX, and from the National Institutes of Health (RO-1-DK-38432; P30 CA015704).
References
- Zager RA, Baltes LA, Sharma HM, et al. (1984) Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int 26: 689-700.
- De Paula LJC, Uchida AH, Rezende PC, et al. (2022) Protective or Inhibitory effect of pharmacological therapy on cardiac ischemic preconditioning: A literature review. Curr Vasc Pharmacol 20: 409-428.
- Koti RS, Seifalian AM, Davidson BR (2003) Protection of the liver by ischemic preconditioning: A review of mechanisms and clinical applications. Dig Surg 20: 383-396.
- Lee JC, Shin BN, Cho JH, et al. (2018) Brain ischemic preconditioning protects against moderate, not severe, transient global cerebral ischemic injury. Metab Brain Dis 33: 1193-1201.
- Anttila V, Haapanen H, Yannopoulos F, et al. (2016) Review of remote ischemic preconditioning: From laboratory studies to clinical trials. Scand Cardiovasc J 50: 355-361.
- Meybohm P, Bein B, Brosteanu O, et al. (2015) A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med 373: 1397-1407.
- Hausenloy DJ, Candilio L, Evans R, et al. (2015) Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med 373: 1408-1417.
- Hausenloy DJ, Mwamure PK, Venugopal V, et al. (2007) Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: A randomised controlled trial. Lancet 370: 575-579.
- Pierce B, Bole I, Patel V, et al. (2017) Clinical outcomes of remote ischemic preconditioning prior to cardiac surgery: A meta-analysis of randomized controlled trials. J Am Heart Assoc 6: e004666.
- Zarbock A, Schmidt C, Van Aken H, et al. (2015) Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: A randomized clinical trial. JAMA 313: 2133-2141.
- Zager RA, Johnson AC, Frostad KB (2016) Combined iron sucrose and protoporphyrin treatment protects against ischemic and toxin-mediated acute renal failure. Kidney Int 90: 67-76.
- Zager RA (2022) Oxidant-induced preconditioning: A pharmacologic approach for triggering renal 'self defense'. Physiol Rep 10: e15507.
- Johnson ACM, Zager RA (2018) Mechanisms and consequences of oxidant-induced renal preconditioning: An Nrf2-dependent, P21-independent, anti-senescence pathway. Nephrol Dial Transplant 33: 1927-1941.
- Johnson ACM, Delrow JJ, Zager RA (2017) Tin protoporphyrin activates the oxidant-dependent NRF2-cytoprotective pathway and mitigates acute kidney injury. Transl Res 186: 1-18.
- Zager RA, Johnson ACM, Renibus T (2021) Iron sucrose ('RBT-3') activates the hepatic and renal HAMP1 gene, evoking renal hepcidin loading and resistance to cisplatin nephrotoxicity. Nephrol Dial Transplant 36: 465-474.
- Zager RA (2015) Marked protection against acute renal and hepatic injury after nitrited myoglobin + tin protoporphyrin administration. Transl Res 166: 485-501.
- Pillinger NL, Kam P (2017) Endothelial glycocalyx: Basic science and clinical implications. Anaesth Intensive Care 45: 295-307.
- Liu H Q, Li J, Xuan CL, et al. (2020) A review on the physiological and pathophysiological role of endothelial glycocalyx. J Biochem Mol Toxicol 34: e22571.
- Alphonsus CS, Rodseth RN (2014) The endothelial glycocalyx: A review of the vascular barrier. Anaesthesia 69: 777-784.
- Svennevig K, Hoel T, Thiara A, et al. (2008) Syndecan-1 plasma levels during coronary artery bypass surgery with and without cardiopulmonary bypass. Perfusion 23: 165-171.
- Passov A, Schramko A, Salminen US, et al. (2021) Endothelial glycocalyx during early reperfusion in patients undergoing cardiac surgery. PLoS One 16: e0251747.
- Pesonen E, Passov A, Andersson S, et al. (2019) Glycocalyx degradation and inflammation in cardiac surgery. J Cardiothorac Vasc Anesth 33: 341-345.
- Gonzalez Rodriguez E, Ostrowski SR, Cardenas JC, et al. (2017) Syndecan-1: A quantitative marker for the endotheliopathy of trauma. J Am Coll Surg 225: 419-427.
- Puskarich MA, Cornelius DC, Tharp J, et al. (2016) Plasma syndecan-1 levels identify a cohort of patients with severe sepsis at high risk for intubation after large-volume intravenous fluid resuscitation. J Crit Care 36: 125-129.
- Salmito FTS, Mota SMB, Holanda FMT, et al. (2024) Endothelium-related biomarkers enhanced prediction of kidney support therapy in critically ill patients with non-oliguric acute kidney injury. Sci Rep 14: 4280.
- Miyazaki A, Hokka M, Obata N, et al. (2024) Perioperative serum syndecan-1 concentrations in patients who underwent cardiovascular surgery with cardiopulmonary bypass and its association with the occurrence of postoperative acute kidney injury: A retrospective observational study. BMC Anesthesiol 24: 154.
- Dixon A, Kenny JE, Buzzard L, et al. (2024) Acute respiratory distress syndrome, acute kidney injury, and mortality after trauma are associated with increased circulation of syndecan-1, soluble thrombomodulin, and receptor for advanced glycation end products. J Trauma Acute Care Surg 96: 319-325.
- De Melo Bezerra Cavalcante CT, Castelo Branco KM, Pinto Junior VC, et al. (2016) Syndecan-1 improves severe acute kidney injury prediction after pediatric cardiac surgery. J Thorac Cardiovasc Surg 152: 178-186.
- Xu J, Jiang W, Li Y, et al. (2021) Association between syndecan-1, fluid Overload, and progressive acute kidney injury after adult cardiac surgery. Front Med 8: 648397.
- Bruegger D, Schwartz L, Chappell D, et al. (2011) Release of atrial natriuretic peptide precedes shedding of the endothelial glycocalyx equally in patients undergoing on- and off-pump coronary artery bypass surgery. Basic Res Cardiol 106: 1111-1121.
- Bruegger D, Rehm M, Abicht J, et al. (2009) Shedding of the endothelial glycocalyx during cardiac surgery: On-pump versus off-pump coronary artery bypass graft surgery. J Thorac Cardiovasc Surg 138: 1445-1447.
- Lamy A, Chertow GM, Jessen M, et al. (2024) Effects of RBT-1 on preconditioning response biomarkers in patients undergoing coronary artery bypass graft or heart valve surgery: A multicentre, double-blind, randomised, placebo-controlled phase 2 trial. E Clinical Medicine 68: 102364.
- Finkelstein DM, Schoenfeld DA (1999) Combining mortality and longitudinal measures in clinical trials. Stat Med 18: 1341-1354.
- Pocock SJ, Ariti CA, Collier TJ, et al. (2012) The win ratio: A new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J 33: 176-182.
- Junqueira DR, Zorzela L, Golder S, et al. (2023) CONSORT Harms 2022 statement, explanation, and elaboration: updated guideline for the reporting of harms in randomised trials. BMJ 381: e073725.
- Zager RA, Johnson AC, Naito M, et al. (2008) Maleate nephrotoxicity: Mechanisms of injury and correlates with ischemic/hypoxic tubular cell death. Am J Physiol Renal Physiol 294: F187-F197.
- Johnson AC, Zager RA (2021) Catalytic iron mediated renal stress responses during experimental cardiorenal syndrome 1 ("CRS-1"). Transl Res 237: 53-62.
- Cruz DN, Gaiao S, Maisel A, et al. (2012) Neutrophil gelatinase-associated lipocalin as a biomarker of cardiovascular disease: A systematic review. Clin Chem Lab Med 50: 1533-1545.
- Sivalingam Z, Erik Magnusson N, Grove EL, et al. (2018) Neutrophil gelatinase-associated lipocalin (NGAL) and cardiovascular events in patients with stable coronary artery disease. Scand J Clin Lab Invest 78: 470-476.
- Ngo V, Duennwald ML (2022) Nrf2 and oxidative stress: A general overview of mechanisms and implications in human disease. Antioxidants (Basel) 11: 2345.
- Kajita Y, Terashima T, Mori H, et al. (2021) A longitudinal change of syndecan-1 predicts risk of acute respiratory distress syndrome and cumulative fluid balance in patients with septic shock: A preliminary study. J Intensive Care 9: 27.
- Konda M, Kitabatake M, Ouji-Sageshima N, et al. (2023) A disintegrin and mtalloproteinase with thrombospondin motifs 4 regulates pulmonary vascular hyperpermeability through destruction of glycocalyx in acute respiratory distress syndrome. Int J Mol Sci 24: 16230.
- Guo M, Xu J, Zhao S, et al. (2022) Suppressing syndecan-1 shedding to protect against renal ischemia/reperfusion injury by maintaining polarity of tubular epithelial cells. Shock 57: 256-263.
- Chen B, Lu Y, Chen Y, et al. (2015) The role of Nrf2 in oxidative stress-induced endothelial injuries. The role of Nrf2 in oxidative stress-induced endothelial injuries. J Endocrinol 225: R83-R99.
Corresponding Author
Richard A Zager, MD, Renibus Therapeutics, Southlake Tx; Fred Hutch Cancer Centre, Seattle, WA; and the University of Washington, Seattle, 1551 Eastlake Ave, E, Seattle, WA 98102, USA, Tel: (206)-890-8633.
Copyright
© 2025 Johnson ACM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.