An Interesting Case of Incidentally Detected Left Ventricular Subepicardial Pseudoaneurysm
Abstract
Introduction: Left ventricular pseudoaneurysm is uncommon but can be potentially life threatening which results from breach that is contained by scar tissue. It carries a significant risk of rupture due to its propensity to grow rapidly.
The symptoms are nonspecific and diagnosis is often delayed.
Case report: We had a 65-year-old hypertensive gentleman who was admitted with complaints of angina and diagnosed to have posterolateral wall myocardial infarction.
Coronary angiogram showed triple vessel disease. After taking informed consent, he was planned for Coronary Artery Bypass Graft surgery.
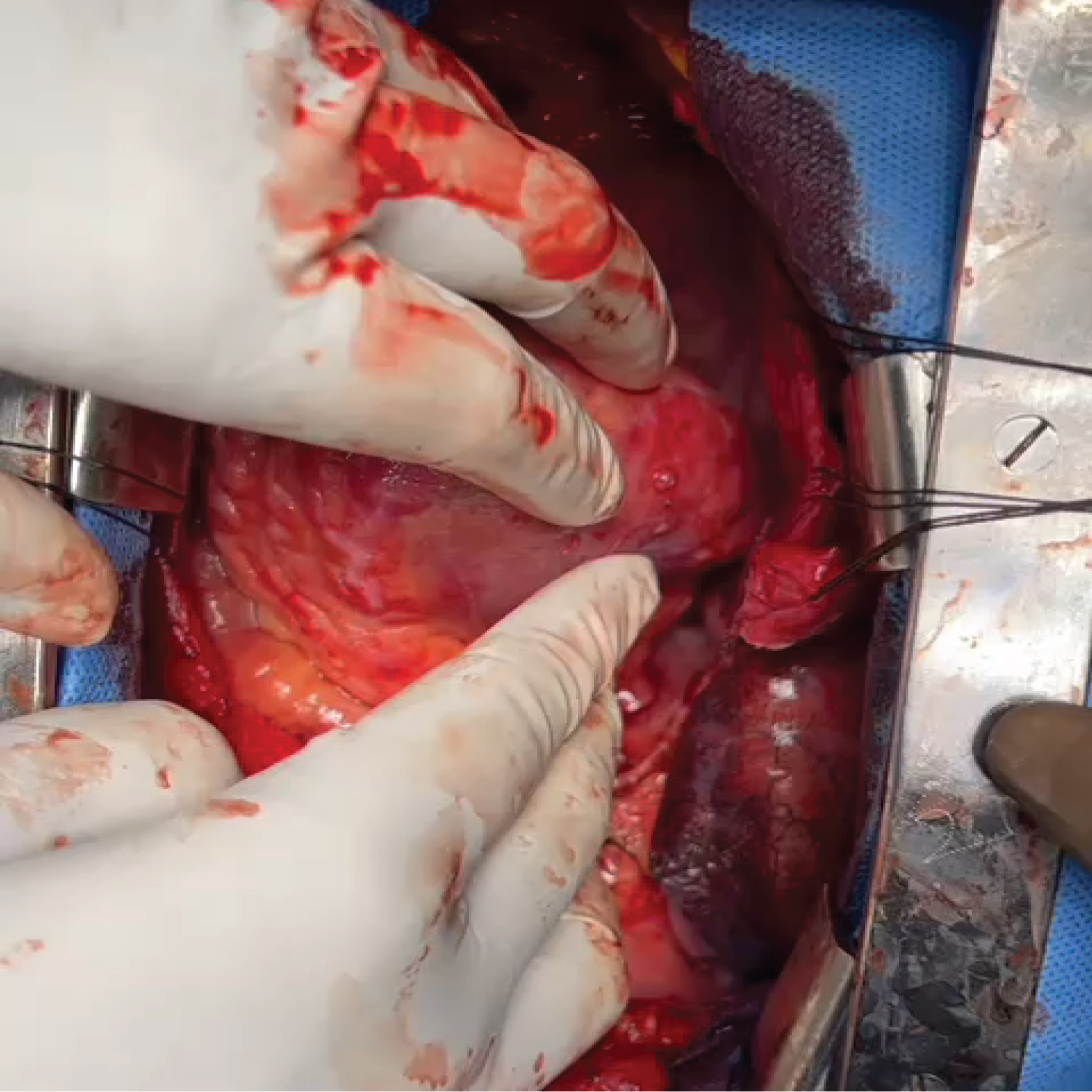
On table, while lifting the heart to check for targets, a subepicardial aneurysm was noted in the lateral wall (OM territory).
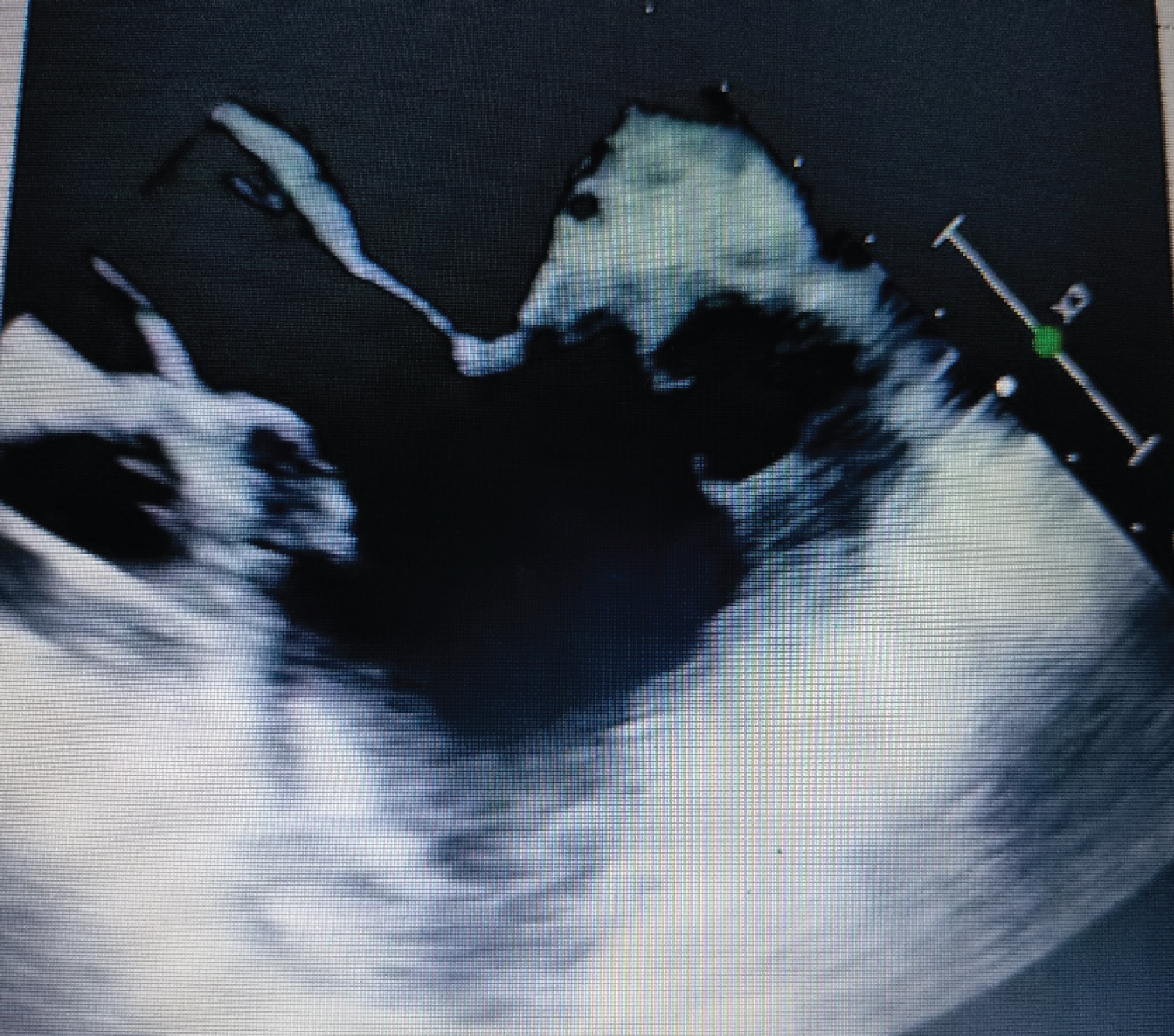
Transesophageal echocardiogram confirmed the same, also revealing that an impending rupture was imminent. Hence it had to be repaired.
Coronary artery bypass graft was done with Left Internal Mammary artery sequentially anastomosed to Diagonal and Left Anterior Descending arteries and Saphenous vein graft to Distal Right Coronary Artery. Cardiopulmonary bypass was initiated. Right Internal mammary to OM anastomosis done. After cardioplegic arrest, the aneurysm was opened. Clots were evacuated.
The defect was closed with bovine pericardial patch and reinforced with Bioglue.
Postoperative TEE showed the patch in situ. Postoperative period was uneventful. Echocardiogram after 3 months revealed the same finding as the post op period.
Discussion: Detection of this pseudo aneurysm was incidental in this patient. Previous transthoracic echocardiograms failed to detect the same.
Surgical resection is the mainstay of management, especially as this aneurysm showed features of impending rupture.
As is true from this case, they can be missed on echocardiogram, especially the ones on the posterior and lateral walls, unless there is a strong suspicion for them.
Conclusion: In conclusion, MI is the most common reason for patients developing LV pseudoaneurysm. Surgery should be recommended as the first option. Conservative management is only applicable for a handful of patients but they should be under regular follow up for increase in size of the aneurysm or any new symptoms developing due to the same.
Keyword
Pseudoaneurysm, post myocardial infarction, Sub-epicardial aneurysm
Introduction
Left ventricular pseudoaneurysm is uncommon but potentially life threatening. Timely diagnosis is essential for early surgical repair. The pseudoaneyrusm results from rupture that is contained by adherent pericardium or scar tissue, with no myocardial tissue. It carries a significant risk of rupture due to a strong propensity to grow rapidly [1].
Myocardial infarction can lead to the development of such pathologies. Left ventricular aneurysm and pseudoaneurysm are 2 complications of myocardial infarction in which the role of imaging is of utmost importance [2] (Figure 1).
The clinical features are not definitive most of the times. The symptoms are nonspecific and diagnosis is often delayed. The most frequently ruptured orifices are usually lateral and posterior walls of the left ventricle. Surgery is recommended as the first option, and conservative therapy can be considered for appropriate patients [3].
Case Report
We had a 65-year-old hypertensive gentleman who was admitted to the hospital with complaints of angina with sweating. He was diagnosed to have postero lateral wall myocardial infarction. ECG showed acute changes. Chest X-ray was normal and did not show any significant abnormality. Echocardiogram revealed an ejection fraction (EF) of 50%.
Coronary angiogram was done which showed critical triple vessel disease with total occlusion of the left circumflex artery. He underwent ballon angioplasty of the left circumflex artery and later came to us for further management.
After performing all relevant preoperative investigations and taking informed consent, he was planned for Coronary Artery Bypass Graft surgery subsequently.
He was put under general anesthesia. On table, while lifting the heart to check for targets, a subepicardial aneurysm was noted in the lateral wall (OM territory).
Immediately, a transesophageal echocardiogram was performed which confirmed the same. It also showed that an impending rupture was imminent. Hence it had to be repaired (Figure 2).
Coronary artery bypass graft was done with Left Internal Mammary artery (LIMA) sequentially anastomosed to Diagonal and Left Anterior Descending arteries and Saphenous vein graft to Distal Right Coronary Artery.
Cardiopulmonary bypass initiated prior to the Obtuse Marginal (OM) graft using Aorta-Right Atrial cannulation. Right Internal mammary to OM anastomosis done. After cardioplegic arrest, the aneurysm was opened. Clots and debris were evacuated.
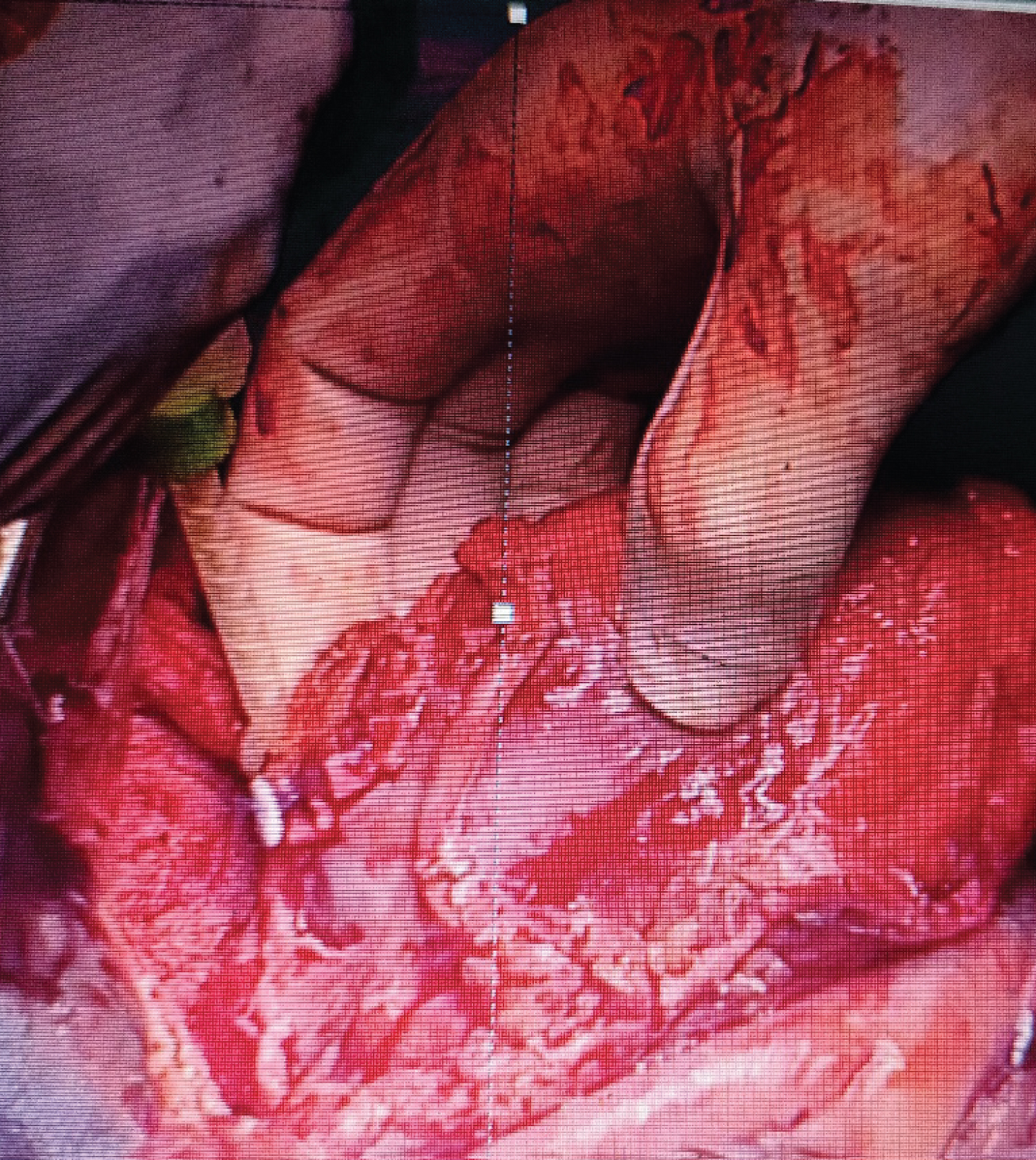
The neck of the aneurysm was identified. This confirmed that it was a pseudoaneurysm. The defect was closed with bovine pericardial patch with 5-0 pledgetted prolene sutures in 2 layers. It was reinforced with application of Bioglue over the suture lines.
Once over, transesophageal echocardiogram was repeated and the patch was seen in situ. No residual shunt was noted. EF was preserved at 50%.
Post-operative period was uneventful. By the first post-operative day, he was extubated. He was discharged home by the sixth post-operative day. He was asked to come for follow up review after 3 months. Echocardiogram done then also revealed the same finding as the early post op period. The patch was noted without any residual shunt.
Discussion
Detection of this pseudoaneurysm was incidental in this patient. Previous transthoracic echocardiograms failed to detect the presence of this aneurysm.
Having noted this intraoperatively, surgical resection of the same is the mainstay of management, especially in this case as the aneurysm showed features of impending rupture.
As is true from this case, they can be missed on echocardiogram, especially the ones on the posterior and lateral walls, unless there is a strong suspicion for them.
For low incidence of LV pseudoaneurysm, only sporadic cases and a few cases series have been reported in the past [3-7].
Usual etiological factors leading to the formation of such LV pesudoaneurysm are transmural MI, surgery, trauma, and infection. Differentiation between pseudoaneurysm and true aneurysm can be difficult because most patients may be asymptomatic or have nonspecific symptoms (Figure 3).
Conclusion
In conclusion, MI was the most common reason for patients developing LV pseudoaneurysm. Lateral and posterior walls of the left ventricle were the most predominant sites, notorious for rupture. Transthoracic echocardiogram is a preferred diagnostic tool followed by cardiac magnetic resonance imaging. Surgery should be recommended as the first option. Conservative management is ojnly applicable for a select handful of patients but they should be under regular follow up for increase in size of the aneurysm or any new symptoms developing due to the same.
References
- Inayat F, Ghani AR, Riaz I, et al. (2018) Left Ventricular Pseudoaneurysm: An overview of diagnosis and management. J Investig Med High Impact Case Rep 6: 2324709618792025.
- Caldeira A, Albuquerque D, Coelho M, et al. (2019) Left ventricular pseudoaneurysm. Circ Cardiovasc Imaging 12: e009500.
- Meng X, Yang Y-K, Yang K-Q, et al. (2017) Clinical characteristics and outcomes of left ventricular pseudoaneurysm. A retrospective study in a single-center of China. Medicine (Baltimore) 96: e6793.
- Frances C, Romero A, Grady D, et al. (1998) Left ventricular pseudoaneurysm. J Am Coll Cardiol 32: 557-561.
- Tuan J, Kaivani F, Fewins H (2008) Left ventricular pseudoaneurysm. Eur J Echocardiogr 9: 107-109.
- Dachman AH, Spindola-Franco H, Solomon N (1981) Left Ventricular Pseudoaneurysm, its recognition and significance. JAMA 246: 1951-1953.
- Duan Q-J, Duan C-T, Yang W-J, et al. (2021) Conservative treatment of left ventricular pseudoaneurysm after mitral valve replacement due to early left ventricular rupture: A case report. J Cardiothorac Surg 16: 69.
Corresponding Author
Dr. Paulami Sen, MBBS, DNB Registrar in Cardiovascular and Thoracic Surgery, Apollo Hospital, Tamil Nadu - 600006, India.
Copyright
© 2023 Sen P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.