Bilateral Carotid Artery Cannulation before Resternotomy to Maintain Cerebral Perfusion in Reoperative Aortic Aneurysm Surgery
Abstract
Objective: Aortic pseudoaneurysm is an uncommon complication following major aortic surgery. Surgical intervention is challenging due to risks of pseudoaneurysm rupture, causing increased morbidity and mortality. Maintaining cerebral perfusion during circulatory arrest is therefore vital to improve patient outcomes.
Methods: This case series involves two patients where a novel technique to maintain cerebral perfusion was used before resternotomy. Both patients underwent emergency repair of expanding aortic pseudo aneurysms following acute symptomatic presentation, on backgrounds of aortic dissection repair surgery.
Results: The novel technique involves direct cannulation of both common carotid arteries via a single neck incision prior to sternotomy. Skin incision was performed on the left side, medial to the sternomastoid muscle to expose the left common carotid artery. The right common carotid artery was exposed via the same incision. Each was directly cannulated and connected to the cardiopulmonary bypass circuit. Circulatory arrests with continuous antegrade cerebral perfusion were commenced simultaneously on pseudoaneurysm breach. Both patients had uneventful rapid recoveries with no adverse neurological events.
Conclusions: The described technique for carotid cannulation is safe, reproducible, and does not need other specialist input. From removing air embolism risk and maintaining brain perfusion throughout the operation this technique makes reoperative aortic surgery significantly safer.
Introduction
Aortic arch surgery has undergone significant development since its inception, and while endovascular techniques are gaining popularity the gold standard remains an open sternotomy method. Reoperative aortic surgery aims to correct unsuccessfully managed conditions or complications of previous operations and is becoming much more common. This is because of improved primary surgery outcomes and a follow-up capacity meaning potential complications are found earlier at treatable stages [1]. In aortic reoperation there is however significant morbidity and mortality, most notably through the impaired neurological function of a stroke and perioperative death from exsanguination [2]. This is due to increased patient comorbidity, surgical complexity for a less characteristic pathology, and that adhesions potentially involving the aorta, its branches, and the heart itself, mean gaining access via resternotomy is significantly harder [1]. The primary surgical indication in this case series is pseudoaneurysm development post-operatively, that is defined as abnormal dilatation of the aorta alongside a haematoma bound by only tunica adventitia. Their reoperation has high mortality, and safe resternotomy is vital for success because pseudoaneurysms are large, often compress other structures, and may adhere to the sternum, meaning high risk of rupture and exsanguination upon incision [3,4].
Given such significance, resternotomy in aortic pseudoaneurysm surgery requires a different strategy [5]. Cardiopulmonary bypass is commonly established before resternotomy through the axillary or femoral vasculature [4,5]. This gives more time to safely negotiate chest entry, provides clearer operative space, and with early circulatory control mitigates potential complications of pseudoaneurysm rupture [3,5]. But, although this common practice is important and effective, it is vital to specifically consider maintaining cerebral perfusion. Unlike for cardiopulmonary bypass there is no general consensus for optimising it, with some research suggesting bypass alone suitably maintains perfusion until a direct carotid canulation is performed through the chest [3,6].
This report describes our approach to minimise such complications through a new direct technique of bilateral carotid cannulation through a neck incision prior to sternotomy.
Case Summaries
This report describes two similar cases of reoperative aortic arch surgery performed at the University Hospitals Plymouth NHS trust in 2021 that are outlined separately.
Case one 59-year-old male
Presentation: They presented to the emergency department with a two-week history of worsening pleuritic chest pain, without dyspnoea or syncope. On examination an enlarging and pulsatile 5 cm × 5 cm mediastinal mass was found in their fourth intercostal space.
Past medical history: This patient had a surgical replacement of their aortic valve, root, and arch for type A dissection nine years ago. A further repair of aortic arch pseudoaneurysm was performed three years after the initial surgery.
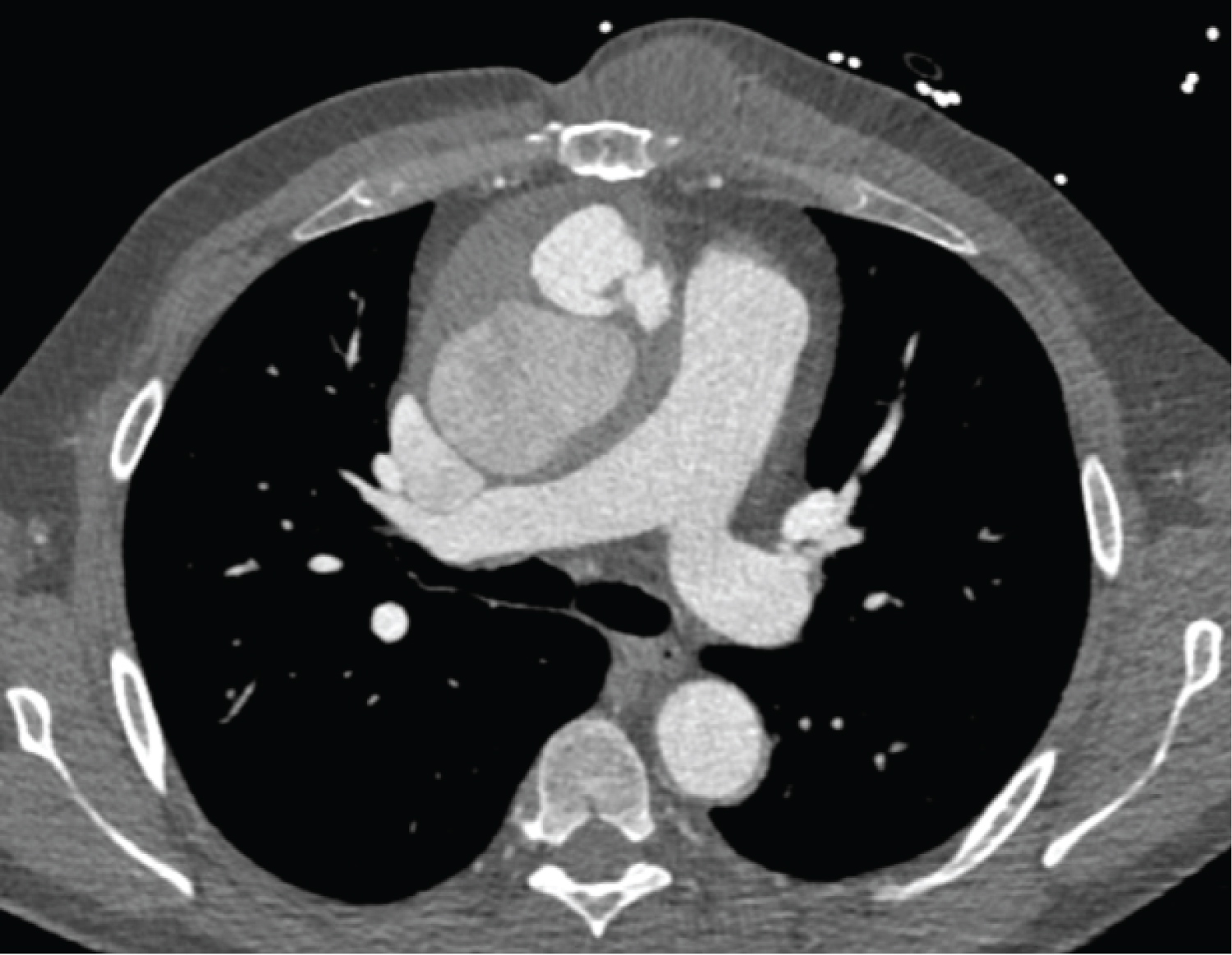
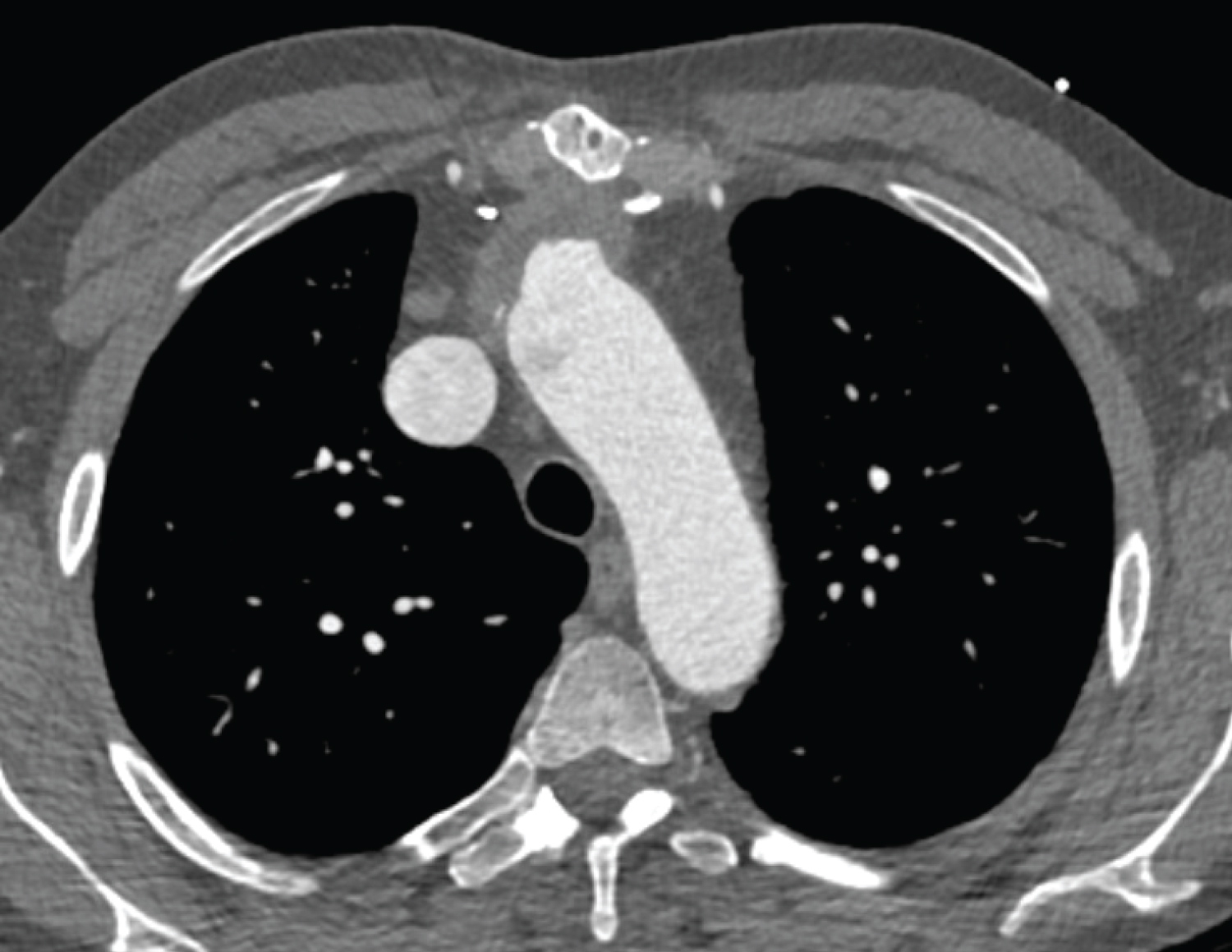
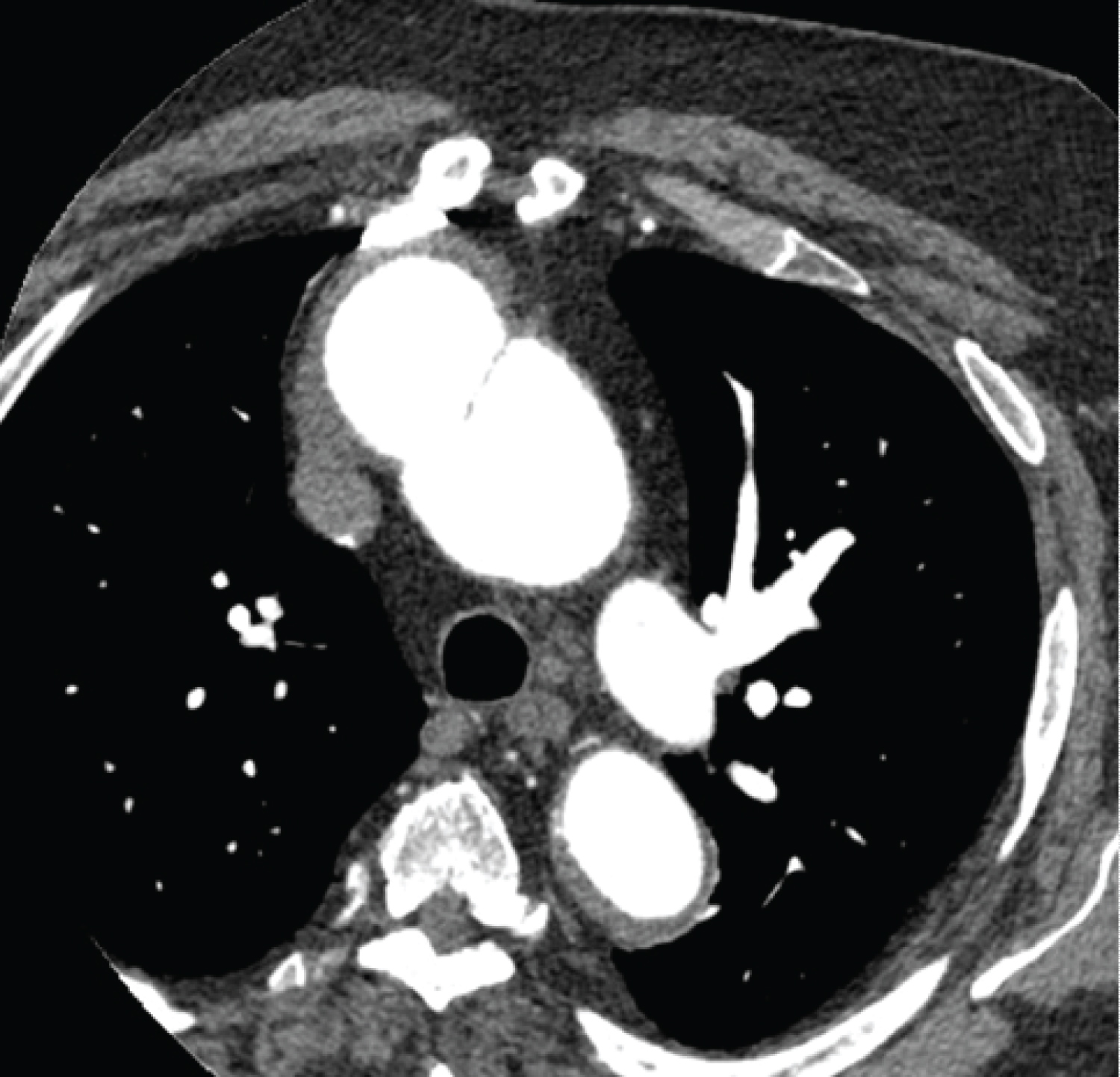
Investigation findings: Cardiac CT scan showed pseudoaneurysm in the distal aortic root measuring 59 mm and contrast extravasation. Echocardiogram showed a dilated left ventricle with mild systolic dysfunction, mitral regurgitation (Figure 1, Figure 2 and Figure 3).
Surgical approach: Cardiopulmonary bypass (CPB) was established before resternotomy by cannulating the right femoral artery and vein. Then, by a newly proposed left-sided direct cervical approach, a medial incision along the sternocleidomastoid muscle was made to form a small flap and allow early cannulation of the right and left common carotid arteries. As deep hypothermic circulatory arrest was reached at 20 °C, resternotomy, graft and pseudoaneurysm resection, and deployment of the ThoraflexTM hybrid prosthesis into the arch were performed. ThoraflexTM arms were anastomosed with the left common carotid and right in nominate arteries. Proximal graft to graft anastomosis was closed, and a bovine pericardium layer separated the sternum from underlying tissues. After warming and haemostasis closure was successfully completed.
Outcome: The patient recovered well and had no significant complications before discharge.
Case two 70-year-old male
Presentation: They presented with prolonged history of worsening fatigue, dyspnoea upon minimal exertion, and presyncopal episodes. Alongside this they recently had developed mild intermittent chest pain and lumbar back pain on exertion. Examination showed ankle oedema bilaterally.
Past medical history: This patient underwent surgical replacement of their aortic arch for type A dissection eleven years previously. Suffered a stroke seven years prior and has limited mobility.
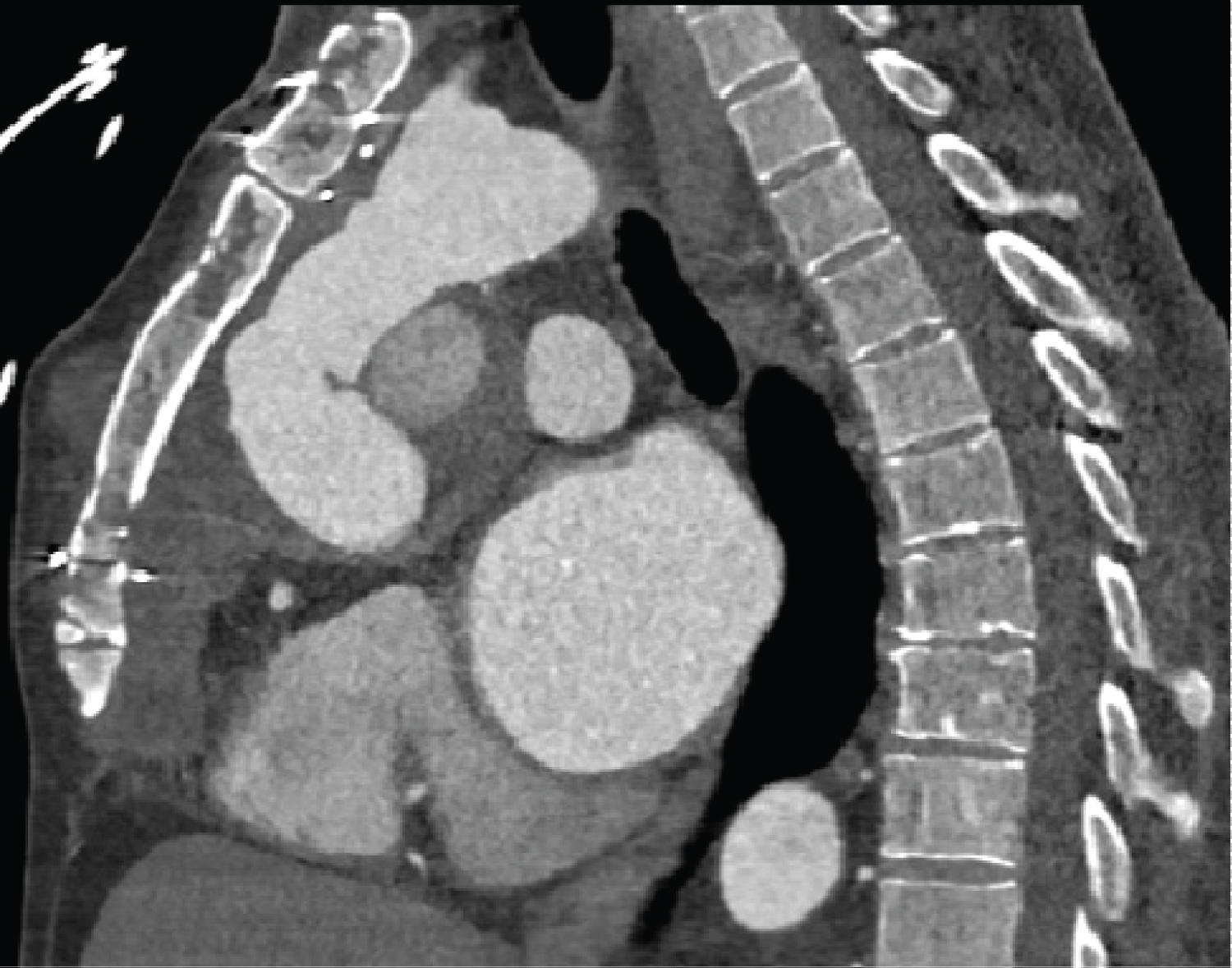
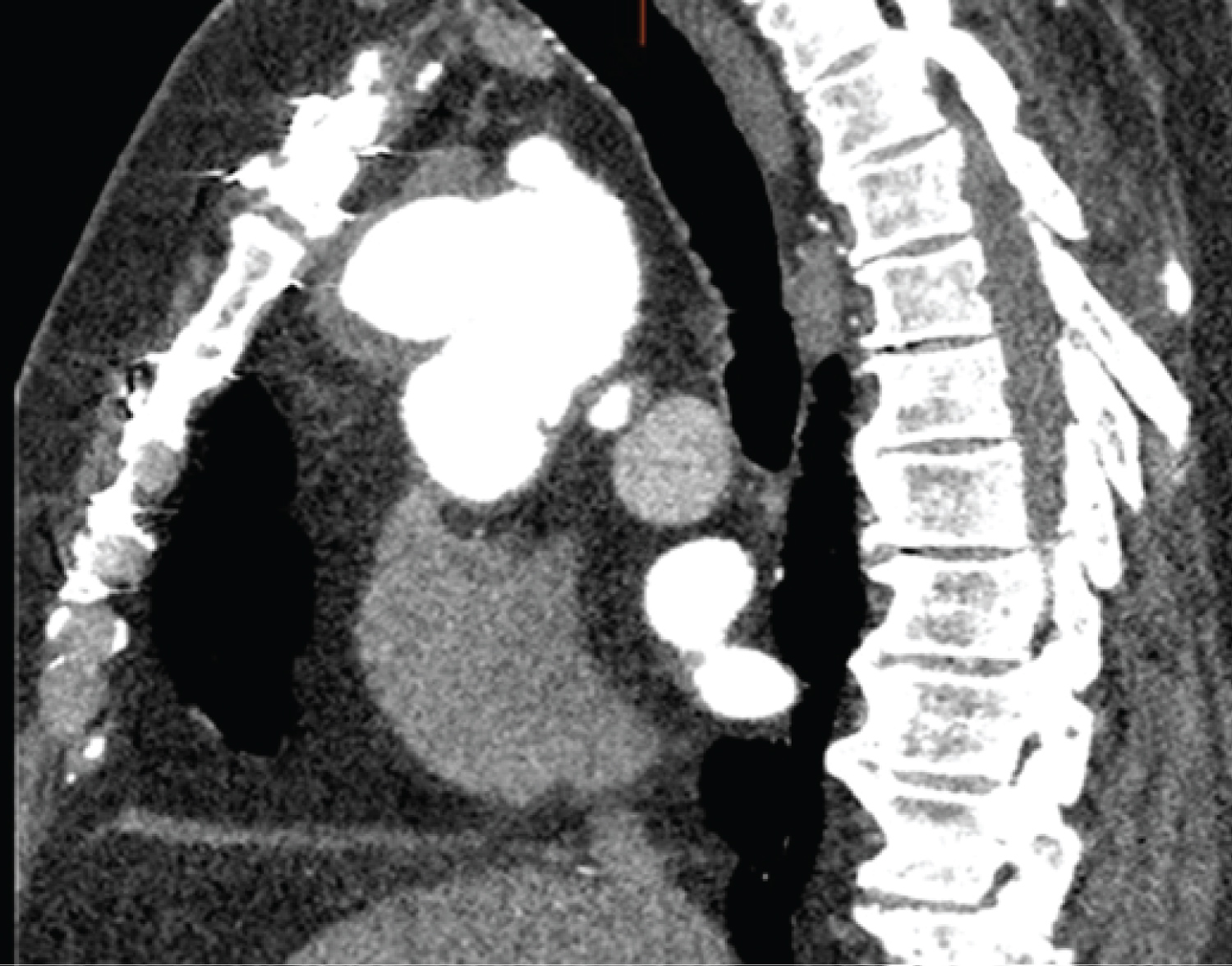
Investigation findings: Cardiac CT scan showed a proximal aortic arch pseudoaneurysm measuring 43 mm. Their chest X-ray and echocardiogram were normal, with a 75% ejection fraction (Figure 4 and Figure 5).
Surgical approach: CPB was established before resternotomy by cannulating the right axillary artery and right femoral vein. Like before, a left-sided cervical incision allowed early cannulation of both common carotid arteries before resternotomy to establish antegrade cerebral perfusion. Once cooled to 20 °C resternotomy was performed, with dehiscence of the previous grafts' distal anastomosis, attachment of a new graft to the distal hemiarch, and an intermediate graft to graft anastomosis. Once off CPB the patient developed ventricular fibrillation so received manual internal defibrillation and rhythm pacing. After haemostasis and rewarming chest closure was completed successfully.
Outcome: The patient recovered well after surgery initially, but was complicated by bradycardia and associated falls before discharge for community rehabilitation.
Surgical approach
Establishing cardiopulmonary bypass: Axillary and femoral vasculature were first cannulated to establish a cardiopulmonary bypass circuit, with temperature set at 22 °C for deep hypothermic circulatory arrest.
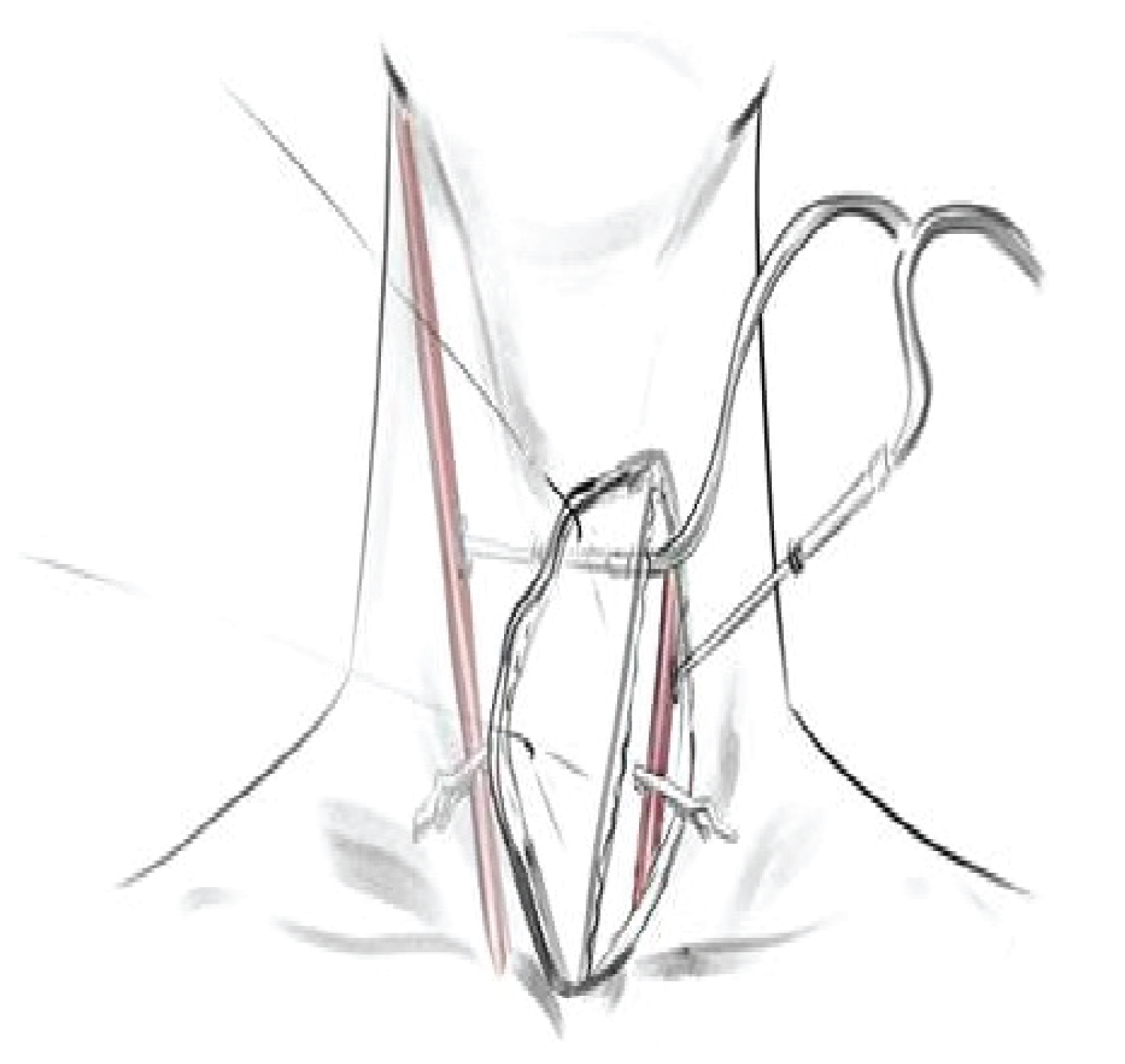
Establishing cerebral protection: A left-sided incision medial to the border of the sternocleidomastoid muscle was performed. The left common carotid artery was exposed and directly cannulated. Deeper dissection was performed medially, and through creating a flap the right common carotid artery was exposed and directly cannulated using an extended catheter. Both catheters were then connected to cardiopulmonary bypass via the right axillary artery.
Resternotomy: After establishing safe protection of the general and cerebral circulation, chest access was gained by careful resternotomy considering risk of aorta rupture. On breaching the pseudoaneurysm carotid arteries were clamped in the cervicotomy and continual antegrade cerebral perfusion was provided at a rate of 10 ml/kg/min (Figure 6).
Pseudoaneurysm repair: The pseudoaneurysm and existing aortic grafts were resected, and new prosthetic structures were deployed and anastomosed with no complications.
Procedure cessation: After safe pseudoaneurysm repair the common carotid arteries were unclipped, the cannulae were removed, and their sites were sealed. Cardiopulmonary bypass was safely removed, with only case two having the complication of ventricular fibrillation so received manual internal defibrillation and rhythm pacing. After haemostasis and rewarming chest closure was completed successfully for both patients.
Discussion
Maintaining safe reliable cerebral perfusion throughout aortic arch surgery is vital, as despite reducing metabolic demand through deep hypothermic circulatory arrest, the risk of cerebral malperfusion and emboli remains high [6]. The potential of neurological injury from vessel rupture is of greater risk in pseudoaneurysm reoperation because of its more complex anatomy and adhesions [4]. This must be minimised, and methods to effectively maintain cerebral perfusion significantly impact positive outcomes [2]. To establish it before resternotomy importantly ensures complete risk coverage across the surgery, and means focus is solely on careful resternotomy and dissection. But, approaches for this vary greatly, and thus it is important to review the literature for comparison to our technique.
One common method utilised axillary artery cannulation to give antegrade cerebral perfusion [7,8]. This usefully establishes cerebral circulation before resternotomy, and carries lower risk of atherosclerotic complication than aortic or femoral cannulation [7]. However, being an indirect cannulation method circulating back into the in nominate artery and up the right common carotid artery, it may not provide adequate cerebral perfusion for patients with an incomplete Circle of Willis, or the complicated anatomy from pseudoaneurysm disruption [4,7].
Therefore, further to modifying cardiopulmonary bypass itself, newer methods aim to directly cannulate their carotid arteries before resternotomy. Bachet, et al. [4], Cappocia, et al. [9], and Mohammadi, et al. [10] all describe success through this approach but utilise slight variations in technique. All used a bilateral cervicotomy incision to access both the right and left common carotid arteries, that Cappocia, et al. [9] state needed input of vascular surgery [4,10]. All together, they describe that using bilateral carotid cannulation offers significant benefit allowing harmless resternotomy, reduced cardiopulmonary bypass time, permanent safe controllable cerebral protection during surgery, and an improved surgical exposure by placing carotid perfusion outside the direct operative field [4,9,10]. However, despite such improvements potential issues of implementing this may include more complex surgical team work through vascular surgery involvement, introduction of new open surgical wounds for associated complications, the resulting visible neck scars, and interference with venous lines using bilateral cervical access.
This case series also implemented carotid cannulation as a method to simplify aortic reoperation surgery procedures, whilst importantly minimising potential neurological complications from them. This method of bilateral carotid cannulation builds upon its advantages and tackles the previously discussed disadvantages. It presents a novel approach that is performed quicker through one access point by the cardiac surgery team, that also simplifies team involvement and operative continuity. Moreover, with a single access point there is less interference with venous instruments, reduced risk of complications from multiple open surgical sites, and lastly improved appearance of scarring on recovery. Thus, by simultaneously addressing limitations of the literature, and making the process of successfully establishing direct cerebral perfusion before resternotomy more efficient, this novel technique enhances current practice.
Conclusion
Altogether, with the increasing need for reoperative aortic arch surgery being driven by improved outcome and resultant high-quality monitoring of primary aortic surgery, it is vital attention turns to optimise reoperative approaches. Where pseudoaneurysm is an indication for it there is a significant risk of rupture on resternotomy leading to a serious ischaemic neurological complication. Establishing cerebral protection prior to resternotomy removes this risk, and our new proposed technique for a direct cervical approach is a simple reproducible method offering multiple advantages over current practice. Direct carotid cannulation is completely reliable. The external route ensures a clearer surgical site to reduce interference and improve chest access to navigate a more complex anatomy, and its simple unilateral technique can be quickly performed without further specialty input. Future work trialling this with other approaches would be useful to highlight comparative effectiveness and establish a standard practice to optimise reoperative aortic surgery outcomes.
References
- Chiesa R, Bertoglio L, Kahlberg A, et al. (2014) Redo surgery in ascending aorta and aortic arch. J Cardiovasc Surg (Torino) 55: 803-812.
- Bajona P, Quintana E, Schaff HV, et al. (2015) Aortic arch surgery after previous type A dissection repair: Results up to 5 years. Interact Cardiovasc Thorac Surg 21: 81-85.
- Inaba Y, Ito T, Hayashi S, et al. (2016) Surgical strategy for thoracic aortic pseudoaneurysm with sternal adherence. Ann Vasc Dis 9: 235-239.
- Bachet J, Pirotte M, Laborde F, et al. (2007) Reoperation for giant false aneurysm of the thoracic aorta: How to reenter the chest? Ann Thorac Surg 83: 1610-1614.
- Roselli EE (2011) Reoperative cardiac surgery: Challenges and outcomes. Tex Heart Inst J 38: 669-671.
- Lee TY, Safi HJ, Estrera AL (2011) Cerebral perfusion in aortic arch surgery: Antegrade, retrograde, or both? Tex Heart Inst J 38: 674-677.
- Harrington DK, Fragomeni F, Bonser RS (2017) Cerebral perfusion. Ann Thorac Surg 83: S799-S804.
- Di Eusanio M, Savini C, Folesani G, et al. (2013) Improved brain and myocardial protection during complex aortic reinterventions. Ann Thorac Surg 95: 358-359.
- Capoccia M, Nienaber CA, Mireskandari M, et al. (2021) Alternative approach for cerebral protection during complex aortic arch and redo surgery. J Cardiovasc Dev Dis 8: 86.
- Mohammadi S, Bonnet N, Leprince P, et al. (2004) Reoperation for false aneurysm of the ascending aorta after its prosthetic replacement: surgical strategy. Ann Thorac Surg 79: 147-152.
Corresponding Author
Nader Moawad, MBBCh, MRCS (Eng), MSc, PGCert (Clinical education), Plymouth University Hospitals, Plymouth, UK
Copyright
© 2022 Moawad N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.