Analyzing Arterial Pressure Waveform for Cardiac Output Measurement in Valvular Heart Surgery Patients
Abstract
Thermodilution via pulmonary artery catheter (PAC) is commonly accepted as gold standart method for cardiac output measurements. The new method Flotrac/Vigileo™ system calculates cardiac output (CO) by analyzing arterial pressure waveform and has recently been marketed which have the ability to monitor CO noninvasively. In this study we compared Flotrac/Vigileo™ (FV) in patients undergoing valvular heart surgery with pulmonary artery cardiac output measurement.
Nineteen patients, who would undergo isolated valve replacement surgery were included into the study. All patients were monitored with PAC and FV monitoring systems. Cardiac outputs were measured at four different stages of the operation: After induction of anesthesia (T1), before cannulation (T2), after cardiopulmonary bypass (T3) and after sternal closure (T4).
There was no difference between demografic data of patients. CO measurements obtained with the FV system were higher for the T1, and T4 measurements; lower for the T2 and T3 than did those obtained with the PAC. For all measurement stages, good agreements were found between two methods as shown by Bland-Altman statistics.
Although many studies are reported for the evaluation of the FV in the operating theatre we could find no studies that have examined this method in isolated valvular heart surgery patients. We compared two different CO measurement methods: FV and PAC in valve replacement surgery patients. The CO values provided by FV were similar to the PAC values that may be considered as gold standart. We concluded that FV is easier to use and less invasive than PAC and would be a good choice in determining CO in patients who will have valvular cardiac surgery.
Keywords
Cardiac output, Pulmonary artery catheter, Flotrac/Vigileo™
Introduction
Thermodilution via pulmonary artery catheter (PAC) is commonly accepted as gold standart method for cardiac output measurement. The FV system is a new less invasive method that calculates cardiac output (CO) by analyzing arterial pressure waveform and pretends to show a reliable performance for variable arterial tones. This system reveals the relationship between the arterial pulse pressure wave and stroke volume [1].
The validity of the CO measurements obtained by FV is mostly studied in coronary artery bypass graft (CABG) surgery or heterogenous groups of cardiac surgery patients and its reliability was found comparable to PAC [2-5]. As there is a little evidence about its use in valvular heart surgery; we compared FV system with PAC to assess its validity in isolated valvular heart surgery patients.
Materials and Methods
Nineteen patients undergoing isolated valve replacement surgery (mitral or aortic valve) were assigned into study after local ethic committee approval and obtaining informed consents. Patients were aged between 40-70 and ASA II-III as health status.
The patients whose ejection fraction was below 40%; who had tricuspid failure more than first degree, arterial fibrillation, serious obstructive lung disease, significant kidney and liver diseases, illnesses related to central nervous system or peripheral blood vessels as well as those with tumoral or immune diseases were not included in the study. Patients who developed cardiac arrhythmia during surgery or needed intra-aortic balloon pump after the bypass were also excluded from the study. The night before the surgery, 0.1 mg/kg diazepam p.o. and 30 minutes before the operation, 0,1 mg/kg morphine sulphate i.m. were applied for premedication. Patients were monitored with electrocardiography (ECG), capnography and pulse oximeter in the operating room. After the radial arterial catheterization, arterial lines were connected to the preprocessor FV. All the transducers were zeroed to atmospheric pressure at the mid-axillary level.
0.1 mg/kg midazolam, 5-7 μgr/kg fentanil and 0.16 mg/kg pancuronium bromide were administered intravenously for an aesthesia induction. After tracheal intubation the patients was ventilated with a tidal volume of 8-10 ml/kg, with an I: E ratio of 1: 2, at a rate of 8-12 breaths/min of 50% oxygen with air and a positive end-expiratory pressure of 5 cm H2O during the surgery. Bolus of 5 μgr/kg fentanil, 0.02 mg/kg midazolam ve 0.02 mg/kg pancuronium bromide were administered as anesthesia maintenance. During the CPB, as a 2.4 lt/min/m2 flow output was being obtained with a rotating pump, the pressure of non-pulsatile perfusion was kept at 60-90 mmHg. As for the intra-operative liquid infusions, they were arranged as follows: Cardiac index > 2 lt/min/m2, central venous pressure 4-10 mmHg, pulmonary arterial closing pressure 8-12 mmHg and urinary extraction 1-2 ml/kg/hour. During the bypass, at most a 30 °C hypothermia was applied to the patients.
PAC and cardiac output measurements
A modified 7.5‐French gauge PAC for CO was inserted via an introducer (8.5 Fr Baxter Edwards Laboratories, Irvine, CA, USA) into the right internal jugular vein using the Seldinger technique. CO was calculated from the thermodilution (TD) curves using the Stewart-Hamilton principle. A bolus CO was calculated as the average of three consecutive measurements performed randomly in the respiratory cycle over several minutes, each using 10 ml of 5% dextrose at room temperature.
FV and cardiac output measurements
FV monitor, generated by Edwards Lifesciences, Irvine, CA, USA, uses arterial pressure waveform analysis to determine the CO, without the need for prior calibration. It consists of a special transducer that attaches to an existing arterial cannula and then connects to a processing/display unit (Vigileo™). The patients' parameters such as age, sex, height, weight were recorded to the Vigileo monitor (Software Version 1.10).
Central venous, systolic, diastolic and mean arterial pressure were monitored, and blood samples was drawn in order to determine the blood gases, electrolytes, and ACT (Activated Coagulation Time). Cardiac outputs were measured at four different stages of the operation: after induction of an aesthesia (T1), before cannulation (T2), after cardiopulmonary bypass (T3) and after sternal closure (T4). In these stages of the operation, cardiac output, beat volume and heart rate measured by thermodilution and Vigileo monitor were recorded.
Statistical analysis
We have performed data analysis by using SPSS for Windows, version 11.5 (SPSS Inc., Chicago, IL, United States). Distributions of continuous variables were analysed with Shapiro9Wilk test. Data were expressed as mean ± standard deviation for continuous variables. PAC was assumed as gold standard and FV was considered as alternative technique. Bland Altman method was used for comparing two methods of measurements in term of limits of agreement. A bias is the mean difference between the two methods of measurement and represents the systematic error; single standart deviation (SD) is a measure of precision, and the limits of agreement, defined as the mean (2 SD), represent the range in which 95% of the differences between methods are expected to lie [mean bias (2 SD)].
Results
After obtaining ethics committee approval and informed concents; 22 patients (ASA II-III) aged between 40-70, who would undergo isolated valve replacement surgery (mitral or aortic valve) were included into the study. Three patients were excluded because of inability to obtain data. The demographic data of the 19 patients are shown in (Table 1, Figure 1).
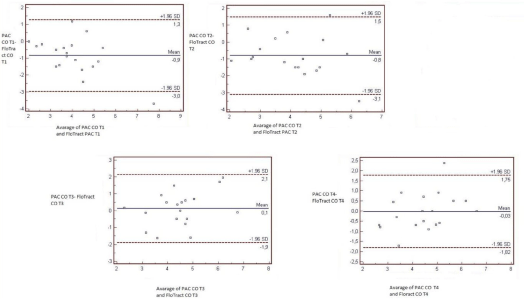
In the four measurement stages, all cardiac outputs determined by FV, and PAC are evaluated. In order to show whether both techniques were methodologically harmonious or not, a confidence interval of %95 was used.
CO measurements obtained with the FV system were higher for the T1, and T4 measurements; lower for the T2 and T3than did those obtained with the PAC.
At T1, mean CO determined by FVwas0.859 l/min higher than the value determined by PAC (95% limits of agreement-2.989, 1.27) when analysed byBland Altman. At T2mean CO determined by FV 0.813 l/min lower than the value determined by PAC (95% limits of agreement-2.989 and 1.27). At T3, the measurements of CO made through FV were 0.124 l/min lower in average than those made with the PAC method (95% limits of agreement-1.893 and 2.141). At T4, mean CO determined by FV was0.030 l/min higher than the value determined by PAC (95% limits of agreement -1.815 and 1.754).
Discussion
The Swan Ganz PAC, using the thermodilution method, has been commonly accepted as a gold standard forcardiac output measurement especially in patients with preserved left ventricular function. In the last few years, many devices have been developed to obtain haemodynamic information with progressively less invasiveness. There is an increasing interest about the systems based on the arterial waveform analysis with no need for external calibration [1-3].
Many studies are reported for the evaluation of the FV in the operating theatre especially during the liver transplant procedure, cardiac surgery and CABG but we could find no studies that have examined this method in isolated valvular heart surgery patients. [6-9]. In this study, we compared the cardiac output measurements obtained from the less invasive FV and PAC in different stages of isolated mitral and aortic valvular surgeries and assess the level of accuracy and reliability of this relatively new and noninvasive system. The CO values provided by FV was similar to the PAC values that may be considered as the gold standart.
Yatin Mehta, et al. [7] compared FV system and thermodilution method in CABG surgery and found good agreement between the CO values obtained by the arterial pressure waveform analysis and TD techniques throughout the intraoperative period.
Cannesson, et al. [3] studied 11 CABG patients in the operating room to compare the accuracy of the FV system vs. pulmonary artery catheter standard bolus thermodilution and found clinically acceptable agreement between two methods in this setting. Cannesson also studied the accuracy of this system by assessing the ability of another parameter, stroke volume variation (SVV), for predicting fluid responsiveness in CABG patients and concluded that SVV derived from FV system predicts fluid responsiveness with an acceptable sensitivity and specificity [10]. Mayer, et al. [11] performed intraoperative goal-directed therapy with FV in high-risk patients undergoing major abdominal surgery and reported a reduced length of hospital stay, a lower incidence of complications compared to a standard management protocol. Another study also compared FV device and PAC for CO measurement in obese patients undergoing CABG surgery and reported an adequately agreement between two systems. All of these studies evaluates the reliability and accuracy of FV system in different patient populations and support present study in which this less invasive system is compared with PAC in valvular heart surgery patients who have not been studied before.
Zimmermann, et al. [12], also studied the accuracy of FV in CABG surgery patients by comparing it with PAC and interpreted their results from different points of view. They reported that cardiac output values derived from the system were beyond acceptable limits under narrow conditions regarding clinical demands (± 20% criteria). Nevertheless, in this initial study they did not exclude patients with rhythm disorders, aortic balloon pump support, excessive tricuspid failure, pulmonary arterial hypertension and whose ejection fraction < 40. But the point is that; the exclusion of these patients would hinder the answer of the exact question because clinically, cardiac output should be measured in haemodynamically unstable patients. Compton, et al. [13] also re-evaluated FV system in haemodynamically compromised patients after the introduction of 1.10 software version and concluded that the updated FV system also could not be recommended to replace more invasive CO monitoring systems in the intensive care unit (ICU). They consider that tracking of individual CO changes is more important than absolute CO values, such as the evaluation of a haemodynamically unstable patient.
Zimmerman conducted another study in patients undergoing CABG surgery with FV system with 1.10 software; finded 69% of data pairs within the ± 20% limits and reported an improvement in the accuracy of the system [14]. Recently, Saraceni, et al. [15] assessed the accuracy of CO measurements obtained by FV, software version 1.07 and version 1.10 compared with CO measurements obtained by bolus thermodilution by PAC in the intensive care setting. They conclude that both software versions of FloTrac/Vigileo did not still provide reliable estimation of CO in ICU setting. In our study we used FV, software version1.10 and excluded the patients with unstable haemodynamic status preoperatively. Further studies are needed to evaluate the accuracy of FV system in haemodynamically unstable valvular heart patients to implement its benefits.
Arterial pressure wave is composed of a forward pressure wave by the ejected blood from the left ventricle and a backward pressure wave. The interplay of several additional physical variables like the arterial compliance and impedance counteracting the pulsatile blood inflow also influence arterial pressure wave and as a result the analysis of this waveform [16]. This may explain our finding of lower correlation between the two measurement methods after the weaning from CPB which is a stage characterized by volume infusion, vasoactive treatment and rapid thermal changes. The measurements obtained after the sternal closure were also more consistent like the first two stages' measurements.
Conclusion
Our data show a good level of concordance between measurements provided by the two methods. Although there is need for further studies for more complicated situations; we conclude that FV system may be a good choice for measuring CO in haemodynamically stable isolated valvular heart surgery patients with the advantages of easy use and less invasiveness.
Conflict of Interest
There is no conflict of interest.
References
- Manecke GR (2005) Edwards flotrac sensor and Vigileo monitor: Easy, accurate, reliable cardiac output assessment using the arterial pulse wave. Expert Rev Med Devices 2: 523-527.
- Jansen JRC, Schreuder JJ, Mulier JP, et al. (2001) A comparison of cardiac output derived from the arterial pressure wave against thermodilution in cardiac surgery patients. Br J Anaesth 87: 212-222.
- Canesson M, Lattof Y, Rosamel P, et al. (2007) Comparison of Flotrac™ cardiac output monitoring system in patients undergoing coronary artery bypass grafting with pulmonary artery cardiac output measurements. Eur J of Anaesthesiol 24: 832-839.
- Mehta Y, Chand RK, Sawhney R, et al. (2008) Cardiac output monitoring: Comparison of a new arterial pressure waveform analysis to the bolus thermodilution technique in patients undergoing off-pump coronary artery bypass. J Cardiothorac Vasc Anesth 22: 394-399.
- D Button, L Weibel, O Reuthebuch, et al. (2007) Clinical evaluation of the FV system and two established continuous cardiac output monitoring devices in patients undergoing cardiac surgery. Br J Anaesth 99: 329-336.
- Matthieu B, Nouette-Gaulain K, Cottenceau W, et al. (2008) Cardiac output measurements in patients undergoing liver transplantation: Pulmonary artery catheter versus uncalibrated arterial pressure waveform analysis. Anaesth Analg 106:1480-1486.
- Biancofiore G, Critchley LA, Lee A, et al. (2009) Evaluation of an uncalibrated arterial pulse contour cardiac output monitoring system in cirrhotic patients undergoing liver surgery. Br J Anaest 102: 47-54.
- Mayer J, Boldt J, Wolf MW, et al. (2008) Cardiac output derived from arterial pressure waveform analysis in patients undergoing cardiac surgery: Validity of a second-generation device. Anesth Analg 106:867-872.
- Opdam HI, Wan L, Bellomo R (2007) A pilot assessment of the FloTrac cardiac output monitoring system. Intensive Care Med 33: 344-349.
- Cannesson M, Musard H, Desebbe O, et al. (2009) The ability of stroke volume variations obtained with Vigileo/FloTrac system to monitor fluid responsiveness in mechanically ventilated patients. Anesth Analg 108: 513-517.
- J Mayer, J Boldt, R Buschmann, et al. (2009) Uncalibrated arterial pressure waveform analysis for less-invasive cardiac output determination in obese patients undergoing cardiac surgery. Br J Anaesth 103: 185-190.
- Zimmermann A, Kufner C, Hofbauer S, et al. (2008) The accuracy of the Vigileo/FV continuous cardiac output monitor. J Cardiothorac Vasc Anesth 22: 388-393.
- Compton FD, Zukunft B, Hoffmann C, et al. (2008) Performance of a minimally invasive uncalibrated cardiac output monitoring system (Flotrac™/Vigileo™) in haemodynamically unstable patients. Br J Anaesth 100: 451-456.
- Zimmermann A, Steinwendner J, Hofbauer S, et al. (2009) The accuracy of the Vigileo/FloTrac system has been improved--follow-up after a software update: A blinded comparative study of 30 cardiosurgical patients. J Cardiothorac Vasc Anesth 23: 929-931.
- Saraceni E, Rossi S, Persona P, et al. (2011) Comparison of two methods for cardiac output measurement in critically ill patients. Br J Anaesth 106: 690-694.
- Romano SM, Pistolesi M (2002) Assessment of cardiac output from systemic arterial pressure in Humans. Crit Care Med 30:1834-1841.
Corresponding Author
Dilek Kazancı, Ankara Şehir Hastanesi, Yoğun Bakım Kliniği, Turkey, Tel: +905057793148
Copyright
© 2021 Kazancı D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.