Microvascular Obstructions Measured by Cardiac Magnetic Resonance, and their Role as a Predictor of Outcomes in Acute Myocardial Infarction
Abstract
Background: Acute myocardial infarction (AMI) is one the main causes of morbidity and mortality in Chile and the world. Cardiac magnetic resonance (CMR) is gaining evidence as a tool to determine prognosis in patients who have suffered an AMI. Microvascular obstructions (MVO) represent non-irrigated areas of the myocardium after reperfusion, and it is determined by CMR. After reestablishment of cardiac blood flow, MVOs could be used as a new marker to determine the heart injury due to AMI and reperfusion damage.
Method and materials: A retrospective analysis of a clinical trial was performed (ISRCTN registry: 56034553). 67 patients suffering ST-segment elevation AMI (STEMI), undergoing a percutaneous coronary angioplasty were studied with a CMR from 7 to 15 days after the intervention. MVOs, infarct size, left ventricular ejection fraction, and left ventricular end-systolic volume indexed to body surface area were determined by three radiologists, blinded to clinical information.
Results: The development of MVOs was associated with a bigger infarction size (p < 0.05), a lower ejection fraction (p < 0.05) and a bigger end systolic volume (p < 0.05) in patients with AMI.
Conclusion: MVOs can be accurate predictors for morphological and functional complications posterior to AMI.
Keywords
Microvascular obstruction, Acute myocardial infarction, Cardiac magnetic resonance, Clinical trial, Retrospective analysis
Introduction
Acute myocardial infarction (AMI) is one of the leading mortality and morbidity causes in the world [1], being associated with high health expenses and diminished life quality in survivors.
In common clinical practice, several scores are usually used to determine the prognosis of a patient who has suffered an acute coronary event, which consider clinical, electrocardiographic and echocardiographic data [2,3]. In the last couple of years there has been a need to find more precise prognosis predictors, and magnetic resonance imaging (CMR) is gaining attention. This technique allows a more detailed evaluation in myocardial morphofunctional parameters, that is easily reproducible [4]. Some of the parameters that can be determined in CMR are transmural necrosis, fibrotic areas, ventricular volume, among others which have been recently implemented such as microvascular obstructions (MVOs) [5]. MVOs can be seen in CMR as low gadolinium catching areas 1 to 2 minutes after the administration of the contrast media, being considered as persistent MVOs when the gadolinium hypocaptation persists after 10 to 20 minutes [5]. MVOs can be a consequence of ischemic damage, reperfusion injury, distal embolization or interpatient variation [6]. These areas are the representation of failed reperfusion and predict worse clinical outcomes and lack of functionality posterior to an AMI [6,7].
Due to the relationship between MVOs, ischemic muscle areas, reperfusion damage and worse functional performance, MVOs detected by CMR have been proposed as a myocardial damage predictor, evaluated with specific morphologic and functional parameters such as infarct size, left ventricle ejection fraction (LVEF), left ventricular end-systolic volume indexed to body surface area; in patients with AMI treated with percutaneous coronary angioplasty (PCA).
Materials and Methods
A retrospective study of the randomized controlled trial PREVEC was performed. It aimed to evaluate whether antioxidant vitamins C and E reduce infarct size in patients with STEMI [8]. 67 patients with STEMI completed follow-up after percutaneous coronary angioplasty was performed. The study took place in three cardiovascular units of Santiago, Chile. During angiography the following parameters were determined: identification of occluded artery causing AMI, the percentage of stenosis and initial TIMI myocardial perfusion grade (TMPG) flow, and myocardial biomarkers.
Cardiac magnetic resonance imaging
Sixty-seven patients underwent CMR between 7 to 15 days after admission into the enrollment. The CMR equipment specifications were Siemens Sonata 1.5 Tesla. Coil: 4-channel array coil equipment. Image protocol consisted in a cine balanced Solvent Saturation Transfer to Proteins (SSTP) sequence and an Investment Recovery Phase-Sensitive Sequence (PSIR), with previous infusion of a dose of 2 mmol/kg of intravenous gadolinium. In all sequences that included slides, short axis planes from the base to the apex, and the 2nd and 4th chamber planes, 7 mm thick slides were used. Ventricular volumes, left ventricular ejection fraction, infarct size and MVOs were determined. All parameters were determined semi-automatically by Segmento 2.0 software, manually delineating areas of interest and measuring signal intensity.
Image interpretation was performed by three thoracic radiologists, who were blinded to clinical information of patients. Each radiologist proposed a score for all assessed parameters. For statistical analysis, the average between the three scores was calculated. All parameters were obtained using the following definitions:
• Infarct size: Expressed as a percentage of the infarcted mass in relation to the total ventricular mass.
• MVO: Manually delineated tissue zones that were not perfused by gadolinium [9,10]. Each radiologist evaluated the presence or absence of MVOs, and assigned a score in relation to signal intensity, obtaining an average result.
• LVEF: Manually delineated chambers volumes by semi-automatic method.
• Left ventricular end-systolic volume indexed to body surface area (ESVI): Measured by semi-automatic method and later normalized by body surface area of patient.
Statistical analysis and ethics
Shapiro-Wilk test was used to assess whether the variables distributed normally. Results are expressed as mean ± standard deviation (X ± SD). Mann-Whitney U-test was used to evaluate statistical differences for non-parametric variables and t-Student test for parametric variables. Pearson or Spearman tests were used to estimate the correlation according to the distribution of each variable. A statistical difference was considered significant if p-value < 0.05. Stata 10.1 and GraphPad Prism 5.0 were used for statistical analysis. This project was approved by the ethics committee of the Faculty of Medicine of University of Chile and by ethics committees of each hospital.
Results
A number of 67 patients completed the study protocol and follow-up. Initial clinical characteristics for the patients are described in Table 1.
Infarct size, LVEF, ESVI and MVO presence
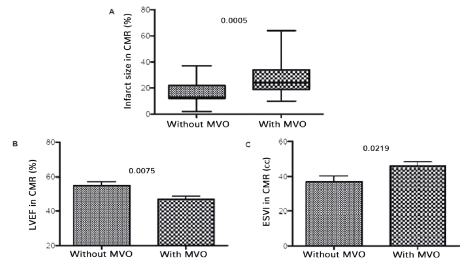
The patients who presented MVOs had a significatively higher infarct size (p = 0,0005) (Figure 1), developed a lower mean LVEF measured by CMR (p = 0,0075) and higher left ventricular end-systolic volume indexed to body surface area (p = 0,0219), when compared to patients that did not present MVOs.
Correlation between mean MVO score and infarct size, LVEF, and ESVI
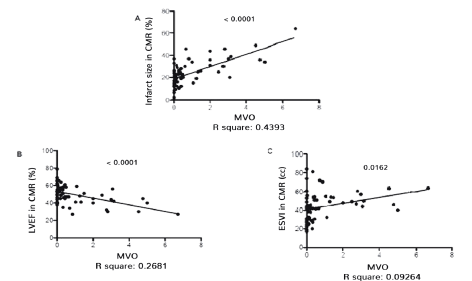
A statistically significant correlation has been observed between infarct size and mean MVO score (R2: 0,4893, p < 0,0001) (Figure 2) and between left ventricular end-systolic volume indexed to body surface area and MVO mean score (R2: 0,0162, p < 0,05). A significant inverse correlation was found between ejection fraction values and MVOs mean score (R2: 0,2681, p < 0,0001).
Discussion
Nowadays there is ongoing investigation focused on finding new markers that can help to determine prognosis posterior to an AMI, future complications and short and long-term mortality [2,3]. This is how CMR interpretation has become a relevant instrument to evaluate the cardiac morphology and functionality posterior to an AMI, since it is a tool capable of detecting early alterations and predicting future ones. This is mainly due to the high reproducibility and detailed cardiac description that it gives [4].
This is how the mere presence of a MVO in a patient who has suffered an AMI and was treated with reperfusion therapy, is a predictor of grim outcomes [6,7], such as higher mortality rates [11], and pathological heart remodeling in the next 6 months posterior to the AMI [12]. The pathophysiology underneath MVOs is complex and multifactorial [6,13,14], including microvascular dysfunction prior to reperfusion, ischemia reperfusion damage, distal embolization, and individual susceptibility. In a nutshell, MVOs represent a higher -myocardial damage.
Infarct size and MVOs
Infarct size can be estimated through different techniques, like ultrasound and positron emission tomography computed tomography (PET-CT). However, currently one the most validated ways to measure infarct size in clinical trials is CMR [15], since it is more accurate than ultrasound or clinical scores, and better at determining left ventricular ejection fraction [16]. Previously, studies have shown similar results, finding a positive correlation between MVO presence and infarct size [17]. This encourages the establishment of CMR as a guide for follow up in patients that have suffered an AMI.
Ventricular function and MVOs
CMR is a valid, strong and reproducible technique for assessed functional parameters as cardiac chambers volumes and LVEF [18]. Lower LVEF is an early marker of morbidity and mortality [7]. While ESVI correlates directly with pathological remodeling of the infarcted area [19]. A retrospective study of a clinical trial that used similar methodology as the present study, concluded a negative correlation between LVEF and MVOs at 90 days [20]. Nevertheless, these results are still controversial since other studies have not found similar associations [21]. Nijveldt R, et al. [7] found a statistically significant association between MVOs and lower LVEF but did not conclude an association between MVO and ESVI. On the other hand, a study with a low number of participants did not find a significant correlation between MVOs and LVEF [17].
Study limitations
Our study limitations include it being a retrospective study of a randomized controlled trial, in which, part of the group of patients studied were given antioxidant drugs, which limits the suitability of the study to obtain conclusions. Despite the above, other studies have evaluated similar parameters using a methodology equivalent to ours [20-22]. Another factor to consider is our exclusion criteria, that does not evaluate patients with bad prognosis, for example, patients with TMPG ≤ 2, and only one reperfusion therapy was evaluated (PCA), leaving behind other reperfusion techniques such as thrombolysis, and patients that presented with non ST Segment Elevation (NSTEMI) at the emergency department.
Our study concluded that presence of MVOs measured by CMR is an accurate predictor of functional and anatomic complications 1-2 weeks after STEMI. Specifically, patients who developed MVO had a larger infarct size, a lower left ventricular ejection fraction and end-systolic volume indexed to body surface area; markers that are associated with pathological cardiac remodeling. Some unresolved questions about different clinical scenarios require new prospective clinical studies that can provide evidence regarding MVOs and its clinical utility. Studying the close relationship between MVOs, infarct size, and ventricular heart function post AMI, could make MVOs a key factor that can be easily implemented in the clinical management of patients.
Acknowledgement
We would like to address the financial support from the grant FONDEF ID 15L10285 that made this study possible.
References
- Roth GA, Johnson C, Abajobir A, et al. (2017) Global, Regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 70: 1-25.
- Damman P, Beijk MA, Kuijt WJ, et al. (2011) Multiple biomarkers at admission significantly improve the prediction of mortality in patients undergoing primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. J Am Coll Cardiol 57: 29-36.
- Morrow DA, Antman EM, Charlesworth A, et al. (2000) TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 102: 2031-2037.
- Pontone G, Guaricci AI, Andreini D, et al. (2017) Prognostic stratification of patients with ST-Segment-elevation myocardial infarction (PROSPECT). Circ Cardiovasc Imaging 10: e006428.
- Perazzolo Marra M, Lima JA, Iliceto S (2011) MRI in acute myocardial infarction. Eur Heart J 32: 284-293.
- Niccoli G, Scalone G, Lerman A, et al. (2016) Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J 37: 1024-1033.
- Nijveldt R, Beek AM, Hirsch A, et al. (2008) Functional recovery after acute myocardial infarction: Comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol 52: 181-189.
- Ramos C, Brito R, Gonzalez-Montero J, et al. (2017) Effects of a novel ascorbate-based protocol on infarct size and ventricle function in acute myocardial infarction patients undergoing percutaneous coronary angioplasty. Arch Med Sci 13: 558-567.
- Hombach V, Grebe O, Merkle N, et al. (2005) Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J 26: 549-557
- Nijveldt R, Beek AM, Hofman MB, et al. (2007) Late gadolinium-enhanced cardiovascular magnetic resonance evaluation of infarct size and microvascular obstruction in optimally treated patients after acute myocardial infarction. J Cardiovasc Magn Reson 9: 765-770.
- Lombardo A, Niccoli G, Natale L, et al. (2012) Impact of microvascular obstruction and infarct size on left ventricular remodeling in reperfused myocardial infarction: a contrast-enhanced cardiac magnetic resonance imaging study. Int J Cardiovasc Imaging 28: 835-842.
- De Waha S, Desch S, Eitel I, et al. (2010) Impact of early vs. late microvascular obstruction assessed by magnetic resonance on long-term outcome after ST-elevation myocardial infarction: A comparison with traditional prognostic markers. Eur Heart J 31: 2660-2668.
- Niccoli G, Burzotta F, Galiuto L, Crea F (2009) Myocardial no-reflow in humans. J Am Coll Cardiol 54: 281-292.
- Niccoli G, Kharbanda RK, Crea F, et al. (2010) No-reflow: Again prevention is better than treatment. Eur Heart J 31:2 449-455.
- Ibanez B, Heusch G, Ovize M, et al. (2015) Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol 65: 1454-1471.
- Eitel I, de Waha S, Wohrle J, et al. (2014) Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol 64: 1217-1226.
- Yan A, Gibson C, Larose E, et al. (2006) Characterization of microvascular dysfunction after acute myocardial infarction by cardiovascular magnetic resonance first-pass perfusion and late gadolinium enhancement imaging. J Cardiovasc Magn Reson 8: 831-837.
- von Knobelsdorff-Brenkenhoff F, Schulz-Menger J (2012) Cardiovascular magnetic resonance imaging in ischemic heart disease. J Magn Reson Imaging 36: 20-38.
- Kim HW, Farzaneh-Far A, Kim RJ (2009) Cardiovascular magnetic resonance in patients with myocardial infarction. J Am Coll Cardiol 55: 1-16.
- Ezekowitz JA, Armstrong PW, Granger CB, et al. (2010) Predicting chronic left ventricular dysfunction 90 days after ST-segment elevation myocardial infarction: An Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) Substudy. Am Heart J 160: 272-278.
- Grover S, Bell G, Lincoff M, Jeorg L, et al. (2015) Utility of CMR markers of myocardial injury in predicting LV functional recovery: results from PROTECTION AMI CMR Sub-study. Heart Lung Circ 24: 891-897.
- Stiermaier T, Jobs A, de Waha S, et al. (2017) Optimized prognosis assessment in ST-Segment-Elevation myocardial infarction using a cardiac magnetic resonance imaging risk score. Circ Cardiovasc Imaging 10: e006774.
Corresponding Author
Ramón Rodrigo, Faculty of Medicine, Molecular and Clinical Pharmacology Program, University of Chile, Independencia 1027, PC 8380453, Santiago, Chile, Phone: +56 2 229786126
Copyright
© 2021 Valenzuela G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.